WAXES, POLICOSANOLS AND ALDEHYDES IN SUGARCANE (Saccharum officinarum L.) AND OKINAWAN BROWN SUGAR
(KOKUTO)
YONATHAN ASIKIN
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
I hereby declare that this thesis is my own work and to the best of my knowledge
it contains no material previously published or written by another person, nor
materials which to a substantial extent has been accepted for the award of any
degree or diploma at any university, except where due acknowledgement has been
made.
Bogor, August 2008
Yonathan Asikin
YONATHAN ASIKIN. Waxes, Policosanols and Aldehydes in Sugarcane (Saccharum officinarum L.) and Okinawan Brown Sugar (Kokuto). Under direction of RIZAL SYARIEF and HANIFAH NURYANI LIOE.
Waxes, long chain alcohols and aldehydes were found in several variants of sugarcane (Saccharum officinarum L.) and Kokuto, a non-centrifuged Okinawan cane brown sugar. Long chain alcohols, policosanols, have been reported to have beneficial effect to human health. The composition of wax in sugarcane was analyzed using HPLC with an evaporative light scattering detector. Sugarcane wax composed of 55–60% aldehydes, sterol esters and wax esters, 32–40% alcohols, and small amounts of triacylglycerols, acids and sterols. Extraction of policosanols performed effectively with hexane and methanol (20:1 v/v), while that of long chain aldehydes was with chloroform and methanol (2:1 v/v). Their composition was determined using GC with a flame ionization detector, whereas their compounds were identified using GC-MS. Sugarcane rinds contained up to 500 mg policosanols and 600 mg aldehydes per 100 g sample of Ni 22 cultivar. The content of policosanol and long chain aldehyde in Kokuto was influenced by its production systems. Compositional analysis of the end product confirmed the presence of policosanols and aldehydes up to 85 mg and 8 mg respectively per 100 g sample of Kokuto A, a product of brown sugar manufacture with open pan heating system. Octacosanol and octacosanal were found to be the major wax
components in both sugarcane and Kokuto samples. This study revealed
YONATHAN ASIKIN. Wax, Polikosanol dan Aldehida pada Tebu (Saccharum officinarum L.) dan Gula Coklat Okinawa (Kokuto). Dibimbing oleh RIZAL SYARIEF dan HANIFAH NURYANI LIOE.
Wax tebu (Saccharum officinarum L.) telah menjadi perhatian bagi banyak orang, karena aplikasinya pada berbagai bidang industri dan sifat fungsional dari salah satu senyawa yang terkandung di dalamnya, yaitu alkohol rantai panjang. Senyawa alkohol rantai panjang (C20–C30) atau polikosanol telah banyak diteliti dan dilaporkan terkait dengan dampak positifnya bagi kesehatan manusia seperti menurunkan agregasi platelet, menurunkan kadar LDL dalam darah, dan menghambat sintesis kolesterol. Dalam penelitian ini kandungan dan komposisi kimiawi wax, polikosanol dan aldehida rantai panjang diteliti dari beberapa varietas tebu dan Kokuto, gula coklat non-sentrifugasi dari Okinawa, Jepang.
Tujuan penelitian ini adalah untuk mengetahui komposisi wax, polikosanol dan aldehida rantai panjang dengan analisis TLC, HPLC-ELSD, FID dan GC-MS. Secara khusus, analisis ditujukan untuk mengetahui pengaruh metode dan waktu ekstraksi; pengaruh bagian-bagian batang tebu, varietas dan umur panen tebu; serta pengaruh cara produksi dan jenis Kokuto terhadap kandungan wax, polikosanol dan aldehida rantai panjang pada tebu dan Kokuto.
Sampel yang digunakan dalam penelitian ini adalah bagian-bagian batang tebu varietas Ni 15, bagian kulit dari tujuh varietas tebu (Ni 13, Ni 17, Ni 22, NiF 8, NCo 310, F 161 dan F 177), serta tujuh jenis Kokuto (tipe A–G). Komposisi wax tebu dianalisis secara kualitatif dengan teknik TLC dan dikuantifikasi dengan
HPLC yang dilengkapi detektor evaporative light scattering. Kandungan dan
komposisi senyawa polikosanol dan aldehida rantai panjang dianalisis oleh GC dengan detektor flame ionization, sedangkan struktur senyawanya diidentifikasi oleh GC-MS. Pengaruh setiap percobaan dianalisis secara statistik dengan rancangan acak lengkap satu faktor.
Studi ini memberikan informasi mengenai senyawa fungsional polikosanol yang terkandung pada tebu dan gula coklat. Polikosanol dapat diekstrak secara efektif dengan pelarut heksana dan metanol (20:1 v/v), sedangkan aldehida rantai panjang dengan pelarut kloroform dan metanol (2:1 v/v). Identifikasi senyawa polikosanol dengan GC-MS terlihat pada pola fragmen massa trimetilsilil-eter dari ion target, sedangkan senyawa aldehida rantai panjang teridentifikasi dari pecahan fragmen spesifik dari senyawa aldehida. Oktakosanol dan oktakosanal merupakan komponen penyusun utama dari wax tebu dan Kokuto.
Studi ini mengungkapkan bahwa bagian-bagian tebu, varietas dan umur panen tebu berpengaruh nyata terhadap komposisi dan kandungan wax, polikosanol dan aldehida rantai panjang pada tebu. Komposisi wax tebu yang dianalisis dengan HPLC-ELSD adalah 55–60% campuran senyawa aldehida, sterol ester dan wax ester, 32–40% alkohol, dan sejumlah kecil triasilgliserol, asam dan sterol.
Senyawa polikosanol dan aldehida rantai panjang pada bagian kulit tebu
yang dipisahkan dengan Cane Separation System ditemukan lebih banyak
sampel.
Pengupasan kulit tebu secara manual membuat wax epidermis masih melekat pada sampel, sehingga total polikosanol dan aldehida yang terkandung di dalamnya mencapai 500 mg dan 600 mg per 100 g sampel tebu varietas Ni 22. Total kandungan wax pada kulit tebu tersebut dipengaruhi oleh jenis varietas tebu, kondisi pertumbuhan dan umur tanaman tersebut. Kandungan senyawa aldehida pada kulit tebu meningkat lebih besar dibandingkan dengan senyawa polikosanol sejalan dengan meningkatnya umur tanaman terebut.
Pada gula coklat Kokuto, kandungan polikosanol dan aldehida rantai
© Copyright of this thesis belongs to Bogor Agricultural University (Institut Pertanian Bogor), Indonesia and University of the Ryukyus, Japan, 2008.
All rights reserved.
1. Due acknowledgement must always be made of the use of any material
contained in, or derived from, this thesis.
a. Any person, may however, use content of the thesis for the purposes of
education, private study, research, scientific report or review.
b. The use of any material contained in, or derived from, this thesis must not
make negative effect for the University.
2. The written permission from the University is required before any part of the
WAXES, POLICOSANOLS AND ALDEHYDES IN SUGARCANE (Saccharum officinarum L.) AND OKINAWAN BROWN SUGAR
(KOKUTO)
YONATHAN ASIKIN
Thesis
as partial fulfillment of the requirements for the degree of Master of Science
in the Department of Food Science and Technology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Student ID : F251050111
Approved by
Advisory Committee
Prof. Dr. Ir. Rizal Syarief, DESS
Chair Member
Dr. Ir. Hanifah Nuryani Lioe, M.Si.
Acknowledged by
Head of Food Science Dean of Graduate School Study Program
Dr. Ir. Ratih Dewanti, M.Sc. Prof. Dr. Ir. Khairil Anwar Notodiputro, M.S.
I would like to thank God, Lord Jesus Christ, for his mercy and spirit
throughout my study and thesis work in Graduate School of Bogor Agricultural
University, Indonesia. He always helps and protects me. I can do everything
through Christ who strengthens me.
This thesis owes its existence to the help, support and inspiration of many
people. Firstly, I would like to express my sincere appreciation to Prof. Dr. Rizal
Syarief as Chair of Advisory Committee for his support and encouragement
during my study in Bogor Agricultural University. I am very grateful to Dr.
Hanifah Nuryani Lioe as Member of Advisory Committee for her advice and
supervision during the thesis work. I am also indebted to Dr. Feri Kusnandar as
Non-committee Examiner for his constructive comments on this thesis.
I would like to thank University of the Ryukyus, Japan for the Short Term
Regular Program (STRP) and for giving me the opportunity to conduct my
research in Laboratory of Food Chemistry, Department of Bioscience and
Biotechnology, Faculty of Agriculture, University of the Ryukyus; and the Japan
Student Service Organization (JASSO) for the scholarship.
