ACE INHIBITION AND ANTIOXIDANT ACTIVITIES OF
COLLAGEN HYDROLYSATE FROM SKIN OF
SNAKEHEAD FISH (Channa striata)
SILVIE FEBRIANA WIBAWA
POSTGRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT LETTER OF THESIS AND SOURCE OF
INFORMATION
I declare that this thesis entitled ACE Inhibition and Antioxidant Activities of Collagen Hydrolysate from Skin of Snakehead Fish (Channa striata) is my own work with guidance of the advisors and has not been submitted in any form at any college, except Bogor Agricultural University. Sources of information derived and quoted from published and unpublished works of other authors mentioned in the text are listed in the Bibliography at the end of this thesis.
Hereby I transfer the copyright of this thesis to Bogor Agricultural University.
Bogor, September 2015
Silvie Febriana Wibawa
SUMMARY
SILVIE FEBRIANA WIBAWA. ACE Inhibition and Antioxidant Activities of Collagen Hydrolysate from Skin of Snakehead Fish (Channa striata). Supervised by MAGGY THENAWIDJAJA SUHARTONO and PUSPO EDI GIRIWONO.
Snakehead fish (Channa striata) is one of fresh water fish consumed by Asian people to maintain good health due to its high content of protein, essential fatty acids and minerals. The skin of this fish which contains collagen (approximatelly 16.57 %) is a byproduct of albumin supplement production. Thus, it is potential to become a collagen source which is used widely in food and other needs including for producing bioactive peptides. The aims of this research were to produce peptides with Angiotensin-I Converting Enzyme (ACE) inhibition and antioxidant activities from snakehead fish by using collagenase from local collagenolytic bacteria such as Bacillus licheniformis F11.4 (isolated from shrimp waste in Palembang) and Stenotrophomonas sp. (isolated from red oncom in from both bacteria used produced peptides with molecular weights of 18-267 kDa. Semi-purified Stenotrophomonas sp. collagenase generated peptides with lower molecular weights (14-152 kDa) than peptides generated from semi-purified
Bacillus licheniformis F11.4 collagenase (15-267 kDa). Stenotrophomonas sp.
collagenase was able to hydrolyze -chain of collagen. Collagen hydrolysates had better ACE inhibition and antioxidant activities that collagen. Stenotrophomonas
sp. collagenase produced collagen peptides that have higher ACE inhibition and antioxidant activities than peptides generated by Bacillus licheniformis F11.4 Collagenase. Collagen hydrolysate generated from Stenotrophomonas sp. crude collagenase after 90 minutes of incubation had the highest ACE inhibitory (13.32 % inhibition/µg protein) effect and reducing power among the other collagen hydrolysate. In addition, collagen hydrolysate generated from semi-purified
Stenotrophomonas sp. collagenase after 90 minutes of incubation showed the highest ability to scavenge free radical such as DPPH (73.22 % inhibition/50µg protein). Based on this data, this collagen hydrolysate may become potential for developing medicine or functional food for the alleviation of hypertension.
RINGKASAN
SILVIE FEBRIANA WIBAWA. Hidrolisat Kolagen dari Kulit Ikan Gabus (Channa striata) yang Menunjukkan Aktivitas Penghambatan ACE Antioksidan. Dibimbing oleh MAGGY THENAWIDJAJA SUHARTONO dan PUSPO EDI GIRIWONO.
Ikan gabus merupakan salah satu ikan air tawar yang sering dikonsumsi oleh orang Asia untuk menjaga kesehatan akibat tingginya kandungan protein, asam lemak esensial, dan mineral pada ikan tersebut. Kulit ikan gabus yang mengandung sekitar 16.57 % kolagen merupakan limbah dari produksi suplemen albumin. Oleh karena itu, kulit ikan gabus berpotensi menjadi sumber kolagen sehingga kolagennya dapat digunakan di industri pangan maupun non-pangan termasuk untuk memproduksi peptida bioaktif. Tujuan dari penelitian ini adalah untuk memproduksi peptida dengan aktivitas penghambatan Angiotensin-I Converting Enzyme (ACE) dan antioksidan dari ikan gabus dengan menggunakan kolagenase dari Bacillus licheniformis F11.4 (diisolasi dari limbah kulit udang di Palembang) dan Stenotrophomonas sp. (diisolasi dari oncom merah di Bogor).
Konsentrasi protein dari kolagen larut asam yang diekstrak dari kulit ikan gabus adalah 0.4 mg/mL. Analisis SDS-PAGE menunjukkan bahwa kolagen yang ini dapat dikategorikan menjadi kolagen tipe I ini mengandung rantai , α1, αβ dengan berat molekul 267, 152, 136 kDa berturut-turut. Kolagenase kasar dari kedua bakteri yang digunakan mampu menghasilkan peptida dengan berat molekul 18-267 kDa. Kolagenase semi-murni dari Stenotrophomonas sp. Menghasilkan peptida dengan berat molekul yang lebih rendah (14-152 kDa) dibandingkan peptida yang dihasilkan oleh kolagenase semi-murni dari Bacillus licheniformis F11.4 (15-267 kDa). Kolagenase Stenotrophomonas sp. Mampu menghidrolisis rantai pada kolagen. Kolagen hidrolisat menunjukkan aktivitas penghambatan ACE dan antioksidan yang lebih baik daripada kolagen. Kolagenase dari Stenotrophomonas sp. Mampu memproduksi peptida kolagen dengan aktivitas penghambatan ACE dan antioksidan yang lebih tinggi dibandingkan peptida yang dihasilkan oleh kolagenase dari Bacillus licheniformis
F11.4. Hidrolisat kolagen yang dihasilkan oleh kolagenase kasar dari
Stenotrophomonas sp. setelah diinkubasi selama 90 menit inkubasi menunjukkan aktivitas penghambatan ACE (13.32 % penghambatan/µg protein) dan kekuatan mereduksi yang paling baik diantara hidrolisat kolagen lainnya. Selain itu, hidrolisat kolagen yang diproduksi oleh kolagenase semi-murni dari
Stenotrophomonas sp. setelah diinkubasi selama 90 menit menunjukkan kemampuan menetralkan radikal bebas DPPH yang paling tinggi (73.22 % penghambatan/50µg protein). Berdasarkan data penelitian ini, kolagen hidrolisat ini berpotensi untuk dikembangkan menjadi obat atau pangan fungsional untuk penderita penyakit darah tinggi.
© Hak Cipta Milik IPB, Tahun 2015
Hak Cipta Dilindungi Undang-Undang
Dilarang mengutip sebagian atau seluruh karya tulis ini tanpa mencantumkan atau menyebutkan sumbernya. Pengutipan hanya untuk kepentingan pendidikan, penelitian, penulisan karya ilmiah, penyusunan laporan, penulisan kritik, atau tinjauan suatu masalah; dan pengutipan tersebut tidak merugikan kepentingan IPB
Thesis
in the partial fulfillment of the requirement for degree of Master of Science
at
Food Science Master Program
ACE INHIBITION AND ANTIOXIDANT ACTIVITIES OF
COLLAGEN HYDROLYSATE FROM SKIN OF
SNAKEHEAD FISH (Channa striata)
POSTGRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
Title : ACE Inhibition and Antioxidant Activities of Collagen Hydrolysate from Skin of Snakehead Fish (Channa striata)
Name : Silvie Febriana Wibawa Student ID : F251140666
Approved by Advisory Committee
Date of Thesis Examination: September 17th, 2015
PREFACE
Praise to The Lord Jesus Christ for His mercy, blessings, and guidance
throughtout the research and finishing this thesis. The research entitled “ACE inhibition and antioxidant activities of collagen hydrolysate from skin of snakehead fish (Channa striata)” was carried out in Bogor Agricultural University
from September 2014 until April 2015.
By completion of this research and thesis, the author would like to express great appreciation and sincere thanks to Prof. Dr. Ir. Maggy T. Suhartono and Puspo Edi Giriwono, PhD as supervisors for their guidances, help, and advices during completing this research and thesis. The author would like to express her thanks to her examiner in thesis examination, Dr. Siti Nurjana STP, MSi, for her time, corrections, and input in improving this thesis.
