No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior written permission of the publisher, Addison-Wesley Publishing . Company, Inc., Advanced Book Program, Reading, Massachusetts 01867, U.S.A. An updated and expanded version of lecture notes prepared for students at the Feinberg Graduate School of the Weizmann Institute of Science, Rehovoth, Israel. This book provides an updated and expanded version of lecture notes that I began to prepare in early 1974, in the wake of the October 1973 Middle East War.
In preparing the e c t u r e notes for publication, I was greatly encouraged by the many students, friends and colleagues, who not only shared with me the feeling that there is an urgent need for such a book, but also helped me with your comments and suggestions. I encourage you to consult some of the books and articles listed in the references. The name carbohydrate was originally assigned to compounds thought to be hydrates of carbon, with the general formula Cn (H20)n.
Chitin is the main organic component of the exoskeleton of arthropods such as insects, crabs and lobsters. They are the main products that harness the sun's energy and convert it into a form that can be used by humans and other animals, as well as by many other organisms.
Even in the most modern textbooks, there is very little, if any, mention of the many other essential roles that carbohydrates fulfill, some of which I will discuss in detail in this course. They have become interesting molecules to think about in relation to the life of the cell. The turn of the century witnessed Emil Fischer's groundbreaking carbohydrate work; about fifty years ago C.
Approx. 100 mg of the pure syrupy compound was obtained from 400 avocados whose weight was 93 kg. In connection with the study of the role of these intermediates in the biosynthesis of complex carbohydrates, new p r i n c i p l e s for the assembly of b i o l o g i c a l polymers have been discovered. A completely different reason for the new wave of interest in carbohydrates stems from the fact that many of the human genetic diseases for which the molecular bases have been elucidated are defects in carbohydrate metabolism.
The best concise description of the subject, a must for anyone who wants to take this course seriously. Nobel Prize lecture by the man who, perhaps more than anyone else, contributed to the return of
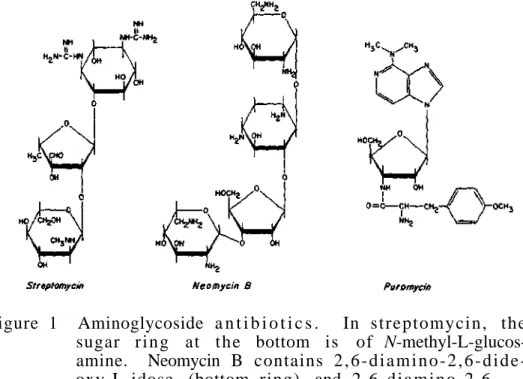
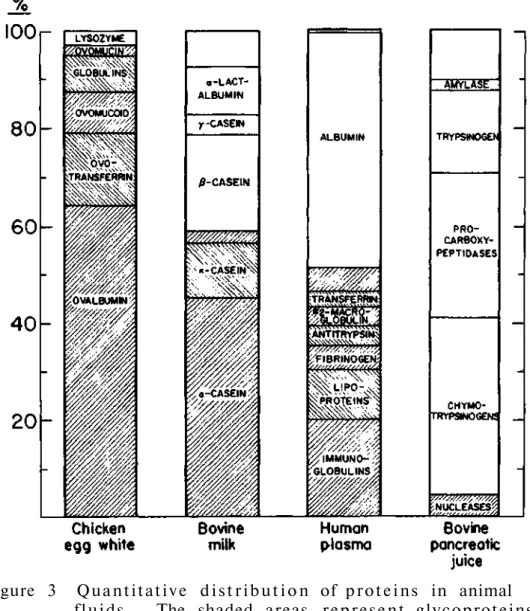
It is possible that there are glycoproteins with even higher sugar content, as there is evidence that glycogen contains small amounts of protein. Therefore, it can be seen that the carbohydrate spectrum of glycoproteins is significantly expanding. 34 "true" proteins such as lysozyme, ribonuclease A and concanavalin A, which do not contain sugar in their molecules.