I would like to express my sincere appreciation to my supervisor during
STRP period, Prof. Dr. Koji Wada, Head of Laboratory of Food Chemistry,
Faculty of Agriculture, University of the Ryukyus for his advice and support
throughout the research. I gratefully thank Dr. Kensaku Takara for valuable
discussions during my research work. I also would like thank Okinawa Prefectural
Agricultural Research Center, Japan for providing sugarcane and Kokuto samples.
I wish to thank all lecturers and colleagues, especially IPN 2005, at the
Food Science Study Program of Graduate School of Bogor Agricultural
University. It has been a pleasure to work with you.
Last but not least, many thanks to my parents, brother and sisters for their
true and endless love, for never-failing patience and encouragement.
Bogor, August 2008
Yonathan Asikin was born in Karawang, West Java Province, Republic of
Indonesia, on November 24, 1981. After graduating from Karawang State Senior
High School (SMUN 1 Karawang) in 1999, he entered Bogor Agricultural
University, Indonesia, from 1999 to 2003 where he obtained a Bachelor (Sarjana)
of Agricultural Technology degree in Food Technology in 2003.
He entered Graduate School of Bogor Agricultural University, majoring
food science (Master Program), in 2005. During school he attended Short Term
Regular Program (STRP) in University of the Ryukyus, Japan, from April 2007 to
March 2008 as research student. He conducted his thesis research in Laboratory
of Food Chemistry, Department of Bioscience and Biotechnology, Faculty of
x
Page
LIST OF TABLES ... xi
LIST OF FIGURES ... xii
LIST OF APPENDIX ... xiv
LIST OF ABBREVIATIONS ... xv
INTRODUCTION... 1
LITERATURE REVIEW ... 4
Sugarcane (Saccharum officinarum L.) ... 4
Brown Sugar ... 7
Plant Wax ... 8
Wax Compositional Analysis... 10
Policosanol in Human Health ... 14
MATERIALS AND METHODS ... 17
Materials... 17
Methods ... 19
Wax compositional analysis by TLC and HPLC-ELSD ... 21
Policosanol and long chain aldehyde analysis by GC-FID ... 24
Policosanol and long chain aldehyde identification by GC-MS ... 27
Statistical analysis ... 28
RESULTS AND DISCUSSION ... 30
Sugarcane Wax Composition ... 30
GC Chromatogram and Mass Spectrum of Policosanols and Long Chain Aldehydes... 38
Policosanols and Long Chain Aldehydes in Sugarcane Rind ... 43
Policosanols and Long Chain Aldehydes in Kokuto ... 50
CONCLUSION ... 57
REFERENCES ... 59
xi
Page
1 Scientific classification of sugarcane ... 4
2 Sugarcane producer countries in 2005 ... 5
3 Mixture composition of aliphatic alcohols (policosanols) ... 12
4 Content and composition of policosanols in some materials ... 13
5 Wax compositions of rind part of Okinawan sugarcane cultivars, obtained by HPLC-ELSD ... 34
6 Mass fragmentation pattern of policosanols and long chain aldehydes ... 42
7 Policosanol and long chain aldehyde contents of rind part of Okinawan sugarcane cultivars ... 45
xii
Page
1 Kokuto, Okinawan brown sugar ... 8
2 Rind (a) and (b) pith parts of sugarcane separated by CSS ... 17
3 Cane Separation System (CSS) ... 18
4 Experimental designs of sugarcane wax, policosanol and long chain aldehyde analysis ... 19
5 Production lines of Kokuto ... 20
6 Experimental designs of policosanol and long chain aldehyde analysis in Kokuto ... 21
7 Thin layer chromatography of waxes of several sugarcane cultivars ... 30
8 HPLC chromatogram of sugarcane rind of Ni 15 cultivar ... 32
9 Standard curve of triacylglycerol ... 32
10 HLPC chromatograms of wax composition standards ... 33
11 Policosanol and long chain aldehyde contents in sugarcane Ni 15 cultivar, obtained by GC-FID... 36
12 Typical gas chromatogram of (a) policosanol and long chain aldehyde standards, (b) Kokuto A, (c) sugarcane rind of Ni 15 cultivar ... 39
13 Standard curve of octacosanol ... 40
14 Mass spectrum of trimethylsilyl ether of C28 of standard ... 41
15 Mass spectrum of trimethylsilyl ether of C28 of Kokuto ... 41
16 Mass spectrum of C28 aldehyde of sugarcane rind of standard ... 42
17 Mass spectrum of C28 aldehyde of sugarcane rind of Ni 15 cultivar ... 42
18 Influence ofsoxhlettimes on policosanol and long chain aldehyde extraction of sugarcane rind of Ni 15 cultivar ... 43
xiii 21 Product derivates from sugarcane ... 49
22 Influence of methods and solvent types on policosanol and long chain aldehyde extraction of Kokuto A ... 51
23 Influence ofsoxhlettimes on policosanol and long chain aldehyde extraction of Kokuto A ... 52
24 Composition of (a) policosanols and (b) long chain aldehydes in Kokuto A . 53
xiv
Page
1 Mass spectrum of trimethylsilyl derivates of policosanols ... 66
2 Mass spectrum of long chain aldehydes ... 69
xv
Abbreviation Term
CSS Cane Separation System
ELSD Evaporative Light Scattering Detector
HPLC High Performance Liquid Chromatography
GC Gas Chromatography
GC-FID Gas Chromatography Flame Ionization Detector
GC-MS Gas Chromatography Mass Spectra
LDL Low Density Lipoprotein
MSTFA N-Methyl-N-(trimethylsilyl) trifluoroacetamide
MTBE Methyl tert-butyl ether
Sugarcane (Saccharum officinarum L.) wax has been a matter of interest,
due to its industry application and functionality of one of its compound, long
chain alcohols. The surfaces of plants, including sugarcane, are coated with
several layers of lipophilic material, the outermost being the epicuticular wax. It
serves many purposes, for example to limit the diffusion of water and solutes,
while permitting a controlled release of volatiles that may deter pests or attract
pollinating insects. It also provides protection from diseases and insects, and
helps the plants resist drought.
Long chain alcohols, which is well-known as policosanols, is a group of
long chain (C20–C30) aliphatic primary alcohols which is of a great interest due
to their health beneficial effect for human health, such as reducing platelet
aggregation, reducing low-density lipoprotein levels in blood, inhibiting
cholesterol synthesis, and ergogenic properties (Castano et al. 2003; Singh et al.
2006; Taylor et al. 2003). Long chain aldehydes as well as alcohols are one of
main component of natural wax extracted from plant (Adhikari et al. 2006).
Straight chain aldehyde also was known as one of lipid biomarker in leaves and
roots of plant (Jansen et al. 2006).
Recently, research in policosanol analysis with several kinds of materials
and techniques have been well reported (Adhikari et al. 2006; Wang et al. 2007;
Wu et al. 2007). Sugarcane and its wax have been reported contain a number of
policosanols used as major source in commercial product of policosanol (Irmak et
al. 2006; Morrison et al. 2006; Nuissier et al. 2002). However, only a few
sugarcane and its products.
Kokuto, a unique brown cane sugar, has been traditionally produced in
Okinawa, Japan from sugarcane by non-centrifugal method, without molasses
removing process. This product has been reported to contain some antioxidants
and phenolic compounds (Takara et al. 2002, 2003). It is then expected that
Kokuto as one of cane food product contain much wax components, including
policosanol which has beneficial health effect as described above.
Indonesian sugar industry, back to the seventeenth century, was known as
one of the oldest and biggest sugar industry in the world. It reached its zenith in
the early-thirties when 179 factories produced nearly 3 million MT of sugar
annually. Following several up and down conditions in many periods of times,
yet, since 1967, Indonesia has reverted to a net sugar importer position and since
the mid-eighties imports have continued to rise. The present average cane yields
are thus about 7.5% (Hadisaputro et al. 2008). Thus this study would explored
information of functional compounds potentially contained in sugarcane and
brown sugar. Sugarcane might be potent sources for high value added products
of sugarcane derivates, such as cane wax and policosanol.
The main purpose of this study is to determine wax, policosanol and
aldehyde compositions in sugarcane and Kokuto, Okinawan brown sugar, with
TLC, HPLC-ELSD, GC-FID and GC-MS. Some specific aims were applied in
this study, i.e.:
a. to determine the effect of extraction methods and times on the policosanol
and long chain aldehyde contents of sugarcane rinds and Kokuto,
b. to determine the effect of sugarcane cultivars on wax, policosanol and long
c. to determine policosanol and long chain aldehyde contents in different parts
of sugarcane,
d. to determine the effect of sugarcane harvesting time on the policosanol and
long chain aldehyde contents of the sugarcane rinds,
e. to determine the effect of Kokuto types on the policosanol and long chain
aldehyde contents of the Kokuto, and
f. to determine the effect of Kokuto production types on the policosanol and
A. Sugarcane (Saccharum officinarum L.)
Sugarcane is an important crop due to the economic value of its
products. Sugarcane is a tall thick perennial that belongs to the grass
family (Poaceae). It has stout, jointed, fibrous stalk that are rich in sugar.