Sincere thankes is also expressed to Dean of Bogor Agricultural University Postgraduate School and his staff, Head of Food Science Master Program and her staff, Bu Ika Malikha as laboratory technician in Laboratory of Microbiology and Biochemistry PAU IPB, and friends from IPN 2013 and 2014. Thanks to Nesya, Mutiara Pratiwi, Florentina, Kak Diana, Mbak Ino, Kak Novan, and Kak Wenny for the support, advice, and help during the study and completing this research. Thanks to DIKTI for supporting the study through fresh graduate scholarship program.
Last but not least, I would like to say a big thanks to her parents and sister for their support during the study of Food Science, the reseach, and thesis completion. Hopefully this thesis is useful for the readers and gives a contribution in food science development.
Bogor, September 2015
TABLE OF CONTENT
LIST OF TABLES xii
LIST OF FIGURES xiii
LIST OF APPENDICES xiv
1 INTRODUCTION 1
Background 1
Problems 2
Objectives 2
Significance 2
2 LITERATURE STUDY 3
Snakehead fish (Channa striata) 3
Collagen 4
Collagenase and Collagenolytic Bacteria 6
Bioactive Peptides 8
3 RESEARCH METHODOLOGY 13
Time and Place 13
Material and Instruments 13
Methods 13
Statistical Analysis 18
4 RESULT AND DISCUSSION 19
Collagen and Collagen Hydrolysate Profile 19
Inhibition of Angiotensin-I Converting Enzyme (ACE) 22
Antioxidant Activity 24
5 CONCLUSION AND RECOMMENDATION 28
Conclusion 28
Recommendation 28
Acknowledgement 28
BIBLIOGRAPHY 29
APPENDICES 36
LIST OF TABLES
1. Table 1 Peptides with ACE inhibition derived from fish 10
2. Table 2 Peptides with antioxidant activities derived from fish 12
3. Table 3 Protein concentration and percentage of peptides produced of
collagen and its hydrolysates 20
4. Table 4 ACE inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis (F11.4) and Stenotrophomonas sp. (Isolate 11), n=2, different letters indicate significance at p<0.05 22
5. Table 5 DPPH inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis (F11.4) and Stenotrophomonas sp. (Isolate 11), ), n=2, different letters indicate significance at p<0.05 24
LIST OF FIGURES
1. Figure 1 Snakehead fish (Channa striata) 3
2. Figure 2 Collagen structure (a) polypeptide chain (α-chain) (b) triple
helix. -chain is pointed by red arrow (c) tropocollagen unites assembled
to form collagen fibril. - chain is pointed by red arrow (Beeley 2000) 5
3. Figure 3 Angiotensin-I Converting Enzyme (ACE) mechanism of
hydrolysis (Fort 2014) 9
4. Figure 4 Captopril structure and compability to ACE active site
(Horrocks 2015) 9
5. Figure 5 Research flow chart 14
6. Figure 6 SDS PAGE of ASC from skin of snakehead fish and its hydrolysates (A) High molecular weight marker (B) Low molecular weight marker (C) Collagen from skin of snakehead fish (D) Collagen hydrolysate by crude Bacillus licheniformis F11.4 collagenase (E) Collagen hydrolysate by semi-purified Bacillus licheniformis F11.4 collagenase (F) Collagen hydrolysate by crude Stenotrophomonas sp. collagenase (G) Collagen hydrolysate by semi-purified
Stenotrophomonas sp. collagenase. The time of hydrolyis (γ0’, 60’, 90’)
were indicated 20
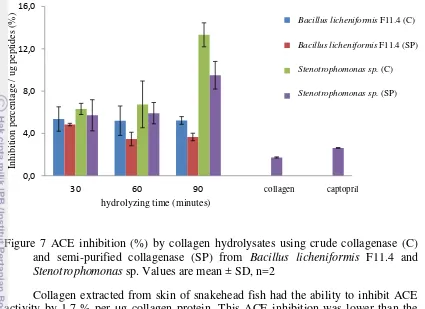
7. Figure 7 ACE inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis F11.4 and Stenotrophomonas sp. Values are mean ± SD,
n=2 23
8. Figure 8 DPPH inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis F11.4 and Stenotrophomonas sp. Values are mean ± SD,
n=2 25
9. Figure 9 Reducing power abilities of collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis F11.4 and Stenotrophomonas sp. Values are mean ± SD,
LIST OF APPENDICES
1. Appendix 1 Reagents for Measuring Enzyme Activity 36
2. Appendix 2 Standar Curve of Bovine Serum Albumin (BSA) 37
3. Appendix 3 Reagents for Bradford Assay 38
4. Appendix 4 Reagents for SDS-PAGE Analysis 39
5. Appendix 5 Composition of Separating and Stacking Gels for
SDS-PAGE Analysis 40
6. Appendix 6 Standard Molecular Weight for SDS-PAGE Analysis 41
7. Appendix 7 Reagents for Measuring Inhibition of Angiotensin-I
Converting Enzyme (ACE) 42
8. Appendix 8 Reagents for Measuring Antioxidant Activity (DPPH
Method) 43
9. Appendix 9 Reagents for Measuring Reducing Power (Fe Reducing
Assay) 44
10.Appendix 10 Statistical Analysis for ACE Inhibitor Data 45
11.Appendix 11 Statistical Analysis for Antioxidant Data (DPPH Method) 46
12.Appendix 12 Statistical Analysis for Antioxidant Activities Data (Fe
1
INTRODUCTION
Background
Snakehead fish (Channa striata) is one of many fresh water fish that is popular among Asia population. This fish is sold in fresh condition and become a main ingredient in processed food in Asia. In South East Asia, people believe that snakehead fish contains essential nutrients such as protein, essential fatty acids, and minerals to maintain good health and recover the lost energy after prolonged illness. Thus, this fish is usually consumed by post-surgery patient to promote wound healing and alleviate post-operative pain, discomfort, and trauma (Lay et al. 2006). The flesh of this fish has already been used commercially as medicine for wound healing due its high content of albumin. The skin (approximately 4 % in total weight of fish) has limited use and becomes waste. The global production of snakehead fish has reached 75.000 tonnes at the end of 2010 (FAO 2011).
Skin of snakehead fish as a waste material can still be utilized as an aternative source of collagen since the skin contains approximately 16.57 % of collagen (See et al. 2010). Collagen is a structural protein which can be found in connective tissues, bone, skin, and animal muscle. Collagen has been used widely in food and non-food industries, cosmetics industries, and for several medical purposes. Collagen derived from skin fish might become a raw material for producing bioactive peptides.
Peptides derived from protein hydrolysis possess bioactivities beneficial for human health and medication. Several researches show that fish protein hydrolysate has functional properties such as antihypertensive, antioxidant, anticancer, and antimicrobial. The fish protein hydrolysate containing peptides can be developed for treatment and medication for degenerative diseases. Proteases from plant, animal, and bacteria have been used widely for producing bioactive peptides.
Data from Indonesian Society of Hypertension shows that the number of hypertension patients in the world reaches 972 millions people in 2000 and it is predicted to increase to 1.56 billion people in 2025 (Indonesian Society of Hypertension 2014). The increasing number of patients with degenerative diseases such as hypertension increases the demand of medicines including the medicines based on bioactive peptides. Information related to bioactive properties of peptides derived from fishes that are easily found in Indonesia is thus very important.
Problems
Fish skin such as skin of snakehead fish (constituted of 4 % of total body weight) is marine waste. Fish skin contains 16.57 % of collagen and thus become potential source of applicative collagen and its derivatives. Collagen can be widely used as food ingredient and medicines due to its bioactivities. Bioactive peptides in collagen hydrolysate can be produced enzymatically by using proteases from collagenolytic bacteria such as Bacillus licheniformis F11.4 isolated from shrimp waste and Stenotrophomonas sp. from Indonesian red oncom. Bioactive peptides can be used for producing functional food and medicines which are needed to overcome degenerative diseases.
Objectives
The objective of this research were to produce peptides with Angiotensin-I Converting Enzyme (ACE) inhibition and antioxidant activities from snakehead fish (Channa striata) by using collagenase from Bacillus licheniformis F11.4 and
Stenotrophomonas sp.
