Although there is some research on age-related changes in sleep spindle activity (Shinomiya et al., 1999; Nicolas et al., 2001), there is little research examining whether sleep spindle characteristics change in response to sleep restriction. Sleep restriction leads to reduction in the duration of stage 2 sleep (Agostini et al., 2017), which is where spindles frequently occur (Rechtschaffen & Kales, 1968). As sleep spindles are argued to play an important role in cognitive functions (Fogel et al., 2007; Fogel. & Smith, 2011), changes in spindle activity in response to sleep loss may be protective for cognitive functioning.
Slow (~11-13Hz) and fast (~13-16Hz) spindles are often investigated separately, given previous research indicating that they are functionally and topographically distinct, as well as having distinct patterns of neural activation (Schabus et al., 2007). Dijk et al., 1993) and slightly longer spindle duration (Dijk et al., 1993) after 40 h of total sleep deprivation. Results regarding changes in spindle amplitude after sleep deprivation are mixed, with one study reporting an increased amplitude (Knoblauch et al., 2003) while another found no difference (Dijk et al., 1993).
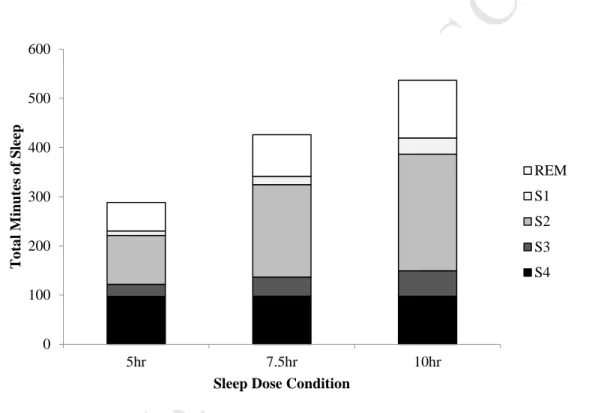
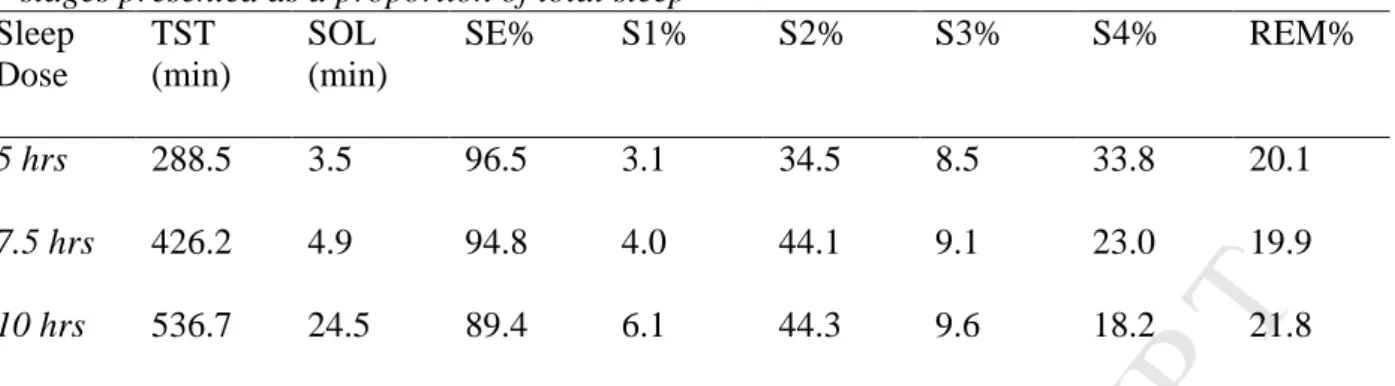
Spindle density is expected to decrease during severe sleep restriction, as shown in previous studies of sleep deprivation in adults (Borbély, et al., 1981; Dijk et al., 1993). Spindle duration is also expected to increase with sleep restriction, given similar findings in studies of sleep deprivation in adults (Dijk et al., 1993). All adolescents had a usual sleep duration of greater than 8 hours, which is within the normative range for Australian adolescents (Short et al., 2013).