The genus Saccharum comprises some different species, such as S.
officinarum, S. barberi, S. sinense, S. edule, S. robustum and S. spontaneumi
(Table 1). Saccharum officinarum and S. spontaneum are thought to be the
ancestors of cultivated sugarcane. Saccharum officinarum was
domesticated in Southeast Asia and originally derived from S. robustum.
All of those species interbreed, and the major commercia
complexet al. 1987).
Table 1 Scientific classification of sugarcane
Kingdom Plantae
Division Magnoliophyta Class Liliopsida Order Poales Family Poaceae
Genus Saccharum L.
Species S. officinarum, S. arundinaceum, S. bengalense, S. edule, S. procerum, S. ravennae, S. Robustum
Sugarcane is a highly productive crop that has high photosynthetic
ability as C4 plant. It requires strong sunlight and abundant water for
months. Sugarcane stalk can grow to heights range 3.05–7.9 m and
measuring 2.54–5.08 cm in diameter. Colors range from white, yellow,
and green to purple.
Sugarcane is mainly cultivated in tropical and subtropical regions.
Indonesia harvested about 436 847 ha and produced 77 MT/ha of cane for
centrifugal sugar (Hadisaputro et al. 2008), of which almost three-quarters
is on Java. Most of the remainder comes from Sumatra, Kalimantan and
Sulawesi. The remainder is cultivated on sugar factory plantations, both
in Java as well as on other islands where the dominant form of sugarcane
cultivation is plantation-style. About 70 percents of the sugarcane areas
are cultivated by farmers, mostly on small to medium sized holdings.
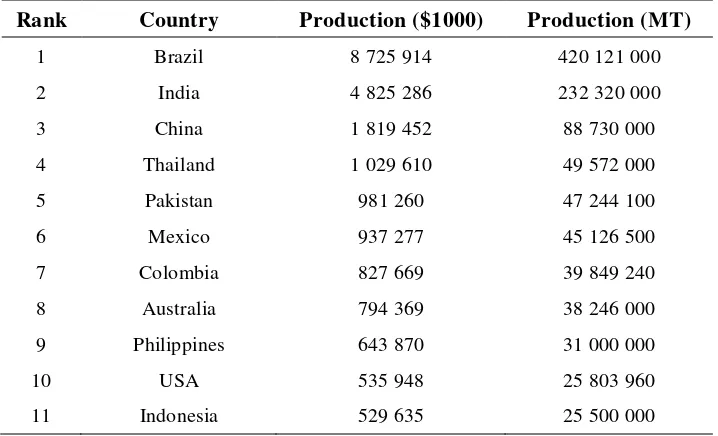
FAO data (2005) mentioned Indonesia in rank 11th of sugarcane producer with 25 500 000 MT or equal to $ 529 635 000 (Table 2).
Table 2 Sugarcane producer countries in 2005
Rank Country Production ($1000) Production (MT)
1 Brazil 8 725 914 420 121 000
2 India 4 825 286 232 320 000
3 China 1 819 452 88 730 000
4 Thailand 1 029 610 49 572 000
5 Pakistan 981 260 47 244 100
6 Mexico 937 277 45 126 500
7 Colombia 827 669 39 849 240
8 Australia 794 369 38 246 000
9 Philippines 643 870 31 000 000
10 USA 535 948 25 803 960
The sugarcane production areas in Japan are limited to the
Southwestern islands, which are located between Taiwan and Kyushu
Island, Japan. The cultivation areas of sugarcane are 20 970 ha in Okinawa
Prefecture and 12 172 ha in Kagoshima Prefecture. Sugarcane occupies
about 50% of agriculture area in Okinawa Prefecture and 60% in
Tanegashima and Amami Oshima regions of Kagoshima Prefecture, and the
crop is considered a key commodity in the region (Takagi et al. 2005).
Sugar is made by some plants to store energy that they do not need
straight away, rather like animals make fat. Scientifically, sugar refers to
any monosaccharide, also called simple sugar, i.e. glucose, fructose and
galactose; or disaccharide, i.e. sucrose (saccharose), maltose and lactose
(Belitz & Grosch 1999). In non-scientific use, the term sugar is used as a
synonym for sucrose, also called table sugar, a white crystalline solid
disaccharide. Many plants produce table sugar although only two are used
commercially and these are commonly known as sugar-cane (Saccharum
officinarum) and sugar-beet (Beta vulgaris). Manufacturing and preparing
food may involve other sugars, including palm sugar and fructose, generally
obtained from fruit.
Typical sugar content for mature cane would be 10% by weight but
the figure depends on the variety and varies from season to season and
location to location. Equally, the yield of cane from the field varies
considerably but a rough and ready overall value to use in estimating sugar
production is 100 MT of cane per hectare or 10 MT of sugar per hectare
B. Brown Sugar
Brown sugar is a sucrose sugar product with a distinctive brown color
due to the presence of molasses resulted from caramelization of sucrose. It
is made up of sucrose with greater amounts of ash, invert sugar and
compounds derived from the process that give the sugar its characteristic
flavor and color. Brown sugar is used in several confections such as
caramels, toffees and butterscotch (Potter & Hotchkiss 1995). In Asia,
Africa and South America non-centrifugal sugars are made for direct
consumption and are known by a range of names: Kokuto in Japan, Gur in
India and Bangladesh, Desi in Pakistan, Jaggery in Africa, and Panela in
South America.
There are two categories of brown sugar: those produced directly
from the cane juice at the place of origin and those that are produced during
the refining of raw sugar. The first type includes a variety of molasses and
syrups. The second type is coated brown or soft sugars and a variety of
refinery molasses and golden syrups. The brown sugar types can be
further divided into those where the crystals are separated (centrifuged) and
those that are not separated (non-centrifuged) from the molasses.
Brown sugar is known to contain antioxidant compounds that have
radical-scavenging activity and related functions such as anticancer effects
and regulation of blood pressure. Takara et al.(2002, 2003) reported that
Kokuto, Okinawan brown sugar (Figure 1) have antioxidative phenolic
glycosides. They are expected to be effective in the prevention of many
polyphenol content and volatile composition in seven cane brown sugars
(four from La Réunion, two from Mauritius and one from France), and the
relation to their unique free radical scavenging capacity.
Figure 1 Kokuto, Okinawan brown sugar.
C. Plant Wax
The term wax is derived from the Anglo-Saxon word weax which was
used to describe the material in the honeycomb of bees. Wax generally
refers to all waxlike solids and liquids found in nature and to those
individual organic substances that crystallize on cooling and melt on heating.
The nature of lipid constituents can vary greatly with the source of the waxy
material, such as hydrocarbons, sterol esters, aliphatic aldehydes, primary
and secondary alcohols, wax esters, diols, ketones, β-diketones,
triacylglycerols, and so on (Dominguez & Heredia 1998).
The surfaces of plants are covered by several layers of lipophilic
hydrophobic barrier of the plant surface, and as such they function primarily
to shed water and prevent nonstomatal water loss (Koch et al. 2006). In
addition, they provide a first line of defense against bacterial and fungal
pathogens and against abiotic stresses such as drought and UV damage
(Reisige et al. 2006). Epicuticular waxes also play a role in plant-insect
communication, by either attracting or deterring insects. This superficial
material is synthesized by specialized cells in the outermost layers of the
plant tissue. The amount of plant cuticular waxes produced is dependent on
growth conditions, whilst chemical composition is less influenced by
environmental factors. In general, wax yield from leaves and fruits of
many species is ranging between 20 and 600 µ g/cm2
Sugarcane wax is the whitish to dark yellowish powdery deposit on the
surface of the stalks of Saccharum officinarum L. During the milling of the
cane, a large portion of this powdery substance is detached and mixed with
the expressed juice. Sugarcane wax has been chemically defined as a
complex and variable mixture of long-chain alkanes, hydrocarbons, fatty
acids, ketones, aldehydes, alcohols, and esters (Nuissier et al. 2002; Purcell
et al. 2005), and steroids such as β-sitosterol, stigmasterol, ketosteroids and
hydroxyketosteroids (Goerges et al. 2006).
(Dominguez & Heredia
1998).
All compounds of plant wax have their own unique roles. However,
only few researches have been investigated wax components functions in
plant and their metabolism to date. Rutherford and Staden (1996) reported
a correlation of high ratio of alcohol to aldehyde and shorter carbon chain
saccharina Walker. Morris et al. (2000) described 1-octacosanal
(C28-aldehyde) as the major component of wheat epicuticular wax that
stimulates oviposition of the Hessian fly, Mayetiola destructor.