Significance
2
LITERATURE STUDY
Snakehead fish (Channa striata)
Snakehead fish is a fresh water fish which can be found in Sri Lanka, Indonesia, Philipines, and China. This fish has an ability to survive during dry season by hiding in the mud of lake, canal, and swamp. In Indonesia, snakehead fish can be found in Sumatra, Java, Bali, Sulawesi, Lombok, Singkep, Flores, Ambon, and Maluku. Data from Food and Agriculture Organization (FAO) shows that global productivity of snakehead fish increases every year and rearches 75.000 tonnes at the end of 2010. Snakehead fish is sold in fresh condition. Besides, processed food from snakehead fish as the main ingredient is also sold in Asia (FAO 2011).
The moisture, carbohydrate, lipid, and protein content of snakehead fish are 78%, 2.2 %, 2.3 %, and 16.2 % consecutively (Wimalasena & Jayasuriya 1996). Percentage of fish skin weight to fish weight is 4 %. Snakehead fish skin contains 36.79 % moisture, 19.26 % protein, 4.21 % lipid and 0.55 % ash. This skin fish also contains 16.57 % of collagen. The isoionic point of this collagen is reported as 9.64 (See et al. 2010). Snakehead fish also has higher content of several minerals such as potassium, sodium, calcium, phosphorus, and iron than other freshwater fish like catfish and nile (Wimalasena & Jayasuriya 1996). Protein of snakehead fish consists of fibrillar and globular protein. Several fibrillar protein that build the fish skin are collagen, keratin, and elastin while albumin and globulin are globular protein which can also be found in the skin. This fish is recently used as medicine for wound healing due to high content of albumin (13.83 – 20.80 %) and minerals in its flesh (Asfar et al. 2014.). Besides, snakehead fish is also consumed by post surgery patients to accelerate wound healing (Lay et al. 2006).
Figure 1 Snakehead fish (Channa striata)
part from skin of snakehead fish also contains protein such as lysozyme, protease, alkaline phosphatase, dan erastase which have antimicrobe properties (Loganathan et al. 2013).
Collagen
Collagen is an extracellular protein which has important role to maintain structural integrity and various physiological function in animal tissue due to fibrillar structure that form extracellular scaffolding. The main characteristic of protein which are grouped as collagen is the presence of three polypeptides in the form of triple helical structure. Collagen contains domain with repetitive of proline rich tripeptides, involved in the formation of its triple helical structure. Glycin and proline are domain amino acids in collagen while the presence of hydroxyproline is also important to form collagen backbone. There are 26 genetically distinct collagen types which have been described. Based on their structure and supramolecular organization, collagens are grouped into fibril-forming collagen, fibril-associated collagen (FACIT), network-forming collagen, anchoring fibrils, transmembrane collagen, basement collagens, and others with unique functions (Gelse et al. 2003).
Type I collagen is a fibril forming collagen which can be found in connective tissue such as tendon, bones, and skin. This collagen contains two α1 polypeptide
Figure 2 Collagen structure (a) polypeptide chain (α-chain) (b) triple helix. β -chain is pointed by red arrow (c) tropocollagen unites assembled to form collagen
fibril. - chain is pointed by red arrow (Beeley 2000)
Collagen is commercially extracted from skin and bones of bovine and pigs. Beside, collagen can also be extracted from marine waste such as fish skin and bones. Research conducted by Nagai & Suzuki (2000) revealed than fish skin and bones contain 50 % and 40-50 % collagen consecutively in dry bases. Several parts of mollusc also contain collagen (Singh et al. 2011). Collagen percentage in fish skin is variying due to the differences of fish species and extraction methods. Skin of
Sepiella inermis contains 16.23 % pepsin soluble collagen and 0.58 % acid soluble collagen (Shanmugam et al. 2012) while skin of catfish (Pangasianodon hypophthalmus) contains 7.7 % pepsin soluble collagen and 5.1% acid soluble collagen (Singh et al. 2011).
extraction and concentration of organic acid solution used for extracting collagen influence the yield of collagen. The yield of collagen increased with the increasing concentration of acetic acid solution and reach optimum yield of collagen by using 0.5 M acetic acid. Longer time of extracting also increases the yield of collagen where higher yield of collagen is obtained after extracting for at least 24 hours (Wang
et al. 2008). Pepsin is used for producing pepsin soluble collagen to cleave cross-linked molecules at telopeptide region resulting in higher solubility of collagen (Nalinanon et al. 2007). The yield of pepsin soluble collagen is higher than the acid soluble collagen. However, using pepsin can cleave collagen during the extraction and storage (Mocan et al. 2011). In this research, collagen will be extracted using acid solution to produce higher yield of non-hydrolyzed collagen.
In the body, collagen has important roles in angiogenesis, tissue scaffolding, tissue morphogenesis, tissue repair (Kadler et al. 2007), cell proliferaton, cell adhesion, and cell migration due to its unique conformation and ability to form agregates (Talwar & Srivastava 2002). Collagen has been used widely in leather and film industries, cosmetics, biomedical material, and food (Ogawa et al. 2004; Kittiphattanabawon et al. 2005). For medical purpose, collagen are used for wound dressing and skin formation (Holmes et al. 2013), vitreous implants (Michael et al. 1971), cardiac catheterization and coronary angioplasty (Eggebretch et al. 2002), tissue repair after surgery (Sullivan et al. 2008) and as carriers for drugs deliveries (Sahithi et al. 2013).
Collagen hydroysates have several functions in human body. Collagen peptides has been commercially used for reducing eye wrinkle formation by stimulating procollagen I, elastin, and fibrillin biosynthesis without giving side effect after 8 weeks of oral consumption (Proksch et al. 2013). Collagen from skin of Allaska polack (Teragra chacogramma) has high ACE inhibition after it was hydrolyzed by alcalase, pronase E, and colllagenase (Byun & Kim 2001). Collagen peptides from skin of tuna and squid with range molecular weight of 1-10 kDa and <1 kDa show antimicrobial activities against Lactobacillus acidophilus, Bifidobacterium animalis
subp. Lactis, Shewanella putrefaciens, and Photobacterium phosphoreum (Gomez-Guillen et al. 2010). Peptides isolated from hoki (Johnius belengerri) skin gelatin capable to enhace the expression of antioxidative enzymes such as glutathione peroxidase, catalase, and superoxide dismutase in human hepatoma cells (Mendis et al. 2005).
Collagenase and Collagenolytic Bacteria
Collagenase is a protease which is produced by animals and several bacteria such as Bacillus cereus and Klebsiella pneumoniae (Suphatharaprateep et al. 2011),
Streptomyces (Jain & Jain 2010), Pseudomonas aeruginnosa (Diener et al. 1973), and
Based in its structure, collagenases are grouped into type I collagenase, type II collagenase, and type III collagenase. These three types of collagenase break triple helical structure of collagen into ¾ and ¼ fragments. Type I collagenase is classic interstitial collagenase, type II collagenase is neutrophile collagenase and type III collagenase is interstitial collagenase from rodent (Rawlings & Salvesen 2013). Collagenase is categorized as Matrix Metalloproteinase (MMP-1, MMP-8, and MMP-13) since the enzyme contains zinc and is active in the presence of calcium (Biljana et al. 2011). MMP is excreted in latent form and its activity is regulated by several inhibitors such as tissue inhibitor metalloproteinases (TIMPs) (Patricia 2005). In this research, collagenases used are produced by Bacillus licheniformis F11.4 and
Stenotrophomonas sp.
Bacillus licheniformis F11.4 is a proteolytic bacteria from Badan Pengkajian dan Penerapan Teknologi (BPPT). This bacteria was isolated from shrimp skeleton in Palembang, Indonesia. The colony of F11.4 isolate has rough surface. Biochemistry characteristics and physical analysis of this bacteria by using API 50 CB, oxidase, catalase, and Voges Proskaues test show that this isolate found is Bacillus licheniformis. In addition, other genetic analysis showed that this isolate has the same loccus with Bacillus licheniformis but lack of chiA. Thus, Bacillus licheniformis F11 does not have an ability to produce chitinase, has potency to deproteinate shrimp waste, and has high protease activity (Waldeck et al. 2006). Bacillus licheniformis
F11.4 is a mutant from Bacillus licheniformis F11 which lacks of forming
polygutamate (∆pga) and chiB operons. This bacteria is lack of chiA due to natural
mutation. Thus, this mutant bacteria has been reported to possess collagenase activity. The molecular weights of its collagenases are 124 and 26 kDa when the bacteria was grown in Luria Bertani (LB) broth with 5 % collagen. The protease shows high activity at pH 6-8 and 50 oC. Activity of this protease is affected of several ions. The presence of Cu2+ and Ca2+ at concertration of 0.001 M enhanced enzyme activity while Fe2+, Co2+, Mg2+, Mn2+, and EDTA at similar concentration decreased the enzyme activity. Km and Vmax value of this protease were 0.26 mg/ml and 0.27 U consecutively (Baehaki et al. 2012).