Gender was included in the analyses, given previous research showing a sexual dimorphism of spindle activity (Carrier, Land, Buysse, Kupfer, & Monk, 2001; Huupponen et al., 2012).
Results 1 Sleep Characteristics
Models specified both slow and fast spindle density, duration, and amplitude as dependent variables, with fully saturated models (all main and interaction effects) for study phase (baseline, experimental phase, recovery), sleep dose condition (5 hours, 7.5 hours, 10 hours TIB), and sex. Gender only made a significant contribution to analyzes of fast spindle amplitude and was therefore removed from subsequent analyzes for all other spindle characteristics. Data for between-subject post hoc analyzes were equated to baseline when making comparisons to control for baseline group differences, while the original values were used for comparisons across study phases within each sleep dose.
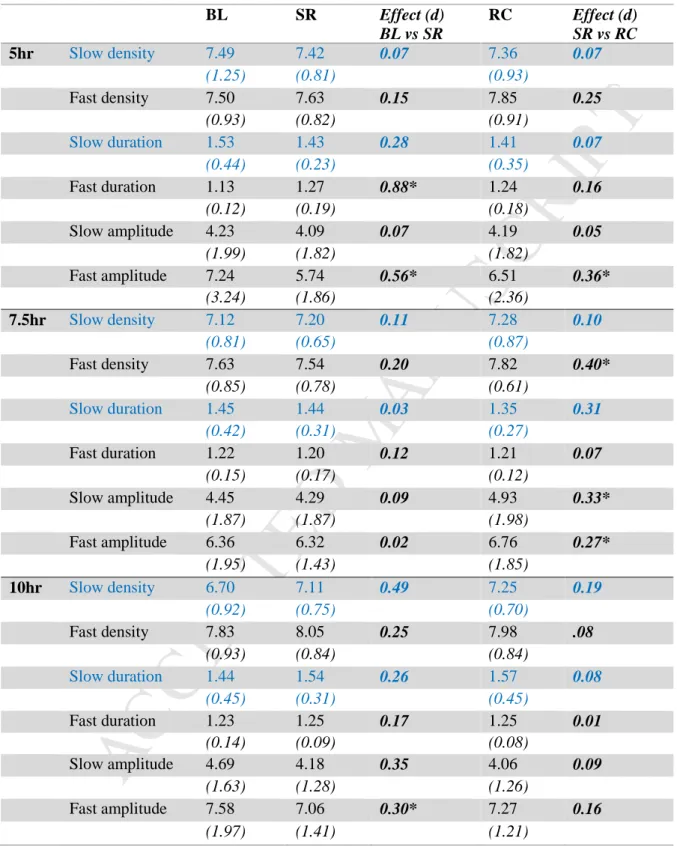
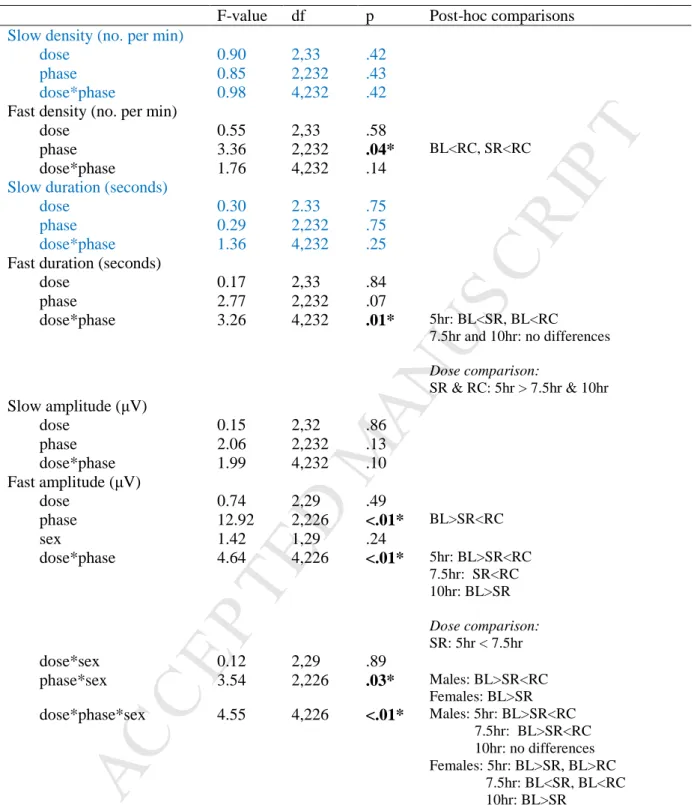
The results of the inferential test are given in Table 4 and the change in the spindle characteristics during the study phase is shown in Figure 4. Main effects and interaction of sleep dose (5 hours, 7.5 hours, 10 hours), study phase (baseline, experimental phase, recovery ) and gender (male, female) on sleep spindle variables. Results are presented per study phase (BL=baseline: 1 night, SR=sleep restriction/experimental phase: average 5 experimental nights, RC=recovery: average 2 nights).