Comparison of the activity of five straight-chain primary aldehydes with
chain lengths from C22 to C30 revealed a relationship between chain length
and the number of eggs laid by female Hessian flies, with 1-hexacosanal and
1-heptacosanal the most active of the aldehydes tested.
Wax is valuable source for many industries such as cosmetic, food
ingredient, lubricant, printing and many other applications. Wax esters,
oxo esters of long-chain fatty acids esterified with long-chain alcohols, can
be used as high pressure lubricants, as replacements for hydraulic oil, and in
the pharmaceuticals, leather, and food industries, as well as in candles and
polishes. Long chain alkanals are known as favorable cosurfactants of
liquids containing charged micelles (Meziani et al. 1997). Lately, long
chain aliphatic alcohols of sugarcane wax have been used as
cholesterol-lowering products (Taylor et al. 2000; Castano et al. 2003).
D. Wax Compositional Analysis
Wax content and composition, including long chain alcohols and
aldehydes, can be analyzed with many techniques and instruments. The
determination of wax composition often requires extensive sample extraction
and preparation prior to instrumental analysis. Cuticular wax from potato
leaf was extracted by dipping and shaking the leaves in dichloromethane
spermaceti, carnauba, candellila and Japan waxes (Regert et al. 2005).
Sorghum wax was extracted from grain sorghum using hot hexane and
precipitating it in a -18°C (Hwang et al. 2002; Adhikari et al. 2006).
Similar extraction concept was applied by Kanya et al. (2007), where
sunflower seed wax was purified from oil refineries through extraction using
solvents and precipitation with chilled acetone. Liquid-liquid extractions
were successfully applied in beeswax, sugarcane and wheat (Irmak &
Dunford 2005; Irmak et al. 2006).
Recently, non conventional methods for wax extraction have been
developed with many techniques. Super critical carbon dioxide extractions
were reported to have higher waxes yields than solvent extractions, i.e.
cuticular wax from flax processing waste (Morrison et al. 2006), beeswax
(Jackson & Eller 2006), and sugarcane crude wax (Lucas et al. 2007).
Solvent-free extraction with high-intensity ultrasound treatment was studied
in rice bran wax. Under sonochemical conditions bran wax could also be
hydrolyzed yielding long chain alcohols (Cravotto et al. 2004). Molecular
distillation was used to increase the purity of octacosanol (C28-alcohol)
extracts from transesterified rice bran wax (Chen et al. 2005, 2007).
Compositional analysis of wax can be conducted with some
instruments, whether qualitative or quantitative analysis. Thin layer
chromatography was widely used to separate wax components (Hwang et al.
2002; Adhikari et al. 2006). Analytical methods of wax quantification
were applied with high performance liquid chromatography (Hwang et al.
2002; 2005). Wax compounds were identified and confirmed by gas
2003; Kanya et al. 2007) and liquid chromatography-mass spectrometry with
atmospheric pressure chemical ionization (Rezanka & Sigler 2006).
Main components of plant waxes were composed of fatty aldehydes,
fatty alcohols, fatty acids, hydrocarbons, wax esters, sterol esters and
triacylglycerols (Hwang et al. 2002; Adhikari et al. 2006), based on HPLC
data. Recently, mixture of long chain aliphatic primary alcohols,
policosanols, has been well investigated with gas chromatography technique
(Cravotto et al. 2004; Irmak & Dunford 2005; Irmak et al. 2006; Wang et al.
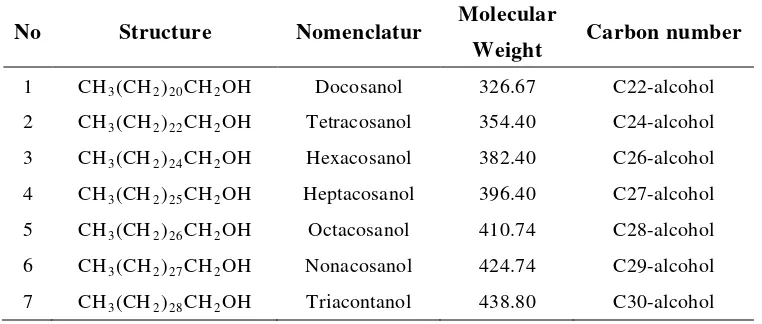
2007). The mixture contains mainly docosanol (C22), tetracosanol (C24),
hexacosanol (C26), octacosanol (C28), and triacontanol (C30), listed in
Table 3.
Table 3 Mixture composition of aliphatic alcohols (policosanols)
No Structure Nomenclatur Molecular
Weight Carbon number
1 CH3(CH2)20CH2OH Docosanol 326.67 C22-alcohol
2 CH3(CH2)22CH2OH Tetracosanol 354.40 C24-alcohol
3 CH3(CH2)24CH2OH Hexacosanol 382.40 C26-alcohol
4 CH3(CH2)25CH2OH Heptacosanol 396.40 C27-alcohol
5 CH3(CH2)26CH2OH Octacosanol 410.74 C28-alcohol
6 CH3(CH2)27CH2OH Nonacosanol 424.74 C29-alcohol
7 CH3(CH2)28CH2OH Triacontanol 438.80 C30-alcohol
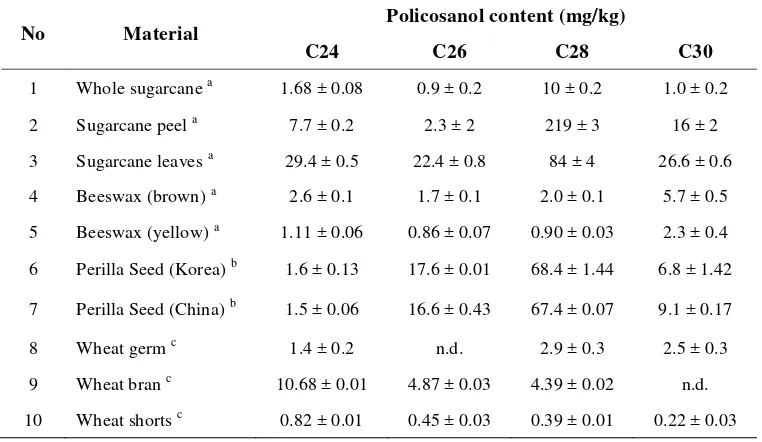
Sugarcane is the major source for the production of commercial
policosanol products. Irmak et al. (2006) reported that sugarcane peel
contained the highest amount of total policosanols, about 270 mg/kg. The
total policosanol contents of sugarcane leaves (181 mg/kg) were quite
compositions of sugarcane plant parts varied significantly, C28 (about 81%)
was the main component in all the sugarcane samples (Table 4).
Policosanols in the perilla seeds were composed of 67–68 % octacosanol,
16–17% hexacosanol, and 6–9% triacontanol. The analysis of
commercially milled wheat grain fractions, germ, bran, shorts, and flour
showed that policosanols where concentrated in the bran. Wheat germ
contained a significant amount of policosanols (10.1 mg/kg). These results
were expected since policosanols are associated with lipids and wax in plant
tissues. About 36% of the total policosanols in the wheat bran fraction was
[image:30.595.134.515.411.632.2]constituted of tetracosanol.
Table 4 Content and composition of policosanols in some materials
No Material Policosanol content (mg/kg)
C24 C26 C28 C30
1 Whole sugarcane a 1.68 ± 0.08 0.9 ± 0.2 10 ± 0.2 1.0 ± 0.2
2 Sugarcane peel a 7.7 ± 0.2 2.3 ± 2 219 ± 3 16 ± 2
3 Sugarcane leaves a 29.4 ± 0.5 22.4 ± 0.8 84 ± 4 26.6 ± 0.6
4 Beeswax (brown) a 2.6 ± 0.1 1.7 ± 0.1 2.0 ± 0.1 5.7 ± 0.5
5 Beeswax (yellow) a 1.11 ± 0.06 0.86 ± 0.07 0.90 ± 0.03 2.3 ± 0.4
6 Perilla Seed (Korea) b 1.6 ± 0.13 17.6 ± 0.01 68.4 ± 1.44 6.8 ± 1.42
7 Perilla Seed (China) b 1.5 ± 0.06 16.6 ± 0.43 67.4 ± 0.07 9.1 ± 0.17
8 Wheat germ c 1.4 ± 0.2 n.d. 2.9 ± 0.3 2.5 ± 0.3
9 Wheat bran c 10.68 ± 0.01 4.87 ± 0.03 4.39 ± 0.02 n.d.
10 Wheat shorts c 0.82 ± 0.01 0.45 ± 0.03 0.39 ± 0.01 0.22 ± 0.03
Note: n.d. = not detected
a
Irmak et al. (2006)
b
Adhikari et al. (2006)
c
E. Policosanol in Human Health
Much research has been done on the potential of policosanols in
lowering blood cholesterol and reducing the development of atherosclerotic
plaques (Rodriguez et al. 1997; Arruzazabala et al. 2002). Studies have
involved a wide range of subjects including experimental animals, healthy
volunteers, and elderly patients with hypercholesterolemia. Several studies
also have reported cardiovascular benefits of policosanols and its major
component octacosanol, without major adverse effects.