Stenotrophomonas sp. used for this research was isolated from red oncom in Bogor (Diana Nur Afifah). Stenotrophomonas bacteria is an aerobic gram negative bacteria which is easily found in soil. This bacteria has important role in nitrogen and sulfur cycles that very useful for plant growth (Ryan 2009). One of species of
Stenotrophomonas bacteria, Stenotrophomonas maltophilia, is a pathogenic bacteria that cause infection on respiratory tract and blood stream (Looney 2009).
nonpathogenic bacteria. The safety of this isolate has been studied by using cell culture (Nailufar, personal communication)
Bioactive Peptides
Protein is known as a food component with nutritional and functional properties. The nutrition properties relate to its amino acid composition. Besides, the functional properties of protein relate to their contribution to the physiochemical and sensory properties of food. Bioactive peptides are food derived components which have physiological effects in the body besides their nutritional value. These peptides are usually consisted of 2-20 amino acids. Bioactive peptides can be obtained from meat and fish by using protease from bacteria, animal, and plants (Ryan et al. 2011). Sources of bioactive peptides are usually protein with one or all of these criterias: 1) a protein-rich food industry byproducts and 2) a protein with specific peptides sequences or amino acid residues that become a particular pharmacological interests (Udeniqwe & Alluko 2012).
In the body, bioactive peptides are absorbed through the intestine and enter the circulatory system intact to exert their physiological effects. Bioavailability of bioactive peptides depends on their physiochemical properties such as charge, molecular size, lipophilicity, and solubility. In addition, bioactive peptides must show stability against gastrointerstinal proteases and peptidases in order to keep their bioactivities. Thus, microencapsulation is usually used to maintain their stability and enhance their absorption. Bioactive peptide have been proven not to exert adverse effect on clinical (blood biochemical, hematology, organ, weight ratios, and hispathological) parameter or mortality in rats (Udeniqwe and Aluko 2012). Several studies revealed that bioactive peptides have physiological functions including antihypertensive, antioxidant, opioid antagonistic, immunomodulatory, antimicrobial, prebiotic, mineral binding, antithrombotic, and hypocholesterolemic effects (Ryan et al. 2011). In this research, bioactive peptides will be examined as antihypertensive and antioxidant.
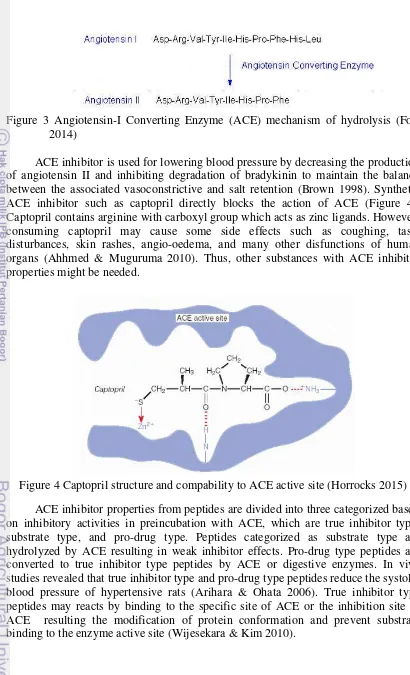
Angiotensin-I Converting Enzyme (ACE) is an enzyme which catalyzes the convertion of angiotensin I into angiotension II by cleaving the C-terminal dipeptide from angiotensin-I (His-Leu). Angiotension-II is a potent vasocontrictor which affects vascular smooth muscle cells directly. Besides, angiotensin-II also plays important role for the expansion of vascular volume via sodium and fluid retention. The combination effects by the presence of angiotensin-II which are vasocontriction and expansion of vascular volume sinergistically increases the blood pressure. ACE also hydrolyzes bradykinin which responsible for uterine and ileal smooth muscle constrictions, enhanced vascular permeability, activation of peripheral, and vasodilatation by advancing the assembly of arrachidonic acid metabolites, nitrit oxide, and endothelium-derived hyperpolazing factor in vascular endothelium (Ryan
Figure 3 Angiotensin-I Converting Enzyme (ACE) mechanism of hydrolysis (Fort 2014)
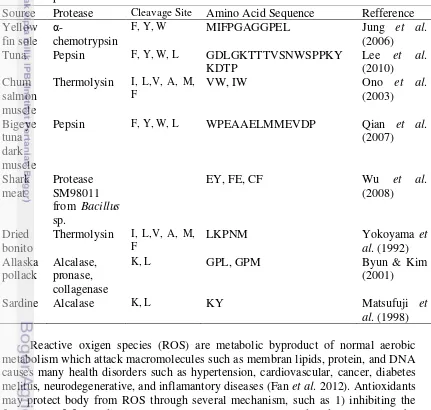
ACE inhibitor is used for lowering blood pressure by decreasing the production of angiotensin II and inhibiting degradation of bradykinin to maintain the balance between the associated vasoconstrictive and salt retention (Brown 1998). Synthetic ACE inhibitor such as captopril directly blocks the action of ACE (Figure 4). Captopril contains arginine with carboxyl group which acts as zinc ligands. However, consuming captopril may cause some side effects such as coughing, taste disturbances, skin rashes, angio-oedema, and many other disfunctions of human organs (Ahhmed & Muguruma 2010). Thus, other substances with ACE inhibitor properties might be needed.
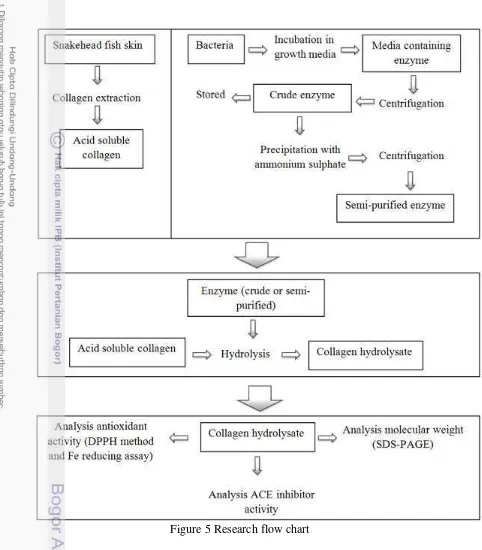
ACE inhibitions from peptides are associated with their structure. Peptides with hydrophobic amino acid residues at C-terminal end are preferable to have ACE inhibition because these residues have crucial role in competitive binding to the active site (Ryan et al. 2011). Other studies also revealed that aliphatic, basic and aromatic residues at the penultimate positions of peptides may interact with the three hydrophobic sub-sites located in the active site of ACE to block the active site of the enzyme. Several researches revealed that fish peptides have antihypertensive properties (Table 1).
Table 1 Peptides with ACE inhibition derived from fish
Source Protease Cleavage Site Amino Acid Sequence Refference Yellow
Tuna Pepsin F, Y, W, L GDLGKTTTVSNWSPPKY
and adduct volatile aldehydes to alter the development of rancidity in saturated oils (Rustad 2011).
Antioxidants also contribute to prevent hypertension by controlling the amount of ROS in the body. ROS have abilities to damage endothelium (Vasdev et al. 2006) and oxidize lipid that cause artherosclerosis (Beg et al. 2011). In the body, ROS are generated from various ways such as an electron leakage in electron transport chain in mitochondria, products from reactions which are catalyzed by NADPH oxidase and uncoupled nitric oxide synthase. Oxidation of Low Density Lipoprotein (LDL) by ROS stimulates the expresion of adhesion molecules and inflammatory cytokines, resulting in platelet aggregation and monocyte adhesion to the endotelium. Oxidized LDL is also acted as ligands to scavenger receptor of macrophages, stimulating LDL uptake and the formation of foam cells which lead to plaque formation. Plaque formation may thicken the cell wall and make vessel loss its elasticity resulting alteration of blood flow that leads to hypertension (Vasdev et al. 2006). Antioxidant is important to control the amount of ROS in the body.