Fast spindle amplitude across the week, formatted as change from baseline (mean and standard error), for men (A) and women (B). There were no main or interaction effects seen for slow spindle density in the present study (Figure 4A). However, there was a significant main effect of study phase on fast spindle density across all groups, with density increasing at recovery compared to both baseline and the experimental phase.
There were no main or interaction effects seen for slow spindle duration in the present study (Figure 4C). Across conditions, the duration of sleep spindles was significantly longer in the 5-h condition compared to the 7.5-h and 10-h conditions, for both the experimental phase and recovery. There were no significant main or interaction effects of sleep dose or study phase on slow spindle amplitude ( Figure 4E ).
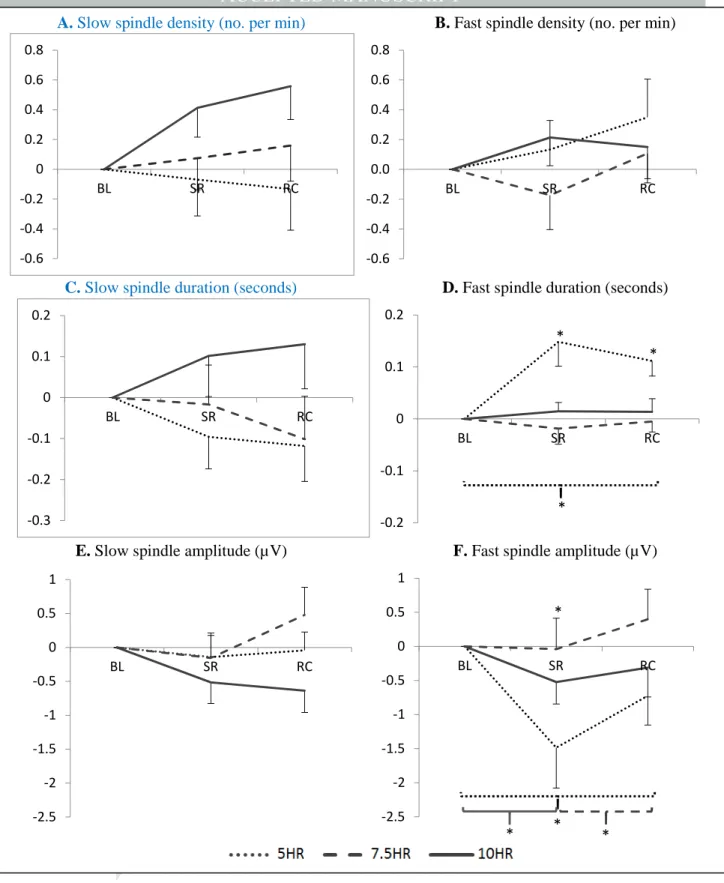
There was a two-way interaction between sleep dose and study phase on fast spindle amplitude, with the amplitude being lower during severe sleep. There was a significant three-way interaction between sleep dose, study phase, and gender for fast spindle amplitude. In men, there was a significant interaction between sleep dose condition and study phase, with fast spindle amplitude decreasing during sleep restriction compared to baseline and recovery for both the 5-hour and 7.5-hour conditions. However, this was more prominent in the 5-hour conditions. hour condition (Figure 5A).