Policosanols may decrease the risk of atheroma formation by reducing
lipid levels, platelet aggregation, endothelial damage, and the development of
foam cells. Octacosanol may decrease cholesterol synthesis in the liver
before the generation of mevalonate. Octacosanol may down regulate the
cellular expression of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA)
reductase. Treatment with octacosanoic acid isolated and purified from
sugarcane wax suppressed HMG-CoA reductase production in cultured
fibroblasts, and this finding suggests a possible depression of de novo
synthesis of the enzyme and cholesterol (Menendez et al. 2001).
Furthermore, Singh et al. (2006) described that policosanol inhibits
cholesterol synthesis in hepatoma cells by activation of AMP-kinase and is
well established to suppress HMG-CoA reductase activity.
Research indicates that octacosanol at a dose of 5 mg/day may
reduce LDL and total cholesterol in patients with borderline to mildly
elevated serum triacylglycerol levels (Castano et al. 2003) and in
Combination treatment policosanol (10 mg/day) and omega-3 fatty acid (1
g/day) has been associated with significant inhibition of platelet
aggregation in rabbits compared with either drug alone (Castano et al.
2006).
Absorption and metabolism of nonesterified policosanol have been
studied by many researchers. Hargrove et al. (2003) mentioned that the
liver may be converting octacosanol to long chain fatty acids, which were
subsequently taken up by muscle. The study identified that the administered
octacosanol was found in sterols and triacylglycerol, which indicated
conversion to fatty acids and esterification. Policosanol metabolism is
linked to fatty acid metabolism via β-oxidation. Menendez et al. (2005)
demonstrated that octacosanoic acid was formed after incubation of fibroblast
cultures with 3H-octacosanol and after oral dosing with policosanol to rats.
In addition, shortened saturated (myristic, palmitic and stearic) and
unsaturated (oleic, palmitoleic) fatty acid were also formed after oral dosing
with policosanol to monkeys.
More rapid onset of effects suggests that oxidation of policosanols to
very long-chain fatty acids may be necessary for their hypocholesterolemic
actions and enhance the breakdown of LDL particles (McCarty 2005). In
another study, dietary octacosanol was thought to increase lipid catabolism
to generate more energy for improvement of motor endurance; and this
action may contribute in reductions of plasma triglyceride levels.
Octacosanol is already recognized as an ergogenic product used to enhance
In an effort to identify the potential mechanism for the
antiatherogenic effects of policosanols, Ng et al. (2005) investigated the
effects of these natural compounds on LDL oxidation and bile acid
excretion. Policosanols showed no antioxidant activity in human LDL
particles but increased bile acid secretion in hamsters.
However, other research groups using policosanol from alternative
sources have failed to reproduce the efficacy of these alcohols observed in
earlier studies. Lin et al. (2004) reported that wheat germ policosanol 20
mg/day had no beneficial effects on blood lipid profiles in subjects with
normal to mildly elevated cholesterol concentrations. Kassis et al. (2007)
mentioned that sugarcane policosanol treatment at a dose of 275 mg/kg diet
had any significant cholesterol-lowering effect on plasma lipid levels in
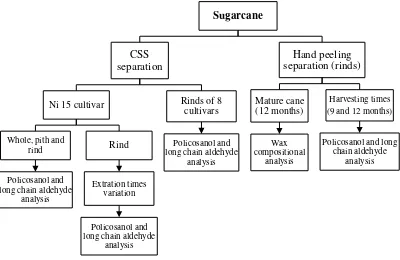
A. Materials
1. Samples
Whole parts of sugarcane stalk of Ni 15 cultivar, rind parts of seven
varieties of sugarcane (Ni 13, Ni 17, Ni 22, NiF 8, NCo 310, F 161 and F
177 cultivars), seven types of Kokuto (type A–G) and two non-Japan
brown sugars (type P from Thailand and type Q from Bolivia) were used
as materials. Rind and pith parts of sugarcane stalk (Figure 2) were
separated by cane separator, known as CSS (Cane Separation System),
from Mitsubishi Engineering Co. Ltd., Japan (Figure 3) and by hand
peeling. Kokuto samples were available products from 2007 to 2008
production year of Kokuto manufacturers in Okinawa Prefecture, Japan.
Cane juice and end product from Kokuto production line were also used in
[image:34.595.169.490.544.686.2]this study.
Figure 2 Rind (a) and (b) pith parts of sugarcane separated by CSS.
Figure 3 Cane Separation System (CSS).
2. Materials and Reagents
The policosanol standards used consist of docosanol (C22),
tetracosanol (C24), hexacosanol (C26), octacosanol (C28), and
triacontanol (C30). They were purchased from Sigma (Sigma Chemical,
St. Louis, MO). Derivatization reagent N-Methyl-N-(trimethylsilyl)
trifluoroacetamide (MSTFA) was purchased from GL Science (Japan).
Pyridinium chlorochromate, from Sigma (Sigma Chemical, St. Louis, MO),
was used for synthesis of long chain aldehyde standards.
Several chemicals were used as standards in analysis of wax
composition. They were triacontane, octacosanol, cholesteryl oleate,
lignoceric acid, methyl palmitate and stigmasterol from Sigma (Sigma
Chemical, St. Louis, MO), synthesized aldehyde (octacosanal), and triolein
(Nakarai Chemicals Ltd., Japan). HPLC grade hexane (Wako Pure
Chemical Industries Ltd., Japan) and methyl tert-butyl ether (Kanto
Chemicals Co. Inc., Japan) were used as mobile phases in HPLC analysis.
All reagents and chemicals were of analytical and HPLC grade, unless
B. Methods
Samples (sugarcane and Kokuto) were prepared and kept dry before
use. Sugarcane and cane juice samples were freeze-dried for 24 h. Then
all samples were crushed and ground with dry blender. Parts of sugarcane
samples were separated by CSS and hand peeling. Those sugarcane samples
were extracted for their wax, policosanol and long chain aldehyde compounds,
and analyzed as shown in experimental design below (Figure 4). Wax
composition of extracted samples were qualitatively separated by thin-layer
chromatography (TLC) and quantified by HPLC-ELSD. Policosanol and
long chain aldehyde contents and compositions were analyzed with GC-FID
and its compounds were identified with GC-MS. All listed quantification
runs were analyzed statistically ANOVA with completely randomized design.
Figure 4 Experimental designs of sugarcane wax, policosanol and long chain aldehyde analysis.
Sugarcane
CSS separation
Ni 15 cultivar
Whole, pith and rind
Policosanol and long chain aldehyde
analysis
Rind
Extration times variation
Policosanol and long chain aldehyde
analysis
Rinds of 8 cultivars
Policosanol and long chain aldehyde
analysis Hand peeling separation (rinds) Mature cane (12 months) Wax compositional analysis Harvesting times (9 and 12 months)
Policosanol and long chain aldehyde
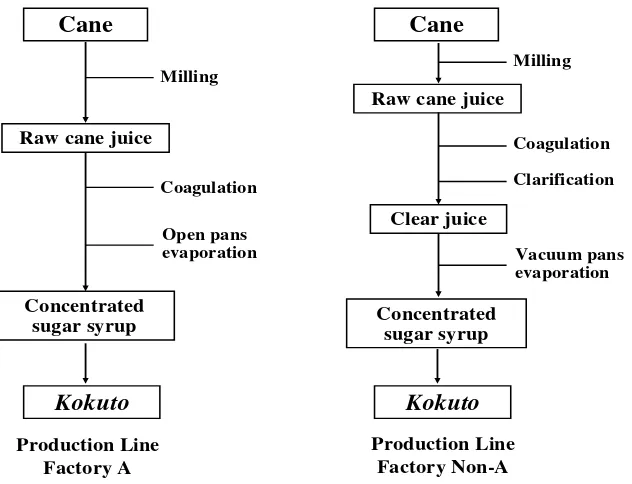
[image:36.595.125.525.434.694.2]Kokuto samples were obtained from two types Kokuto factories
(Figure 5); there were the traditional factory with open pan method (Kokuto A)
and the modern factory with vacuum pan technology (Kokuto Non-A). Raw
cane juice, clear juice and end product of both production lines were compared
to investigate the effect of Kokuto production types on policosanol and long
chain aldehyde contents. Several extraction methods and times were tried as
shown in the experimental design (Figure 6). Samples of Kokuto B–G were
collected from the modern factories (Kokuto Non-A type). Policosanol and
long chain aldehyde contents and compositions were analyzed with GC-FID
and its compounds were identified with GC-MS. All listed runs were
analyzed statistically ANOVA with completely randomized design.