Table 2 Peptides with antioxidant activities derived from fish Source Protease Cleavage Site Amino Acid
Sequence
Catfish Protamex Theodore &
3
RESEARCH METHODOLOGY
Time and Place
This research was conducted from September 2014 until April 2015 in Microbiology and Biochemistry Laboratory, Biological Research Center and Biotechnology, Bogor Agricultural University.
Material and Instruments
Collagen was extracted from skin of snakehead fish. The fish was obtained from traditional markets in Jakarta. Bacterial used for producing collagenase were
Bacillus licheniformis F11.4 and Stenotrophomonas sp. (Isolate 11). Bacillus licheniformis used was from bacterial collection of Microbiology and Biochemistry Laboratory PAU IPB. Stenotrophomonas sp. used which was isolated from Indonesian fermented food, red oncom, was collection of Diana Nur Afifah. These bacterias were grown in Luria Bertani broth (LB) (Oxoid, USA). Chemicals used were in analytical grade. High molecular weight (300-40 kDa) and low molecular weight (40-5 kDa) protein marker from Thermoscientific were used in SDS-PAGE. Bovine Serum Albumine (BSA) and ascorbic acid were used in protein concentration determination and antioxidant activity measurements consecutively.
Instruments used for this research were centrifuge (Tomy Seiko; model MRX-152; Japan), spectrophotometer visible (Novaspec II, Pharmacia), spectrophotometer UV-VIS (Shimadzu, model UV 2100, Japan), incubator (model Certoma WR), waterbath shaker, pH meter, and SDS PAGE apparatus (Bio-Rad Model 1000/500).
Methods
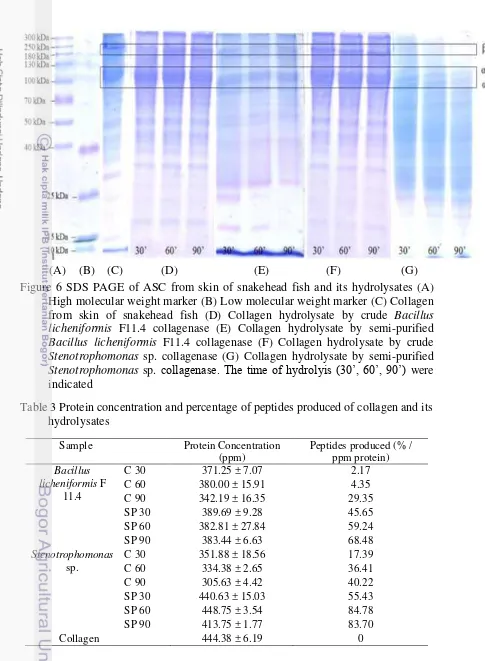
This research was divided into 3 parts, which are 1) Collagen extraction from snakehead fish and production of collagenase from Bacillus licheniformis F11.4 and
Figure 5 Research flow chart Collagen Extraction (Singh et al. 2011)
hours, then it was filtered using double cheesecloth. The aqueous part which contains collagen was precipitated by adding NaCl powder until reaching 2.6 M in the presence of 0.05 M Tris (hydroxymethyl) aminomethane pH 7 then it was centrifuged 4150 g for 1 hour. The pellet was dissolved in 0.5 M acetic acid solution and dialyzed (MCO: 2 kDa) using 0.1 M acetic acid solution and aquadest. All processes were carried out at 4 oC with continuous stirring.
Production and Precipitation of Collagenase from Bacillus licheniformis F11.4 and Stenotrophomonas sp.
Media used for producing collagenase were ½ Luria Bertani Broth (LB) with 5% collagen for Bacillus licheniformis F11.4 and ½ Luria Bertani Broth (LB) with 15% collagen for Stenotrophomonas sp. Bacteria was inoculated first to Luria Bertani Broth and incubated for 24 hours in 37 oC. Then, 10 % (v/v) of LB broth was added to production media and they were incubated for 40 hours in 37oC with 120 rpm agitation. By the end of incubation, the media was centrifuged at 4150 g for 15 minutes at 4 oC to produces cell free supernatant containing collagenase enzyme. Collagenase obtained from this processes was used as crude collagenase. Enzyme was precipitated by adding 50 % (w/v) ammonium sulfate salt to the media. The media was stirred and left overnight. Enzyme was collected by centrifuging the media at 7500 rpm for 30 minutes at 4 oC. Pelet collected was dissolved in 0.02 M phosphate buffer pH 8.5 at ratio 1:1 (w/v). Collagenase obtained from this processes was used as semi-purified collagenase.
Enzyme Activity (Bergmeyer 1983)
Enzyme activity was measured by using modified Bergmeyer Methods (1983). Substrate used was 5 % collagen and the standard was 5 mM tyrosin solution. Fifty µL of enzyme was mixed with 250 µL 0.05 M phosphate buffer pH 8 and 250 µL substrate. The mixture was incubated for 10 minutes at 45 oC (for collagenase from
Bacillus licheniformis F11.4) or 37 oC (for collagenase from Stenotrophomonas sp. Then, 500 µL 0.1 M TCA was added to stop the hydrolyzing process and the solution was incubated again at 37 oC for 10 minutes. After the second incubation, the mixture was centrifuged at 4150 g for 10 minutes at 4 oC. Then, 375 µL of filtrate was taken and mixed with 1250 µL 0.4M Na2CO3 and 250 µL folin ciocalteau at dilution ratio 1:2 (v/v). The mixture was incubated for 20 minutes at 37 oC and the absorbance was measured at 578 nm. Composition of reagents for measuring enzyme activity is listed in Appendix 1. Enzyme activity was calculated by using this following formula:
Protein Concentration (Bradford 1976)
Protein concentration was measured with Bradford assay. The protein standard was Bovine Serum Albumin (BSA) at concentration of 0-600 ppm (Appendix 3). Protein concentration was measured by mixing 50 µL samples (collagen, collagen peptides or enzyme) with 1250 µL Bradford reagent (0.35 g comassie brilliant blue G-250 in 95 % ethanol and 88 % phosphate acid) and 1250 µL aquadest. Then, the mixture was incubated for 2 minutes and the absorbance was measured at 595 nm. Composition of reagents for measuring protein concentration is listed in Appendix 3. Collagen Hydrolysate Preparation (Singh et al. 2011)
Collagen hydrolysates were made by mixing 6 mL acid soluble collagen with 1 mL 0.05 M phosphate buffer pH 8 (for collagenase from Bacillus licheniformis
F11.4) or pH 8.5 (for collagenase from Stenotrophomonas sp.). Then, 100 µL of crude of semi-purified enzymes was added to 300 µL of prepared mixture and it was incubated in 45 oC (for collagenase from Bacillus licheniformis F11.4) or 37 oC (for collagenase from Stenotrophomonas sp.) for 30, 60, and 90 minutes. Hydrolyzing process was stopped by heating the mixture in boiling water for 5 minutes. The collagen peptides were storaged in freezer (-20 oC).
SDS-polyacrylamide Gel Electrophoresis (SDS-PAGE) (Singh et al. 2011)
SDS-polyacrylamide Gel Electrophoresis (SDS-PAGE) was used for analyzing pattern of collagen and collagen hydrolysate. The samples were prepared by mixing 6 mL collagen (0.4 mg/mL) with 1 mL 0.1 M sodium phosphate pH 8 (for collagenase from Bacillus licheniformis F11.4) or pH 8.5 (for collagenase from
Stenotrophomonas sp.) which contained 0.5 % sodium dedocyl sulfate (SDS). The mixture was incubated at 45 oC for an hour. Then, 100 µL of crude or semi-purified collagenase is added to 300 µL of the prepared mixture and incubated at 45 oC (for collagenase from Bacillus licheniformis F11.4) or 37 oC (for collagenase from
Stenotrophomonas sp.) for 30, 60, and 120 minutes. By the end of incubation, 80 µL of sample was mixed with 20 µL sampel buffer SDS-PAGE and heated in boiling water for 5 minutes. The samples were injected into the gel which contained 10 % acrylamide for separating gel and 4 % acrylamide for stacking gel. Composition of gel electrophoresis was listed in Appendix 5. The electrophoresis process is run at 70 Volt and 50 mA for 4-5 hours using electrophoresis (Bio-Rad Model 1000/500). The gel was stained in staining solution for an hour and destained with mixture of 10 % methanol and 10 % acetic acid until the contrast blue bands shown. High molecular weight marker by Themoscientific was used to estimate the molecular weight of protein. Composition of reagents for SDS-PAGE is listed in Appendix 4. Quantitatif analysis of SDS PAGE was performed by using Gel Analyzer 2010.