This also appeared to be a cumulative effect, with fast spindle amplitude decreasing over multiple sleep restriction nights (Table 2). For females, there was also a significant interaction between sleep dose and trial phase (Figure 5B), with fast amplitude decreasing significantly during the trial phase compared to baseline for the 5- and 10-h conditions, with the 5-h condition not returning to baseline levels upon recovery , while fast amplitude increased significantly from baseline during the trial phase and recovery for the 7.5-h condition.
Discussion
This was consistent with expectations that spindle amplitude would decrease with sleep restriction, given its association with sigma power (Tarokh et al., 2014; Purcell et al., 2017), which decreases with sleep loss in adults (Brunner et al., 1990; Finelli et al., 2001) and adolescents (Jenni et al., 2005), although this effect was not seen for slow spindle amplitude. Fast spindles are believed to be more important than slow spindles for the maintenance and encoding of the thalamocortical system (Fogel et al., 2007), as well as learning and complex cognitive skills (Urakami et al., 2012; Bodizs et al., 2014). Along with changes in sleep finger characteristics during severe sleep restriction, there were interesting effects for other conditions.
This is interesting as it suggests that the sleep opportunity typical of Western youth (Lund et al., 2010; Gradisar et al., 2011) does not influence sleep spindles after baseline 'optimal'. While there is increasing evidence that sleep restriction has a negative impact on cognitive functioning in areas such as full scale IQ (Geiger et al., 2010), fluid IQ (Geiger et al., 2010) and memory (Randazzo et al., 1998, Geiger et al., 2010, Vriend et al., 2012) the mechanisms behind these effects are not fully elucidated. If sleep restriction affects these sleep spindle characteristics known to be associated with cognitive function, it is possible that this is a potential mechanism.
It was expected that in the 5-h condition, spindle density would be reduced, as evidenced in previous studies of sleep-deprived adults (Borbély, et al., 1981; Dijk et al., 1993), however, there were no changes clear on sleep axis density in the present adolescent sample. Furthermore, the 5-hour group showed an increase in rapid finger duration compared to the other groups, similar to the adult findings of increased finger duration after total sleep deprivation of 40 hours (Dijk et al., 1993). Past research on adults found gender differences in the relationship between spindles and intellectual performance, with females showing positive relationships between rapid finger amplitude and intelligence, while males showed non-significant negative relationships (Ujma et al., 2014).
Previous research on sleep spindle activity in response to sleep restriction has been conducted primarily in adults and with a single dose of restricted sleep or sleep deprivation. Future research would also benefit from including more baseline nights to obtain reliable estimates of slow spindle density and duration on which to base findings (Reynolds et al., 2018). This is also relevant given the emerging body of research on the relationship between sleep spindles and cognition during adolescence (Geiger et al., 2011; Astill et al., 2014; Bodizs et al., 2014 and Hoedlmoser et al., 2014).
There were significant changes in sleep spindle activity during severe sleep restriction to 5 hours of sleep per night, with fast spindles becoming lower in amplitude and longer in duration compared to baseline. Adolescent sleep spindle activity appears to be affected by severe sleep restriction, and the nature of this effect warrants further investigation. The sleep spindle and slow wave frequency reflect the motor skills of primary school-age children.
The individual adjustment method of sleep fingerprinting: Methodological improvements and roots in the fingerprint paradigm. Circadian morning preference is associated with lower slow-wave sleep axis amplitude and intensity in adolescents.