Production Line Factory Non-A Milling
Open pans evaporation
Kokuto
Production Line Factory A Raw cane juice
Cane
Coagulation
Clear juice
Concentrated sugar syrup
Milling
Vacuum pans evaporation
Kokuto Raw cane juice
Cane
Coagulation
Concentrated sugar syrup
Clarification
[image:37.595.161.477.427.669.2]
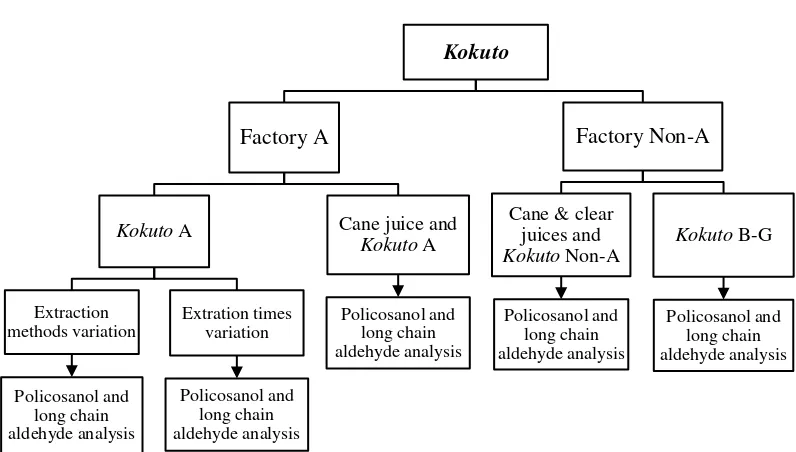
Figure 6 Experimental designs of policosanol and long chain aldehyde analysis in Kokuto.
1. Wax compositional analysis by TLC and HPLC-ELSD
a. Standard preparation
Several compounds were used as standards of wax components:
triacontane (for hydrocarbon), synthesized octacosanal (aldehyde),
cholesteryl oleate (sterol ester), octacosanol (alcohol), triolein
(triacylglycerol), lignoceric acid (acid), methyl palmitate (methyl ester)
and stigma sterol (sterol). Those standards were prepared in toluene for
TLC analysis and in chloroform with different concentrations (0.05–0.50
mg/ml) covering the levels of sample components for quantification of
each wax component by HPLC analysis.
Kokuto
Factory A
Kokuto A
Extraction methods variation
Policosanol and long chain aldehyde analysis
Extration times variation
Policosanol and long chain aldehyde analysis
Cane juice and
Kokuto A
Policosanol and long chain aldehyde analysis
Factory Non-A
Cane & clear juices and
KokutoNon-A
Policosanol and long chain aldehyde analysis
KokutoB-G
b. Sugarcane wax extraction
Briefly, 10 g of freeze-dried rind of sugarcane were placed in
Advantec No. 84 thimble filter and extracted using soxhlet for 4 h with
about 150 ml of hexane, methanol (20:1 v/v). The solvent solution was
removed from the extract with rotary-evaporator under vacuum condition
at 40°C. The amount of dry wax extract was weighed and diluted with
toluene for TLC analysis and with chloroform down to 1–2 mg/ml to the
reach detection level of ELSD used in HPLC analysis.
c. TLC Analysis
Thin-layer chromatography (TLC) of waxy materials extracted
from sugarcane rind samples using a silica gel 60, 20 cm × 10 cm × 250
µm TLC plate. Cholesteryl oleate, methyl palmitate, synthesized
octacosanal, triolein, lignoceric acid, octacosanol and stigma sterol were
used as standards. Samples and standards were eluted with two steps of
developing solvents. The first solvent mixture, comprising hexane,
diethyl ether, acetic acid (95:5:1 v/v/v), was allowed to travel 10 cm
before the plate was removed and the solvent allowed to evaporate.
Once dried, the plate was redeveloped in hexane, diethyl ether, acetic
acid (80:20:1 v/v/v) to the top of the plate. Developed bands were
visualized by spraying the plate with 10% cupric sulfate solution
containing 8% phosphoric acid. Then the TLC plate was dried in heater
d. HPLC-ELSD analysis
Wax components were separated and determined as previously
reported by Adhikari et al. (2006), using HPLC equipped with a Luna
silica column (250 mm × 4.6 mm i.d. × 5 µm film thickness) connected
to a guard column (4 × 3 mm i.d.) supplied by Phenomenex (Torrance,
CA). Two Shimadzu LC-10AD-VP pumps were operated in
combination with a Shimadzu SCL-10A-VP gradient controller. The
column and guard column temperature were kept constant at 40°C by a
Shimadzu CTO-10AC-VP. Shimadzu model LT evaporative light
scattering detector was operated at 50°C with nitrogen pressure 350 kPa.
Mobile phases consisted of a gradient of hexane (solvent A) and methyl
tert-butyl ether containing 0.2% acetic acid (solvent B), with the
following profile: 0–2 min, 100% A; 3–10 min, 95% A; 14 min, 55% A;
23–26 min, 0% A; then 27–40 min, 100% A. Flow rate of mobile phase
was 1 ml/min. Injection volume of samples and standard were 5 µl.
The detection limit of this analysis was 0.01 mg/ml.
Several compounds were used as standards, i.e. triacontane,
cholesteryl oleate, synthesized octacosanal, triolein, lignoceric acid,
octacosanol and stigma sterol. Wax component contents in analyzed
samples were determined based on the relation between peak area and
calibration curve of each standards. Wax contents were calculated in
percentage (%, w/w) and concentration (mg/100 g sample, wet weight
basis) by following equations:
Component A (%) =Peak area of component A
Concentration (mg/100g) =
Content�mgml�∗× Volume of extract (ml) × 100
Sample weight (g)
where content (*) was calculated by calibration curve of its standard.
2. Policosanol and long chain aldehyde analysis by GC-FID
a. Standard preparation
Mixed policosanol standards for policosanol compounds
quantification were prepared in toluene. Aldehyde standards were
synthesized from their alcohols form by oxidation with pyridinium
chlorochromate, as described by Pérez-Camino et al. (2003). Each 1
mM corresponding alcohols standards (19.14 mg hexacosanol, 20.54 mg
octacosanol, 21.94 mg triacontanol) and 9 mM pyridinium
chlorochromate (97.5 mg) were stirred in 50 ml dichloromethane for 1.5
h at room temperature. The reaction mixture was eluted with
dichloromethane through a short column (6 × 2 cm I.D.), packed with
silica gel 60. Then the reaction product was dried by N2 gas and diluted
in toluene. The synthesized long chain aldehyde standards were
checked with GC-FID analysis.
b. Sample extraction
Kokuto A samples were extracted with two extraction methods in
order to investigate the optimum extraction method; they were
liquid-liquid extraction (LLE) by the method of Irmak et al. (2006) and
method was applied to policosanol and long chain aldehyde contents
analysis in sugarcane and other Kokuto samples.
Liquid-liquid extraction (LLE) method. Briefly, 6 g of Kokuto
sample was mixed with 50 ml of 1.0 N NaOH and 50 ml of methanol and
subsequently hydrolyzed by refluxing in a heater for 30 min. After
cooling, the mixture was filtered through Advantec No. 5A filter paper
under vacuum condition. Then Millipore water was added to the filtrate.
The solution was extracted three times with 50 ml diethyl ether.
Combined diethyl ether phases from three extractions were neutralized
with Millipore water until pH of water phase reached 7. The extract
was dried over 50 g of anhydrous sodium sulfate by storing at 4°C for
one night. Then the solvent was removed from the extract with
rotary-evaporator under vacuum condition. The dry extract was diluted
in toluene or chloroform and made up to 2 ml prior to analysis.