Inhibition of Angiotensin-I Converting Enzyme (ACE) (Chusman & Cheung 1971)
buffer. The mixture was incubated at 37 oC for 5 minutes. Then, 50 µL ACE (25 mU/mL) was added and the mixture was incubated again at 37 oC for 30 minutes. Hydrolyzing process was stopped by adding 190 µL HCl (1 N) and 1140 µL of ethyl acetate was added to extract hippuric acid formed. The mixture was then vortexed for 20 seconds and centrifuged at 4150 g for 10 minutes. Supernatant (800 µL) was collected and dried in oven at 95 oC for an hour of until dried. Then 3000 µL of deionized water is added to dissolve the hipuric acid. Sampel absorbance was measured at 228 nm. Composition of reagents for measuring Inhibition of ACE is listed in Appendix 7. Inhibition of ACE was calculated using the following formula.
Ac : control absorbance
As : sampel absorbance Ab : blank absorbance
DPPH-radical Scavenging Activity (Wang et al. 2013)
Antioxidant properties from collagen peptides were examined by using DPPH-methods. Collagen peptide (2 mL) was mixed with 2 mL ethanol 99.5 % and 0.5 mL 0.2 % (w/v) DPPH solution. The mixture was incubated in dark room for 1 hours and the absorbance was measured on 517 nm. The control was conducted in the same manner, except distilled water was used to replace the sample. In blank, DPPH solution was subtituted by ethanol. Ascorbic acid with concentration 15 ppm was used for comparison. Composition of reagents for analyzing antioxidant activity is listed in Appendix 8. Antioxidant activities was calculated by using the following Fe-reducing Assay (Giri et al 2012)
Statistical Analysis
Experimental design used was factorial design with bacteria used and purity level of enzyme as the factors. Statistical differences of Angiotensin-I Converting Enzyme (ACE) inhibition and antioxidant properties between collagen hydrolysates and control used were examined by using one way analysis of variance (ANOVA) in 2 separate determinations. Significant differences between mean values were
determined by Duncan’s multiple range tests and accepted at p < 0.05 using SPSS
4
RESULT AND DISCUSSION
Collagen and Collagen Hydrolysate Profile
In this research, collagen from skin of snakehead fish was extracted by using acetic acid solution to produce acid soluble collagen (ASC). The yield of ASC produced was 0.4 % (w/w) with concentration of 0.444 ± 0.006 mg/mL. The low yield of ASC was caused by incomplete solubilisation of the fish skin in acetic acid solution. The solubility of collagen decreases by the presence of crosslinkings and intermolecular crosslinkings between collagen molecules (Singh et al 2011). The type of acid used to extract collagen also influences solubilization of collagen. The non-ionized acetic acid molecules act as swelling agent by cleaving hydrogen bonds on intermolecular linking of collagen and associate with the carboxyl group of peptide bond (Gomez-Guillen 2003). This collagen was then hydrolyzed by using collagenases from Bacillus licheniformis F11.4 and Stenotrophomonas sp. for 30, 60, 90 minutes. The collagen and collagen hydrolysate profiling by using SDS-PAGE were shown in Figure 6.
Collagen is a protein which has three polypeptides (α-chains) in triple helix form (Gelse et al. 2003). Figure 6A shows the pressence of α1 and αβ-chain with molecular weight of 152 and 136 kDa consecutively. This acid soluble collagen is
catagorized as type I collagen since it contained α1 and αβ-chain at ratio of approximately 2:1. Type I collagen were also extracted from skin of several fish such as tuna abdominal (Han et al. 2011), striped catfish (Singh et al. 2011), and brown stripe red snapper (Jongjareonrak et al. 2005). This collagen also contained -chain with molecular weight of approximately 267 kDa and other protein with molecular weight of 34-69 kDa. Beta chain indicates the presence of intermolecular
(A) (B) (C) (D) (E) (F) (G) Figure 6 SDS PAGE of ASC from skin of snakehead fish and its hydrolysates (A)
High molecular weight marker (B) Low molecular weight marker (C) Collagen from skin of snakehead fish (D) Collagen hydrolysate by crude Bacillus licheniformis F11.4 collagenase (E) Collagen hydrolysate by semi-purified
Bacillus licheniformis F11.4 collagenase (F) Collagen hydrolysate by crude
Stenotrophomonas sp. collagenase (G) Collagen hydrolysate by semi-purified
Stenotrophomonas sp. collagenase. The time of hydrolyis (γ0’, 60’, 90’) were indicated
Profiles of collagen hydrolysates generated by Bacillus licheniformis F11.4 collagenase are shown in Figure 6D and 6E. These crude and semi-purified
collagenase were not able to hydrolyze the -chain. The α1 and αβ-chains were also remained after 90 minutes of incubation. Crude collagenase from this bacteria generated collagen hydrolysate with molecular weight of 18-267 kDa while semi-purified collagenase generated collagen peptides with lower molecular weight (15-267 kDa). Peptides with molecular weight of 18 and 27 kDa were generated after 30 minutes of hydrolyzing by semi-purified collagenase, but these peptides were hydrolyzed by longer incubating time.
Figure 6F and 6G show collagen hydrolysate profiles generated by
Stenotrophomonas sp. collagenase. This semi-purified collagenase was able to hydrolyze the -chain. Figure 6G also show that semi-purified collagenase from this
bacteria completely hydrolyzed α1 and αβ-chains after 90 minutes of incubation.
However, the α1 and αβ-chains were still remained after 90 minutes of hydrolyzing by the crude collagenase. Peptides with molecular weight of 18-267 kDa were shown in collagen hydrolysate produced by crude Stenotrophomonas sp. collagenase. Semi-purified collagenase from this bacteria produced collagen hydrolysate with molecular weight of 14-152 kDa. This data implied that collagenases from bacterias used might have different specificity that influent their ability in hydrolyzing collagen.
The specificity of collagenase was influenced by its protein structure. Collagenase is synthesized as a pre-proenzyme and secreted as an inactive proenzyme which consists of a pro-peptide, catalytic domain, a short linker region rich in proline and a C-terminal hemopexin (Hpx) domain. Collagenase with catalytic and Hpx domains is able to bind the collagen, unwind the rigid structure, and hydrolyze the α -chains. Thus, this collagenase can cleave the or - chains of collagen. Collagenase which lacks of Hpx domain is only able to hydrolyze the unwound collagen (α -chains) (Chung et al. 2004). Figure 6D and 6E shows that -chains were still remained because collagenase from Bacillus licheniformis F11.4 was lack of Hpx domain. This collagenase only hydrolyzed α-chains of collagen which was produced by partial hydrolyzing during extraction. On the contrary, collagenase from
Stenotrophomonas sp. was able to hydrolyze -chain due to the presence of catalytic and Hpx domains on its structure This assumption was proven with ammount of peptides produced after hydrolysis (table 3). Collagen hydrolysates produced by collagenase from Stenotrophomonas sp. contain more peptides than collagen hydrolysates produced by collagenase from Bacillus licheniformis F11.4.