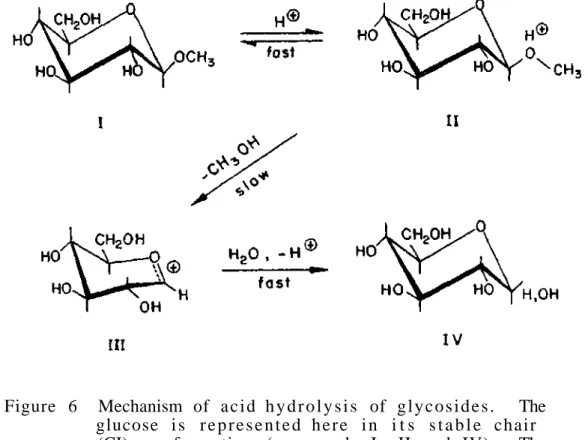
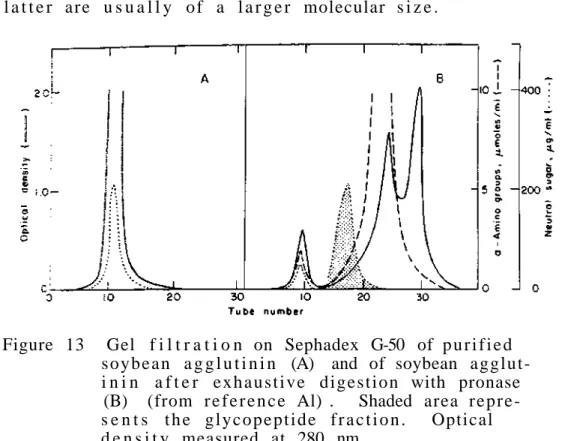
For the is o l a t i o n and p u r i f i c a t i o n of glycoproteins we usually use the same methods as those used in the study of p r o t e i n s without carbohydrates. This destruction is not due to the effect of acid only s i n c e it has been shown t h a t if oxygen is excluded, e x t e n t e .
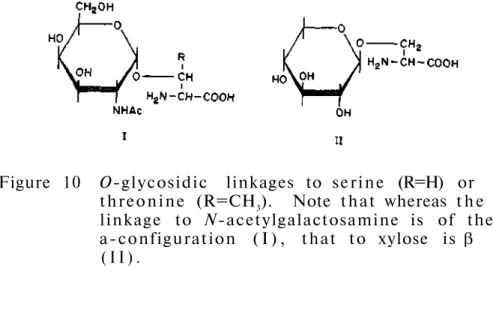
Such a product is formed because the carboxyl function of the alternative uronic acid residues protects the glycosidic oxygen from attack by hydrogen ions. At the other extreme, we have proteins such as sheep submaxillary mucin (molecular weight of about one million), which contains about 800 chains in each mole. Of the approximately twenty amino acids that make up proteins, only five appear to participate in the formation of bonds with carbohydrates.
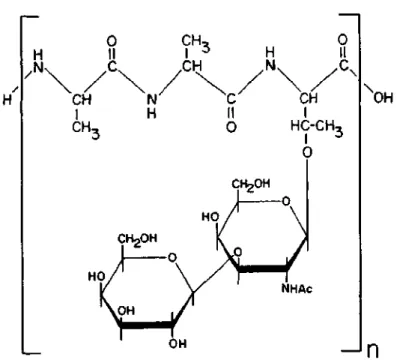
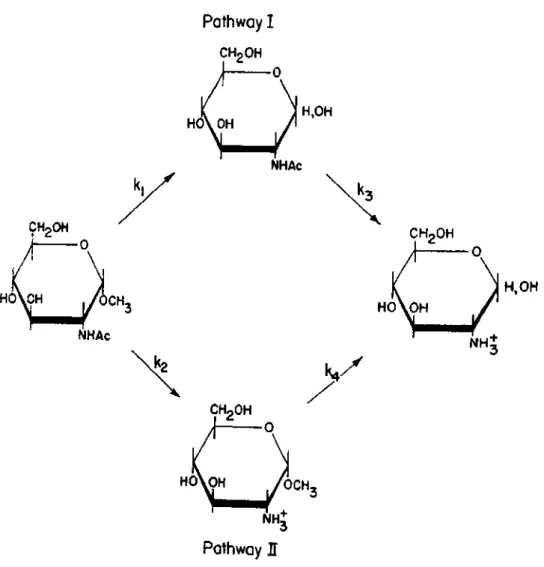
The occurrence of both N-glycosidic and C-glycosidic carbohydrate-peptide bonds in the same molecule has been observed in immunoglobulins, f e t u i n, human choriongonado- t r o p i n, e r y t h r o c y the glomerular membrane and the glomerular membrane. THE N-ACETYGLUCOSAMINYL-ASPARAGINE LINKAGE (3) The first carbohydrate-peptide linkage to be i d e n t i f i e d was the t between N-acetylglucosamine and asparagine (GlcNAc-Asn or Asn-GlcNAc). A l t e r n a t i v e l y, the compound can be considered as a condensation product of the β-glycosylamine of N-acetylglucosamine (or more a c c u r a t e l y 2-aceetamido-2-deoxy- β-D-glucopyranosylamine) d.