Solid-liquid extraction (SLE) method. Briefly, 10 g of
freeze-dried sugarcane or 6 g of Kokuto were placed in Advantec No. 84
thimble filter and extracted using soxhlet with about 150 ml of several
systems of organic solvent. The solvent systems were Soxhlet A
(chloroform, methanol (2:1 v/v)); Soxhlet B (hexane, methanol (10:1
v/v)); Soxhlet C (hexane, methanol (20:1 v/v)); Soxhlet D (hexane,
methanol (30:1 v/v)); and Soxhlet E (hexane). Several extraction times
were also attempted in order to investigate the heat stability and optimum
extraction condition of policosanol and long chain aldehyde compounds
in sugarcane and Kokuto samples. The solvent solution was removed
Then the dry extract was diluted in toluene and made up to 2 ml for
GC-FID analysis, whereas for GC-MS analysis, the extract was diluted in
chloroform.
c. Gas chromatography analysis (GC-FID)
Shimadzu GC 17-A equipped with a fused capillary column (DB
5, 0.25 mm i.d. × 30 m × 0.25 µm film thickness) from J&W Scientific
(Folsom, CA) and a flame ionization detector were used for policosanol
and long chain aldehyde quantitative analysis. The GC injector was set
at 350°C and the flame ionized detector 350°C. The samples (1 µl)
were injected with split ratio of 1:10. The carrier gas was helium with a
flow rate of 1 ml/min. The oven temperature was programmed at
150°C as initial temperature, raised to 320°C at 4°C/min and maintained
at 320°C for 15 min.
By injecting mixture standards of policosanol and aldehyde with
different concentrations covering the levels of sample extracts, relation
between concentration and peak height was plotted for calibration.
Concentrations of standard solutions for the standard curve were
0.05–0.50 mg/ml. The detection limit of this analysis was 0.01 mg/ml.
Policosanol and long chain aldehyde concentrations were calculated as
mg/100 g sample (wet weight basis for sugarcane) by this following
equation:
Concentration (mg/100g) =
Content�mg ml�
∗
× Volume of extract (ml) × 100
Sample weight (g)
3. Policosanol and long chain aldehyde identification by GC-MS
The silylated derivatization was used for mass spectrum analysis of
policosanol, however mass spectrum of aldehyde was analyzed without
silylation. In this case, policosanol was identified as its trimethylsilyl
derivates. N-Methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) was
used as silylation reagent. The derivatization solution was made by mixing
0.5 ml of sample in chloroform with 250 µl MSTFA. The solution was
heated at 50°C for 15 min. The volume of the mixed solution was made up
to 1 ml with chloroform for GC-MS analysis. Derivatization was also
applied for policosanol standard solution at concentration of 0.1 mg/ml.
The analysis was performed using Shimadzu GC-MS QP-2010 with
a fused capillary column DB-5 MS (0.25 mm i.d. × 30 m × 0.25 µm film
thickness) from J&W Scientific (Folsom, CA) under the same GC
conditions. The samples (0.3 µl) were injected with split ratio of 1:10.
For MS detection, the electron impact (EI) ion source and transfer line
temperature were set at 200°C and 280°C. The ionization energy was
mode at 70 eV. The mass acquisition scan range and rate were 30–500
amu and 2 scans/s. Identification of each policosanol or aldehyde was
conducted by comparing its retention time and mass spectrum directly to
those of its respective standard. The mass spectrums were also confirmed
with NIST 2005 Mass Spectral Library by GCMSsolution software
4. Statistical analysis
All extraction runs and analysis were carried out in triplicate and
analyzed statistically using ANOVA with completely randomized design.
The mean values of analysis results were reported. The data were analyzed
by SPSS Version 13.0 for Windows (SPSS Inc., USA, 2003). Univariate
comparisons of the various means were carried out by post hoc test of
Duncan at p = 0.05. Nine experimental designs were attempted with
statistical analysis, there were:
a. The effect of rind parts of sugarcane cultivars on wax composition (3
samples, i.e. NiF 8, Ni 15 and Ni 22 cultivars).
b. The effect of different parts of sugarcane of Ni 15 cultivar on the
policosanol and long chain aldehyde contents (3 samples, i.e. rind, pith
and whole stalk).
c. The effect of extraction times of rind part of Ni 15 cultivar on the
policosanol and long chain aldehyde contents (5 extraction times, i.e. 2, 4,
8, 16 and 24 h).
d. The effect of sugarcane cultivars on the policosanol and long chain
aldehyde contents of the sugarcane rinds (7 samples, i.e. Ni 13, Ni 15, Ni
17, NiF 8, NCo 310, F 161 and F 177 cultivars).
e. The effect of sugarcane harvesting time on the increasing of total
policosanol and long chain aldehyde contents of the sugarcane rinds (5
samples, i.e. NCo 310, Ni 15, Ni 17, NiF 8 and Ni 22).
f. The effect of extraction methods of Kokuto A on the policosanol and
long chain aldehyde contents (6 extraction methods, i.e. a liquid-liquid
g. The effect of extraction times of Kokuto A on the policosanol and long
chain aldehyde contents (5 extraction times, i.e. 4, 8, 16, 24 and 32 h).
h. The effect of Kokuto types on the policosanol and long chain aldehyde
contents of the Kokuto (9 samples, i.e. type A–G, P and Q).
i. The effect of Kokuto production types on the policosanol and long chain
aldehyde contents (5 samples, i.e. raw juice and end product of Kokuto
factory A; and raw juice, clear juice and end product of Kokuto factory
A. Sugarcane Wax Composition
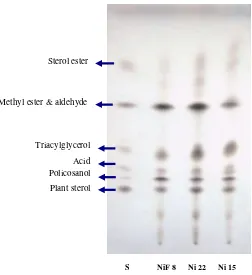
The qualitative separation of most of the major classes of aliphatic
[image:47.595.174.426.407.682.2]wax components could be achieved by silica gel of thin layer chromatography
(Figure 7). Compositions of the waxy materials from sugarcane cultivars
were all similar. They were composed of sterol, alcohol, acid,
triacylglycerol, methyl ester, aldehyde and sterol ester. Thin layer
chromatography had been widely used for qualitative analysis in waxy
materials (Hwang et al. 2002; Adhikari et al. 2006; Webster et al. 2006).
Figure 7 Thin layer chromatography of waxes of several sugarcane cultivars. S: Standard.
S NiF 8 Ni 22 Ni 15
Plant sterol Policosanol Acid Triacylglycerol Sterol ester
Wax components of sugarcane on TLC plate could finally be
visualized by cupric sulfate charring. Each component of waxy materials
was well charred and separated, except for sterol ester which split into two
smear bands. These two separated bands were identified by Adhikari et al.
(2006) as wax ester and sterol ester. Besides this, methyl ester and aldehyde
were appeared as a single spot, while acid and policosanol as closed dense
spots.
Separation of cane wax components by high performance liquid
chromatography (HPLC) were further quantified with evaporative
light-scattering detector (ELSD). Five peaks in HPLC chromatogram were
identified as groups of aldehyde, sterol ester, triacylglycerol, acid, alcohol,
and sterol (Figure 8). Wax component contents in analyzed samples were
determined based on the relation between peak area and calibration curve of
each standards. An example of standard curve of triacylglycerol is shown in
Figure 9, it has a linear equation of y = 1E+07x – 52429. These standards
(Figure 10) had different concentrations covering the levels of components in
samples, i.e. 0.05–0.50 mg/ml.
This result confirmed the same result obtained by TLC. Every peak
represented one group of wax components, except for peak 1 which was a
mixture of aldehyde and sterol ester. However, Hwang et al. (2002) and
Adhikari et al. (2006) identified this peak as mixture of aldehyde, sterol ester
and also wax ester. A very weak respond of hydrocarbon compound
somewhat was detected (after 3 minutes retention), but it was not enough for
Figure 8 HPLC chromatogram of sugarcane rind of Ni 15 cultivar. The chromatogram was obtained by HPLC-ELSD method.
Peak 1 : Aldehyde, sterol ester Peak 4 : Alcohol
Peak 2 : Triacylglycerol Peak 5 : Sterol
[image:49.595.137.497.111.330.2]Peak 3 : Acid
Figure 9 Standard curve of triacylglycerol. y = 1E+07x - 524294
R² = 0.9987
0 1,000,000 2,000,000 3,000,000 4,000,000 5,000,000 6,000,000
0.00 0.10 0.20 0.30 0.40 0.50 0.60
P
ea
k
A
rea
Concentration (mg/ml)
0.0 5.0 10.0 15.0 20.0 25.0 30.0
0.2 0.4 0.6 0.8 1.0
0.0
1
2 3 4
5
[image:49.595.164.490.350.682.2]Figure 10 HLPC chromatograms of wax composition standards. (a) aldehyde, (b) sterol ester, (c) triacylglycerol, (d) acid, (e) alcohol, (f) sterol. Standards concentrations were 0.5 mg/ml (a-d) and 0.4 mg/ml (e-f). The chromatograms were obtained by HPLC-ELSD method.
