P O L E C T R A C T I O N : The percentage ratio of the weight of pol in mixed juice to the weight of pol in the corresponding weight of cane. The average weight per pole will be determined by mutual agreement between the miller and the supplier of the cane.
Imbibition water
When the sugar cane is weighed in slings, the bundle with attached slings must be completely disconnected from the crane. The average weight per pole should be used in conjunction with the total number of poles used per truck to determine the tare amount for poles in each truck.
Mixed juice a. General
The arithmetic mean of the ten check weights shall be determined and if this mean weight differs by more than 0. A u t o m a t i c weighing machines of the variable load type shall be adjusted by the District Assessor or by a person authorized by him to perform such . adjustment.
Bagasse
Final molasses By weighing
Filter cake
Sugar
Burnt lime, Sulphur and Phosphoric acid By weighing
General
T h e second place of the resulting figure must be discarded and the brix of all p r o d u c t s must be recorded to the nearest first decimal place. T he corresponding weights of sucrose in mixed juice and bagasse must be recorded to the third decimal place in tonnes, and their sum, which is the weight of sucrose in cane, to the third decimal place.
Note: Although the weight of bagasse is not assumed to be accurate, the third decimal place must nevertheless be recorded, to make the fundamental e q u a t i o n balance.). Where a decimal place to be discarded is represented by a n u m b e r less than five, the preceding digit (that is, the last digit recorded) will remain as it s t a n d s ; b u t where the number to be discarded is greater than five, one must be added to the preceding figure.
POUNDS OF FIBRE PER CUBIC FOOT ESCRIBED VOLUME [0]
P is the purity of the final molasses (either the expected or actual purity can be used). Sugar Milling Research Institute monthly period calculation sheet The calculation sheet used by the Sugar Milling R e s e a r c h I n s t i t u t e for.
P is the purity of the final molasses (expected or actual purity can be used). the coefficient, which is the same as the expression in the formula for calculating the percentage of crystallized sucrose in the product, depends on the purity of the mixed juice. Thermometers of this type are used to measure the temperatures of solutions to be polarized in the inversion method of analysis. A summary of sampling procedures recommended for routine sampling is included in Table VII.
Cane
In order to obtain correct results, the cleanliness of the sample containers and strict periodic sampling control are essential. Regardless of chokes or other crushing irregularities, a sample of cane should be taken at least every quarter of an hour across the width of the mill discharge as the cane comes from the last two rolls of the mill string. The sample is taken using a small sampler of the correct shape, the length of which should be equal to the width across the discharge of the back chute of the mill.
First expressed juice a. Sampling procedure
At the end of each hour, the sub-samples thus collected shall be thoroughly mixed, shall be quarter mixed. This constitutes the hourly sample for analysis from which 520 g for the pol test a n d 100 g for the moisture test are d r a w n in r a n d o m .
Mixed juice
Sulphited mixed juice
Syrup
Massecuite (from pan)
Molasses
Sugar
T he trier should be dipped into the center of the packet and filled with sugar extracted. Care should be taken not to obtain a surface sample, due to the variations caused by drying or a b s o r p t i o n of moisture. In addition to the n a m e and strength of the reagent, the date of its p a r a t i o n should be indicated on the label.
Clarifying reagents
B A S I C L E A D: Accurately weigh approximately 5 grams and dissolve in 100 ml of carbon dioxide-free water in a 500 ml volumetric flask. Neutralize the filtrate to phenolphthalein, add an excess of 0.25 ml of acetic acid and 0.25 ml of a freshly prepared 10% potassium solution. Solution A: Dissolve 5 grams in 42 ml of water and 3 ml of acetic acid and add 5 ml of sulfuric acid. pickles. I R O N: Was the precipitate of iron and aluminum hydroxides obtained in the previous test sufficient to remove most of the acetate.