Figure 6E and 6G show that collagenase from Bacillus licheniformis F11.4 and
Stenotrophomonas sp. generate peptides with different molecular weight. This might be related to the difference of cleavage site of collagenases used. Butre et al. (2014) reported that protease from Bacillus licheniformis was able to hydrolyze whey protein isolate after glutamate residue. The cleavage site of protease from Stenotrophomonas
Inhibition of Angiotensin-I Converting Enzyme (ACE)
The abilities of peptides to inhibit ACE are also influenced by their structure and amino acid sequence. Generally, collagen is suitable to become a parent protein of ACE inhibitor peptides due to the high amount of proline residues in its sequences. Proline is the most favorable amino acid for binding ACE (Wu et al. 2006). Thus, peptides generated from collagen including collagen from skin of snakehead fish may content proline residue at their C-terminus, antepenultimate, or ultimate positions that make them exert their affinity to ACE. This theory is proven by the discovering of collagen peptides from chicken breast with sequence Gly-Phe-His-Tyr-Pro-Gly-Thr-His-Tyr-Pro-Gly-Leu-His-Tyr-Pro-Gly-Phe. This peptide contains aromatic amino acid at the antepenultimate position and phenylalanine at the C-terminal end which are responsible for inhibiting ACE. The IC50 value of this peptide was 42.4 µM (Saiga 2003). Peptides from marine sources were mostly found to be competitive. Peptides from rainbrow trout muscle (Kim & Byun 2012) and chum salmon muscle (Gobbetti et al. 2000) showed their ACE inhibitory by binding to the active site of ACE to block and prevent Angiotensin-I hydrolysis. Thus, collagen peptides from snakehead fish skin were presumably acting as competitive inhibitor.
Table 4 ACE inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis (F11.4) and
Stenotrophomonas sp. (Isolate 11), n=2, different letters indicate significance at p<0.05
Sample Inhibition percentage/ ug peptides (%)
Figure 7 ACE inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis F11.4 and
Stenotrophomonas sp. Values are mean ± SD, n=2
Collagen extracted from skin of snakehead fish had the ability to inhibit ACE activity by 1.7 % per µg collagen protein. This ACE inhibition was lower than the inhibitory effect by captopril used (2.6 % per µg captopril) but not significantly different at p<0.05. Figure 7 shows that the highest inhibitory effect was shown by collagen hydrolysates by crude collagenase from Stenotrophomonas sp. for 90 minutes (13.3 % per µg peptides). From the data, these collagen hydrolysates except collagen hydrolysate produced by semi-purified Bacillus licheniformis F11.4 collagenase showed inhibitory effects that siqnificantly higher than captopril (table 4). Captopril is a commercial synthetic ACE inhibitor which commonly used as medicine for lowering blood pressure.
Figure 7 implied that Stenotrophomonas sp. collagenase has an ability to produced collagen peptides with higher inhibitory effect in ACE than collagen peptides produced by Bacillus licheniformis F11.4 collagenase. This might be related to the differences of cleavage sites of collagenases used and the ability of collagenase from Stenotrophomonas sp. to unwind the -chains and hydrolyze them to generate peptides with ACE inhibitory effects. Collagenase from Stenotrophomonas was apparently able to produce peptides with structure that more suitable for binding ACE active site. The peptides produced may contain hydrophobic amino acids such as proline at their C-terminus, antepenultimate, or ultimate positions. This hydrophobic amino acid residues are responsible in attaching on ACE active site. Besides, Unwound collagen (α-chains) is known to be a substrate in collagen hydrolysis (Chung et al. 2004). The amount of substrate (α-chains) will increase if the collagenase is able to unwind the -chain. Thus, collagenase from Stenotrophomonas
sp. was able to hydrolyze more α-chains than collagenase from Bacillus licheniformis
F11.4 (table 3) and generated more low molecular peptides. This was shown by SDS-PAGE data where Stenotrophomonas sp. collagenase was able to generate collagen peptides with lower molecular weight than collagen peptides generated from Bacillus licheniformis F11.4. Lower molecular peptides are prefferable as ACE inhibitor. Other research revelead that frame protein from Alaska Pollack (Theragra chalcogramma) with molecular weight of <1 kDa showed ACE inhibition with IC50 value of 14.7 µM (Byun & Kim 2001).
Antioxidant Activity
Table 5 DPPH inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis (F11.4) and
Stenotrophomonas sp. (Isolate 11), ), n=2, different letters indicate significance at p<0.05
Sample DPPH inhibition / 50 ug peptide (%)
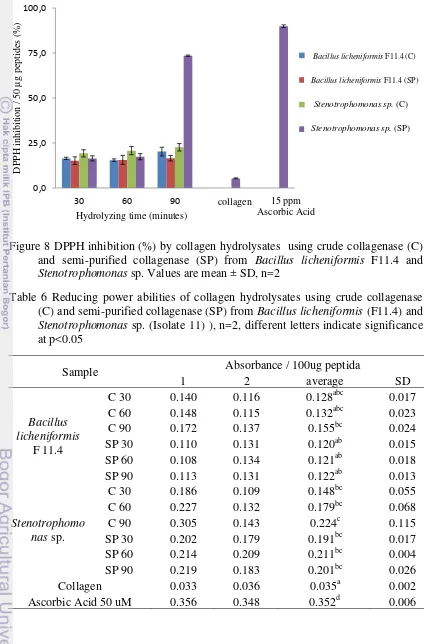
Figure 8 DPPH inhibition (%) by collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis F11.4 and
Stenotrophomonas sp. Values are mean ± SD, n=2
Table 6 Reducing power abilities of collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis (F11.4) and
Figure 9 Reducing power abilities of collagen hydrolysates using crude collagenase (C) and semi-purified collagenase (SP) from Bacillus licheniformis F11.4 and
Stenotrophomonas sp. Values are mean ± SD, n=2
In this research, antioxidant activities of collagen and its hydrolysates were measured by using DPPH method and Fe reducing assay. DPPH method was used to examine the ability of sample as free radical scavenger by donating its hydrogen to form non-toxic species which inhibits the propagation state of lipid peroxidation. In Fe reducing assay, sample reduces Fe3+ in ferrycyanide complex to form a ferrous
complex. Measuring the formation of Perl’s prussian blue at 700 nm can determine
the Fe2+ formed (Giri et al 2012).
hydrolysate at the same concentration was 0.287. The abilities of protein hydrolysates to act as antioxidant were influenced by their concentration where the antioxidant ability increases by the increasing of its concentration (Chi et al. 2014). This explains the differences between antioxidant activity of collagen hydrolysate from snakehead fish skin and other similar research.
In general, crude and semi-purified Stenotrophomonas collagenase generated collagen peptides with higher antioxidant activities than collagen peptides generated by Bacillus licheniformis sp. collagenase. This might be related by the different molecular weights of peptides generated from those collagenases and the amount of peptides generated due to the ability of Stenotrophomonas sp. collagenase to unwind the -chain (table 3). SDS-PAGE data shows that Stenotrophomonas sp. collagenase has an ability to generate peptides with lower molecular weights than Bacillus licheniformis F11.4 collagenase. Peptides with molecular weight of 1.4 kDa generated from mackarel (Scomber austriasicus) by using Protease N were able to quenched DPPH free radical and reduced Fe3+ (Wu et al. 2003).
The abilitiy of collagen peptides as antioxidants are also influenced by its structure. The presence of several glycine residues in a peptide sequence may confer high flexibility on the peptide structure, while the pyrrolidine ring of proline could impose certain conformational constrains in the secondary structure of the peptides (Aleman & Alvarez 2013). In addition, hydrophobic amino acids were reported to contribute to enhace the reduction power of peptides (He et al. 2013). Thus, collagen peptides which mostly contain hydrophobic amino acid such as glycine, proline, and hydroxyproline have reduction power. These assumptions were in aggrement with Mendis et al. (2005) who reported that hoki skin gelatin peptides (His-Gly-Pro-Leu-Gly-Pro-Leu) was able to act as strong radical scavenger.
Based on the data, collagen and collagen hydrolysate with ACE inhibition and antioxidant activities can be obtained from acid soluble collagen extracted from skin of snakehead fish by using collagenase from Bacillus licheniformis F11.4 and
5
CONCLUSION AND RECOMMENDATION
Conclusion
Acid soluble collagen from skin of snakehead fish (Channa striata) is
categorized as type I collagen since it contains α1 (15β kDa) and αβ-chain (136 kDa). Beta chain with molecular weight of 267 kDa and other proteins were also present in this collagen. Collagenase from Bacillus licheniformis F11.4 and Stenotrophomonas
sp. can be used to produce collagen peptides eventhough these collagenase have different specificities. This collagen and its hydrolysates have abilities to inhibit activity of Angiotensin-I Converting Enzyme (ACE), scavenge DPPH free radical, and reduce Fe3+. Stenotrophomonas sp. collagenase generated collagen peptides that have higher ACE inhibition and antioxidant activities than peptides generated by
Bacillus licheniformis sp. collagenase.