(a) (b)
(c) (d)
Wax components in sugarcane rind were composed of mixture of
aldehydes and sterol esters (55.1–60.4%), alcohols (31.8–39.8%),
triacylglycerols (2.3–4.6%), acids (2.0–2.7%), and sterols (0.5–0.9%),
respectively (Table 5, Figure 8). Significant differences (p < 0.05) were
observed in all wax composition of Okinawan sugarcane cultivars. The
highest content of wax component was found in sugarcane rind of Ni 22
cultivar with about 1 g of mixture of aldehydes and sterol esters per 100 g
sample (wet weight basis). Followed with mixture of aldehydes and sterol
esters of NiF 8 and Ni 15 cultivars, i.e. 751.0 and 594.6 mg/100 g. The
alcohol group, policosanols, of Ni 22 cultivar sample was 462.9 mg/100 g;
while sterol group was less abundant and its response became too small for
making a detectable peak.
Table 5 Wax compositions of rind part of Okinawan sugarcane cultivars, obtained by HPLC-ELSD.
Cultivar Aldehyde,
sterol ester* Triacylgycerol Acid Alcohol Sterol
% (w/w)
NiF 8 55.1 ± 1.1 b 3.7 ± 0.4 b 2.7 ± 0.1 a 39.8 ± 4.0 a 0.5 ± 0.03
Ni 15
b
60.5 ± 3.0 a 4.6 ± 0.6 a 2.4 ± 0.4 ab 31.8 ± 2.1 b 0.9 ± 0.2
Ni 22
a
58.5 ± 1.8 ab 2.3 ± 0.2 c 2.0 ± 0.2 b 37.3 ± 1.9 ab n.d.
mg/100 g
‡c
NiF 8 751.0 ± 29.4 q 101.7 ± 10.7 p 62.0 ± 1.6 p 367.5 ± 8.1 q 33.3 ± 0.01
Ni 15
p
594.6 ± 32.6 r 80.2 ± 6.0 q 39.0 ± 1.4 q 216.4 ± 14.7 r 25.3 ± 0.2
Ni 22
q
1027.1 ± 66.5 p 97.0 ± 6.9 p 63.2 ± 3.1 p 462.9 ± 7.6 p n.d.r
*Calculated as aldehyde group.
‡
Not detected.
a
Data are means ±S.D. (n = 3). Means in the same column with the same letter are not
[image:51.595.135.512.485.664.2]Similar separation method and stationary phase of wax component of
plant waxes were applied in grain sorghum (Hwang et al. 2002), perilla and
sesame seeds (Adhikari et al. 2006), potato leaves (Szafranek & Synak 2006),
and calanoid copepod Calanus finmarchicus (Webster et al. 2006). The
amounts and compositions of these waxes were markedly different depended
on their genetics, bio-functions, plant growth conditions, and environments.
Another HPLC technique of wax components separation was using alumina
as stationary phase (Nordback & Lundberg 1999).
Plant waxes are a complex heterogeneous mixture of very long chain
(C20–C34) fatty acids and their derivatives. During wax biosynthesis very
long chain fatty acids are further modified to aldehydes, alkanes, ketones, and
so on. Grain sorghum and carnauba waxes compositions were well
investigated by Hwang et al. (2002). Grain shorghum wax was composed of
46.3% (w/w) fatty aldehydes, 7.5% fatty acids, 41.0% fatty alcohols, 0.7%
hydrocarbons, 1.4% wax esters and sterol esters, and 0.9% triacylglycerols.
Carnauba wax contained of 34.3% wax esters, 5.1% fatty acids, undetermined
amount of fatty alcohols, and 3.0% triacylglycerols, determined by HPLC.
Major components of the waxy materials from Korean and Chinese
perilla seeds were alcohols (25.5 and 34.8%), hydrocarbons (18.8 and 10.5%),
wax esters, steryl esters and aldehydes (53.0 and 49.8%), acids (1.7 and
2.1%), and triacylglycerols (1.0 and 2.9%), based on HPLC data were
reported by Adhikari et al. (2006). The principal components of leaf
cuticular waxes from potato varieties were very long chain n-alkanes,
2-methylalkanes and 3-methylalkanes (3.1–4.6 µg cm-2), primary alcohols
cm-2
In general, sugarcane stalk was composed of sugars 12–16%, water
70–74%, pith fiber 7%, rind fiber 7%, and epidermis 0.1%. Wax would
abundantly found in rind part as epicuticular wax. Figure 11 shows the
comparison of policosanol and long chain aldehyde contents as wax
components in sugarcane stalk. Rind and pith samples were separated by
CSS (Cane Separation System). This study discovered that policosanol and
long chain aldehyde compositions of sugarcane parts varied significantly.
Policosanol and aldehyde compounds in the rind part of sugarcane were
found much abundant than in pith.
), analyzed by GC-FID (Szafranek & Synak 2006). Methyl ketones,
sterols, β-amyrin, benzoic acid esters and fatty acid methyl, ethyl, isopropyl
and phenylethyl esters were found in potato waxes. A new group of
cuticular wax constituents consisting of free 2-alkanols with odd and even
numbers of carbon atoms ranging from C25 to C30 was also identified.
Figure 11 Policosanol and long chain aldehyde contents in sugarcane Ni 15 cultivar, obtained by GC-FID analysis.
Means in the same group with the same letter are not significantly different (p > 0.05).
0 20 40 60 80 100
Rind Pith Whole stalk
A
m
ou
n
t (
m
g/
100 g)
Policosanol
Aldehyde a
p
c r
b
[image:53.595.172.471.503.661.2]The whole stalk of sugarcane Ni 15 cultivar contained 35 mg
policosanols and 24 mg aldehydes per 100 g of wet weight basis. The
policosanol and aldehyde contents in pith part were negligible (about 1
mg/100 g), however, policosanols and adehydes of rind part was found in
high concentration, i.e. 80 mg/100 g. This result is associated with surface
wax present in rind of cane. The surface waxes protect plants from water
lost and environmental stress (Koch et al. 2006; Dominguez & Herdia 1996).
Separation with CSS would make loosing an amount of epicuticular waxes in
rind, while either it separated to epidermis wax chamber or attached in rolls
and blades of CSS. In this way the hand peeled rind samples were also
investigated.
Rutherford and Staden (1996) reported a suggestion of sugarcane
surface wax component towards resistance to borer Eldana saccharina
Walker. In their investigation, a high ratio of alcohol to aldehyde and
shorter chain length appeared to be associated with cane resistance.
Furthermore, Purcell et al. (2005) described epicuticular wax as a potential
genetic marker and predictor of desirable plant traits. According to that a
comprehensive study of wax compositional analysis and eco-geochemistry of
B. GC Chromatogram and Mass Spectrum of Policosanols and Long Chain Aldehydes
Figure 12 shows the typical chromatogram of standard mixture and
sample extracts. All compounds of standard mixture were completely
separated to every single peak (Figure 12a). The retention time of three
synthesized aldehyde compounds (hexacosanal, octacosanal, and triacontanal)
were detected 1 minute faster than their corresponding alcohol compounds.
Quantification of each policosanol and aldehyde compounds in extracted
samples was determined base on the retention time and peak height of each
referred standard.
Derivatization is primarily performed to modify an analyte’s
functionality in order to enable chromatographic separations. The formation
of chemical derivatives to facilitate meaningful analysis has long been a
common practice in gas chromatography. The use of MSTFA as silylation
reagent for derivatization has been reported in policosanol determination
using GC-FID by some researchers (Adhikari et al. 2006; Morrison et al.
2006), however, the use of MSTFA in the pre-study was found in less
effective for policosanol determination comparing to direct analysis.
Besides this, the use of internal standard (i.e. octacosanoic acid) was avoided
due to a number of closed peaks in interest area of chromatogram of samples
(Figure 12b and 12c).
Policosanol and long chain aldehyde contents in analyzed samples
were determined based on the relation between peak height and calibration
curve of each standards. An example of standard curve of octacosanol is
Figure 12 Typical gas chromatogram of (a) policosanol and long chain aldehyde standards, (b) Kokuto A, (c) sugarcane rind of Ni 15 cultivar. The chromatograms were obtained by GC-FID.
Peak 1 : C22-OH Peak 2 : C24-OH
Peak 3 : C26-OH Peak 3a
Peak 4 : C28-OH Peak 4
: C26-CHO
a
Peak 5 : C30-OH Peak 5
: C28-CHO
a
: C30-CHO
0.0 10.0 20.0 30.0 40.0 50.0 (min)
(b) 4 5a 5 3 3a 2 1 4a
0.0 10.0 20.0 30.0 40.0
4a 4 5a 5 (min) 3 3a 2 1 (a)
0.0 10.0 20.0 30.0 40.0 50.0 (min)
(c)
4 5a
[image:56.595.137.526.75.529.2]Figure 13 Standard curve of octacosanol.
Figures 14 and 16 show the mass spectrum of trimethylsilyl ether of
C28, derivate of octacosanol, and C28 aldehyde (octacosanal) as standards.
The policosanol and aldehyde compounds of the samples (Figures 15 and 17)
were identified by direct comparison of their chromatographic retention times