Indicators
The pink color produced shall not exceed that produced by 0.125 mg of copper in an equal volume of solution containing the quantities of reagents used in the test. To a cold, saturated solution of common alum (aluminum potassium sulfate) in water, add ammonium to a slight excess with constant stirring, and allow the precipitate to settle. Pour off the supernatant liquid and wash the precipitate by decanting until the wash water shows only a small sulphate content when tested with barium chloride solution.
Standard acid and alkali solutions
Titrate the released iodine with the thiosulphate solution and when the color has become yellow dilute to a b o u t 200 ml with distilled water. Preparation of N/32 iodine solution: 100 ml of the stock solution diluted to 2 liters form the N / 3 2 solution required for SO2. A d 1 ml of 3 0 % sulfuric acid to 25 ml of N / 3 2 iodine solution pipetted into a 250 ml Erlenmeyer flask and titrated with standardized N / 3 2 thiosulphate solution until a faint yellow shade is obtained.
For the determination of sucrose
Preparation of iodine stock solution, N/1.6 approximately: Weigh 180 g of iodized p o t a s s i u m and transfer to a clean one liter volumetric flask.
For the determination of reducing sugars
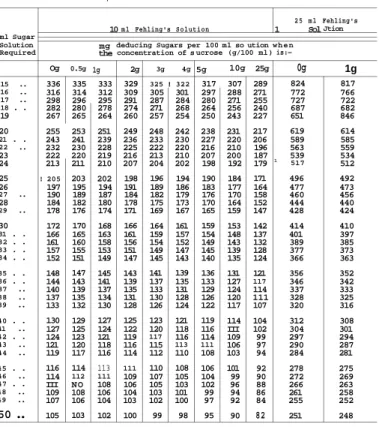
The neutralized invert sugar solution, prepared as described above, is titrated with 25.0 ml of Fehling's solution. If the Fehling solution is of the correct strength, 24.8 ml of inverted solution is used. If less than 24.8 ml of invert sugar solution is required, the Fehling solution is too weak and copper sulphate should be added to solution A until the desired 24.8 ml has been used.
R. sodium thiosulphate crystals plus 0.2 g anhydrous sodium carbonate in distilled water and make up to 1 litre
Juice preservative
L U F F S O L U T I O N : Add solution B slowly and with constant shaking to solution A. Never add solution A to solution B. Cool and top up to 1 litre. potassium iodide in 80 ml of water.
For the determination of calcium and magnesium
Standardization of the ethylenediaminetetraacetic acid solution: T he solution of ethylenediaminetetraacetic acid must be standardized against a calcium chloride solution. To do this, measure 80 ml of tap water (containing magnesium salts), 4 ml of the buffer solution and 0.2 g of Eriochrome black T indicator in a 350 ml Erlenmeyer flask. T he solution of ethylenediaminetetraacetic acid is then added dropwise from a burette until the color of the indicator changes from red to blue.
For the determination of available phosphate
Then, using a pipette, 20 ml of the standard calcium chloride solution is added and after the burette has been filled again to the 0 ml mark, the titration is continued until the end point is reached again. Since the molarity of the calcium chloride solution is known, the strength of the titrating solution can be calculated in terms of millimoles of calcium or magnesium per liter.
For the determination of sulphur dioxide (modified Monier- Williams method)
Temperature
Filtration
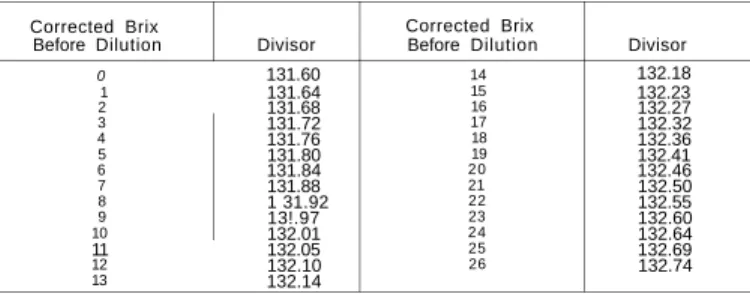
Brix
M a k e to the m a r k with distilled water, add the m i n i m u m amount of dry basic lead acetate required for clarification, shake vigorously and filter. Instead of dry basic lead acetate, a solution of basic lead acetate can be used by a d i n g the m i n i m u m q u a n t i t required for clarification before m a k i n g to the m a r k . If the weight was used, the pol is equal to the saccharimeter reading, but if a multiple or a fraction of the no m a l weight was used, the saccharimeter reading must be multiplied by the normality used.