Recommendation
Further research is recommended to pursue the bioactivities of small peptides generated by Stenotrophomonas sp. collagenase. Determination of ACE inhibition and antioxidant activities of this collagen and its hydrolysate in vivo are also needed. Then, the collagen hydrolysate with the best bioactivities can be fractionated and sequenced to know its true mechanism in inhibiting ACE activity and acting as antioxidant.
Acknowledgement
BIBLIOGRAPHY
Ahhmed AM, Muguruma M. 2010. A review of meat protein hydrolysates and hypertension. Meat Sci. 86: 110 – 118. doi: 10.1016/j.meatsci.2010.04.032.
Ajibola CF, Fashakin JB, Fagbemi TN, Aluko RE. 2011. Effect of peptide size on antioxidant properties of african yam bean seed (Sphenostylis stenocarpa) protein hydrolysate fraction. Int J Mol Sci 12: 6685-6702. doi: 10.3390/ijms12106685. Aleman A, Martinez-Alvarez O. 2013. Marine collagen as a source of bioactive Arihara K, Ohata M. 2006. Advanced Technologies for Meat Processing. New York
(USA): Springer.
Asfar M, Tawali AB, Abdullah N, Mahendradatta M. 2014. Extraction of albumin of snakehead fish (Channa striatus) in producing the fish protein concentrate (FPC).
IJSTR 3(4): 85-88.
Baehaki A, Suhartono MT, Sukarno, Syah D, Sitanggang AB, Setyahadi S, Meinhardt F. 2012. Purification and characterization of collagenase from Bacillus licheniformis F11.4. Afr J Microbiol Res 6(10): 2373 – 2379. doi: 10.5897/AJMR11.1379.
Beeley JA, Yip HK, Stevenson AG. 2000. Chemochemical cariers removal: a review of the techniques and latest development. Br Dent J 188: 427-430. Doi: 10.1038/sj.bdj.4800501
Beg M, Sharma V, Akhtar N, Gupta A, Mohd J. 2011. Role of antioxidant in hypertension. JIACM 12(2): 122-127.
Bergmeyer HU, Bergmeyer J, Gra 1 M. 198γ. Methods of Enzymatic Analysis Vol 2.Weinheim (GER): VerlagChemie.
Biljana E, Boris V, Cena D, Veleska-Stefkovska D. 2011. Matrix metalloproteinases (with accent to collagenases). J Cell Anim Biol 5(7): 113-120.
Bradford MM. 1976. A rapid sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal. Biochem
72: 234-254. doi: 10.1016/0003-2697(76)90527-3
Byun HG, Kim SK. 2001. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 36: 1155–1162. doi: 10.1016/S0032-9592(00)00297-1
Brown NJ, Vaughan DE. 1998. Angiotensin-converting enzyme inhibitors.
Circulation 97: 1411-1420.
Bacillus licheniformis protease. J Agric Food Chem 62 (42): 10230 – 10239. doi: 10.1021/jf503151f.
Charpentier R, Cateora H. 1996. Tumeric: Phytonutrient protection for variety of physiological stress. Nevada (USA): Carcon.
Chi CF, Cao ZH, Wang B, Hu FH, Li ZR, Zhang B. 2014. Antioxidant and functional properties of collagen hydrolysates from spanish mackarel skin as influenced by average molecular weight. Molecules 19: 112111 – 11230. doi: 10.3390/molecules190811211
Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. 2004. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO 23: 3020 – 3030.
Chusman DW, Cheung HS. 1971. Spectrophotometric assay and properties of the angiotensin converting enzyme of rabbit lung. Biochem Pharmacol 20: 1637-1648. doi: 10.1016/0006-2952(71)90292-9
Diener B, Lee Carick JR, Berk RS. 1973. In vivo studies with collagenase from
Pseudomonas aeruginosa. Infect Immun 7(2): 212-217.
Dong SY, Zeng MY, Wang DF, Liu ZY, Zhao YH, Yang HC. 2008. Antioxidant and biochemical properties of protein hydrolysates prepared from Silver carp (Hypophthalmichthys molitrix). Food Chem. 107: 1485–1493. doi: 10.1016/j.foodchem.2007.10.011
Eggebretch H, Michael H, Woertgen U, Schmermund A, Birgelen C, Naber C, Baumgart D, Kaiser C, Olderberg O, Bartel T, Kroeger K, Erbel R. 2002. Systematic use of collagen-based vascular closure device immediatelly after cardiac catheterization procedure in 1,317 consecutive patients. Catheter Cardiovasc Interv 57(4): 486-495. doi: 10.1002/ccd.10254
Fan J, He J, Zhuang Y, Sun L. 2012. Purification and identification of antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) frame protein. Molecules 17: 12836-12850. doi: 10.3390/molecules171112836
[FAO] Food and Agriculture Organization. 2011. Channa striata [internet].
[Accessed on September 16th 2014]. Available at
Adv Drug Deliv Rev 55: 1531-1546. Doi: 10.1016/j.addr.2003/08.002
Ghassem M, Babji AS, Said M, Mahmoodani F, Arihara K. 2014. Angiotensin-I converting enzyme inhibiory peptides from snakehead fish sarcoplasmic protein hydrolysate. J Food Biochem 38(2): 140-149. doi: 10.1111/jfbc.12031.
Giri A, Nasu M, and Ohshima T. 2012. Bioactive properties of japanese fermented fish paste, fish miso, using koji inoculated with Aspergillus oryzae. Int J Food Sci
1(1): 13-22. doi: 10.11648/j.ijnfs.20120102.12.
Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp.
cremoris FT4. Appl Environ Microbiol 66: 3898 3904.
Gomez-Guillen MC, Lopez-Caballero ME, Lopez de Lacey A, Aleman A, Gimenez B, Montero P. 2010. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. In E. Le Bihan and N Koueta (Eds), Sea by-products as a real material: New ways of application (pp.89-115). Kerala (IND): Transworld Research Network Signpost.
Han SH, Y Uzawa, T Moriyama, Y Kawamura. 2011. Effect of collagen and collagen peptides from blue fin tuna abdominal skin on cancer cells. Health 3(3): 129-134.
Harrington DJ. 1996. Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun 64 (6): 1885-1891.
He XQ, Cao WH, Zhao ZK, Zhang CH. 2013. Analysis of protein composition and antioxidant activity of hydrolysate from Paphia undulate. J Food Nutr Res 1(3): 30-36.
Holmes C, Wrobel JS, MacEachern MP, Boles BR. 2013. Collagen-based wound dressings for the treatments of diabetes-related food ulcers: a systematic review.
Diabetes Metab Syndr Obes 6: 17-29. doi: 10.2147/DMSO.S36024
Horrocks M. 2015. The nervous systems [internet]. [Accessed on September 22th 2014]. Available at http://www.4college.co.uk/a/Md/medicine.php
Hsu KC. 2010. Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem. 122: 42–48. doi: 10.1016/j.foodchem.2012.02.013
[ISH] Indonesian Society of Hypertension. 2014. Optimizing hypertension management in primary and referral care for morbidity and mirtality reduction [internet]. [Accessed on June 26th 2014]. Available at
http://www.inash.or.id/upload/event/event_Final_announcement_-_Final123171.pdf
Jain R, Jain PC. 2010. Production and partial characterization of collagenase of Stenotrophomonas exfoliatus CFS 1068 using poultry feather. Indian J Exp Biol
48: 174-178.
Jae JY, Park PJ, Kim SK. 2005. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 38: 45–50. doi: 10.1016/j.foodres.2004.07.005
Jongjareonrak A, S Benjakul, W Visessanguan, T Nagai, M Tanaka. 2005. Isolation and characterisation of acid and pepsin-solubilised collagen from the skin of brownstripe red snapper (Lutjanus vitta). Food Chem 93: 475-484.
Jung WK, Mendis E, Jae JY, Park PJ, Son BW, Kim HC, Choi YK, Kim SK. 2006. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 94: 26–32. doi: 10.1016/j.foodchem.2004.09.048 Kadler KE, Baldock C, Bella J, Raymond P, Handford B. 2007. Collagens at glance.
J Cell Sci 120(12), 1955-1958. doi: 10.1.1.496.1166