One way to form such a bond is by acylation of the β-amine of the sugar with the β-carboxyl of a s p a r t i c acid. We have seen here an example of the use of sodium borohydride to cleave al k a l i - l a b i l e sugar l i n k a g e s. g. glucose) is a par t ial transformation to the C-2 epimeric aldose and the corresponding keto s e. If alkali treatment is excluded in the presence of borohydride, converts the unsaturated amino acid d e r i v a t i v e s to alanine with the DL configuration and 2-aminobutyric acid (also DL), r e s p e c t i v e l ; the latter compounds are stable to acid hydrolysis and can be determined by amino acid analysis.
Treatment with alkali under reducing conditions converts the peptide-bound monosaccharide to the corresponding sugar alcohol and t h e r e f o r e serves to d e n t i f the sugar of the linkage region. Although the anomeric configuration of th i bond is not known, it is described here as a β-form rb i t r a r i l y . The recent finding of glycopeptides containing thio-glycosidic bonds is of some i n t r e s t , p a r t i c u l a r l y since one of the glycopeptides, with a glucose t r i is a membrane-bound cysteine c h a r i d by a membrane cysteine.
The amino acid sequence of this unusual glycopeptide was similar to that of the other glycopeptide with a thioglycosidic bond isolated from urine. The existence of a new type of glycosidic bond, formed between the phenolic hydroxyl group of tyrosine and the glycosidic hydroxyl of sialic acid, was recently suggested. Such a carbohydrate-peptide linking group was reported to be present in chicken ovomucoid, although the evidence for this structure was not very convincing.
In fact, this was why until recently it was believed that all mannose residues in glycoproteins were α-linked, which turned out to be incorrect when highly purified α-mannosidase became available. However, no mannose could be released by prolonged incubation with high concentrations of α-mannosidase, although such removal was easily achieved after incubation of the purified glycopeptide with small amounts of the enzyme. The enzyme works by breaking the bond between the GlcNAc-Asn and the rest of the carbohydrate side chain.
The microheterogeneity of the carbohydrate s i d e chains of glycoproteins may be due to an incomplete biosyn- t e t i c sequence (which we will discuss in d e t a i l l a t sintering of placement or by a complete degradation of carhyd) chains. A common sequence also appears to exist in the core region of the carbohydrate s i d e chains of glycoproteins of. As mentioned in a previous lecture (p.72), this led to complete cleavage of the glucosamine-asparagine bond, with accompanying production of terminal glucosaminitol.
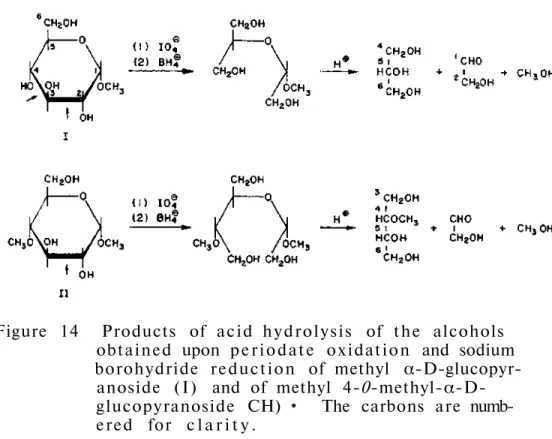
Periodat o x i d a t i o n , NaBH4 reduction and hydrolysis in strong acid of the fragments thus prepared all gave xylosamine t o l . Definitive evidence for the s t r u c t u r e of the isolated product, a f t e r complete removal of mannose from the glycopeptides, was obtained when it was shown that be i d e n t i c a l with the chemically synthesized GlcNAc-β-(l->4)-Glc-NA. Using Smith's degradation method and methylation s t u d i e s , the penultimate glucosamine was found substituted with mannose at the 4 p o s i t i o n .