Sucrose
Then add the required amount of invertase solution (10 ml of invertase concentrate is sufficient) and mix thoroughly. The reading of the first solution is called the direct saccharimeter reading (Dr), and the second reading, which must be corrected for the rotation of the invertase present, is called the inverted saccharimeter reading (Ir). The temperature of the solution at the time of the invert reading should be recorded to the nearest 0.1 °C.
Reducing sugars
Sulphur dioxide
Using tube E, connect the upper end of the condenser to a 100 ml Erlenmeyer flask F, followed by Peligot tube G. Add 250 g of sample directly through the dropping funnel using as little distilled water as possible. as much as possible. After introducing the sample, boil the mixture for 1 hour under a slow stream of nitrogen, stopping the flow of water in the condenser 54 — 1962 Laboratory Manual for S .
The conductivity of solutions of white sugars
This causes the condenser to heat up and float over residual traces of sulfur dioxide that are retained in the condenser.
Calcium and magnesium (by a modification of the Schwarzenbach method)
4 ml of the buffer and about 0.2 g of the indicator are added, and the solution is titrated with ethylenediaminetetraacetic acid until there is no further color change. 20 ml of the standard calcium solution is diluted with 60 ml of distilled water and 5 ml of 6 N caustic soda solution. For the actual determination, 20 ml of the prepared solution is used instead of the standard calcium chloride solution and the titration is carried out as described above.
Moisture
A blank determination should be carried out in 80 ml of distilled water and the blank titer subtracted from the titer obtained in the standardization. The magnesium concentration is found by subtracting the calcium concentration from the total calcium concentration in one day of magnesium found as above. To convert millimoles per liter to milligrams of C or O per liter, multiply by 56.08; in milligrams M g O per liter, multiply by 40.32.
Titrated acidity or alkalinity
Sulphated ash
Heat gently over a flame to drive off the excess acid and then fire in the muffle at 800°C for one hour.
Test for traces of sugar in waters (Skarblom test)
Usually only the weight of cane produced is determined and the weight of juice is calculated as the difference between Laboratory Manual for S. 58 — 1962. This method is usually used by factories to estimate the proportion of fiber in cane crushed over a weekly period. The weighed sample is crushed and the purity of the squeezed juice and the weight, half and moisture content of the bagasse are determined.
Bagacillo
Special care is required to ensure that fine particles are not blown from the tray and do not block the gauze openings.
All juices other than mixed juice
20 ml of the cooled filtrate is measured in duplicate from a burette or pipette into Erlenmeyer flasks. CALCULATE: The formula from Chapter VII, 4, a shows how to convert the saccharimeter value into the pol (apparent sucrose content) of the analyzed solution. The reading of the inverted solution must be corrected for the optical activity of the invertase solution.
Syrup
Example: 10 ml of filtered juice was diluted to 200 ml, and 10 ml of this solution was used for the development of the blue color, the intensity of which was found to correspond to 0.2 mg of P2O5.
Massecuites
Crystal content: The crystal content of a massecuite can be calculated based on the analyzes of the massecuite and its mother liquor (molasses).
Molasses (excluding final molasses) and wash
Final molasses
Reducing sugars: 20 ml of the diluted solution prepared for the determination of sucrose (10, c above) are pipetted into a 200 ml volumetric flask and made up to the mark with distilled water. Reducing sugars according to the Luff-Schoorl method: Weigh accurately 20 g of the sample and transfer it to a 100 ml volumetric flask. Carry out a blank determination with 25 ml of distilled water instead of the sugar solution.