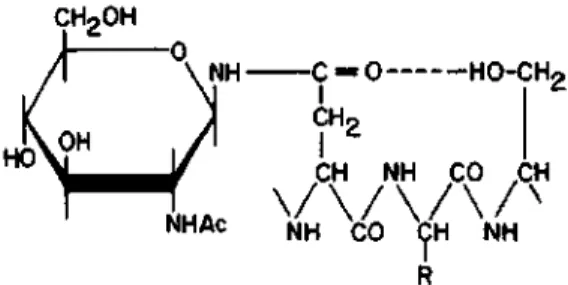
Neuberger suggested in 1968 that in the sequence Asn-X-Ser (or Thr) a hydrogen bond is formed between the carbonyl of the side chain of asparagine and the hydroxyl group of the hydroxyamino acid (Fig. 21).
109 Table 9
This circumstance is not unique: the conversion of p r o l i n e to hydroxyproline by collagen p r o l i n e hydroxylase requires that the amino acids appear in sequence.
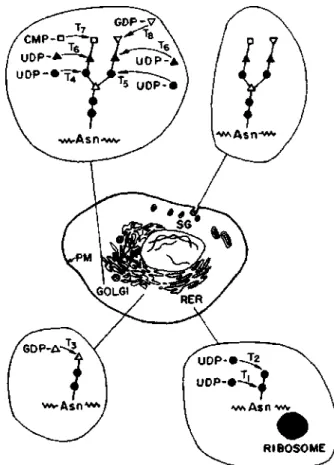
About 10% of the membrane consists of sugar residues, compared to less than 1% in most vertebrate fiber collagens. This is to be expected for sugars that occur only at or near the non-reducing ends of oligosaccharide pro s the groups and are therefore incorporated into glycoprotein in the final stages of the biosynthetic process. The secretory granule membrane fuses with the plasma membrane and the fused portion is then degraded, releasing glycoprotein into the external space.
The first step involves oxidation at C-4 and conversion of the primary alcoholic group at C-6 to a methyl group. It is formed by an oxidation-reduction reaction without the release of intermediate products from the surface of the enzyme. The presence of the 4-axial hydroxyl group introduces a degree of stability into the galactose molecule compared to glucose.
Although written a dozen years ago, it is still a very basic review of the subject with many stimulating ideas. The stereochemistry of t h e k e t o s i d i c bond of s i a l i c acid has been elucidated by a study of the two anomers. The same sequence of r e a c t i o n is involved in the synthesis of the glomerular basement membrane, i.a. a collagen-type glycoprotein (Fig. 3 9.
The subcellular distribution of the glycosyltransferases involved in the assembly of the NANA-Gal-GlcNAc terminal trisaccharide from the GlcNAc-Asn-type carbohydrate units was studied in rat liver. These findings are consistent with the role of the Golgi apparatus in the biosynthesis of glyco. After i n i t i a t i o n has occurred, elongation of the oligosaccharides is mainly controlled by the specificity of the MGT system for the acceptors.
It is therefore not surprising that the structures of the heterosaccharide side chains of glycoproteins do not show the same uniformity that we expect to find in the amino acid sequence of the protein, for the latter is a direct result of the DNA sequence. Thus, porcine liver contains a sialyltransferase with a specificity different from that of the enzyme acting on the GlcNAc-Asn-type prosthesis. In the covalent enzyme-substrate intermediate formed, the configuration of the anomeric bond has been reversed.
The location of the lipid in the membrane allows it to mediate the transfer of low molecular weight hydrophilic compounds that serve as building blocks of macromolecules located within or outside the hydrophobic region. There are now an increasing number of glycoproteins for which we have good evidence or reasonable and sound proposals for the function of the sugar in the molecule.