Understanding HMOs and Implications in Infant
Health
A/Professor Daniel Avi Lemberg Head of Gastroenterology Sydney Children’s Hospital
Conjoint University of New South Wales
My town
My Place
Thank you for having me
Conflict
• Many and varied but currently these talks are sponsored by Nestle
What are HMOs?
BREAST IS BEST
Human breast milk 1,2
•Is the most suitable nutrition for infants
•Has a unique composition
•Offers various health benefits to infants
•Including reduced risk of:
•gastrointestinal infections
•respiratory infections
So Breast is best !
What are HMOs?
Human milk oligosaccharides (HMOs) are specific bioactive compounds present in human milk. 1
The amount and variety of oligosaccharides in human milk is
unique and is not found in the milk of cows or other animals. 2,3,4
1 Kunz, Adv Nutr 2012; 2 Urashima et al. Adv Nutr 2012; 3 Ninonuevo et al. Pediatr Res 2008; 4 Ruhaak et al. Anal Bioanal Chem 2014
Human milk contains two major types of carbohydrates:
Lactose is a major nutrient source and the most abundant solid component of human milk. 1
Oligosaccharides are the third most abundant solid component of human milk, generally
regarded to be non-digestible by the infant due to lack of the necessary enzymes in humans. 2,3
1 Pacheco et al. Annu Rev Abim Biosci 2015; 2 Jantscher-Krenn et al. Minerva Pediatr 2012; 3 Ruhaak et al. Anal Bioanal Chem 2014
Monosaccharides
e.g. glucose 1 monosaccharide
molecule
Disaccharides
e.g. lactose 2 monosaccharide
molecules linked
Oligosaccharides
e.g. HMO
3-10 monosaccharide molecules linked
Polysaccharides
e.g. starch
>10 monosaccharide molecules linked
Carbohydrates (also called saccharides) can be classified into mono-, di-, oligo- and
polysaccharides:
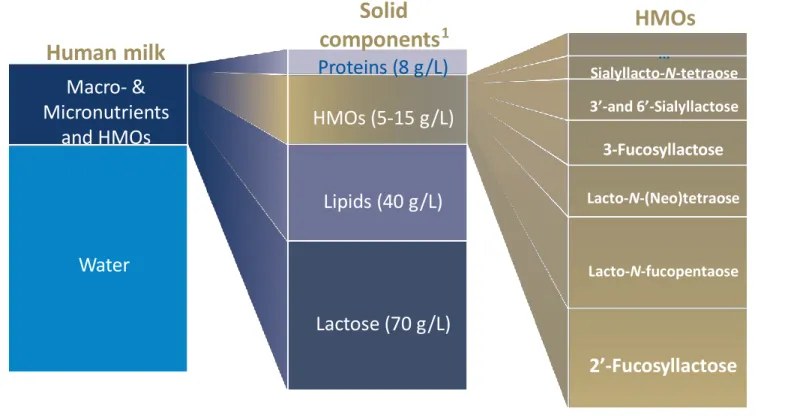
HMOs ARE THE 3 RD LARGEST SOLID COMPONENT IN HUMAN MILK
The HMO fraction is quantitatively larger than that of protein (which is typically around 8g/L) 1,2 ; HMOs and are thus considered a key component of breast milk. 1
HMO concentrations range between 20–25 g/L for colostrum 2 and 5–15 g/L for mature milk. 1
1 Zivkovic et al. PNAS 2011; 2 Bode, Glycobiology 2012; fig. adapted from Zivkovic et al. Functional Food Reviews 2013
HMOs
…
Sialyllacto-N-tetraose 3’-and 6’-Sialyllactose
3-Fucosyllactose
Lacto-N-(Neo)tetraoseLacto-N-fucopentaose
Lactose (70 g/L)
2’-Fucosyllactose Water
Human milk Macro- &
Micronutrients and HMOs
Proteins (8 g/L) HMOs (5-15 g/L)
Lipids (40 g/L)
Solid
components
1HMOs commonly contain a lactose core (= a disaccharide consisting of glucose Glc and galactose Gal) 2,3 which is elongated by one or more of the following 4 monosaccharides: 1
N-acetylglucosamine (GlcNac) Fucose (Fuc)
Sialic acid (N-acetylneuraminic acid (NeuAc)) Galactose
1 Bode, Glycobiology 2012; 2 Bode et al. Adv Nutr 2012; 3 Jantscher-Krenn et al. Minerva Pediatr 2012
HMO CATEGORIES
1 Ninonuevo et al. J Agric Food Chem 2006; 2 Urashima et al. Adv Nutr 2012; 3 Bode, Nutr Rev 2009; 4 Hennet et al. Swiss Med Wkly 2014; 5 Bode et. al. Early Hum Dev 2015; 6 Adapted from
More than 200 HMOs 1,2,3,4 have been identified so far and classified into three different categories 5 :
Fucosylated (= neutral) HMOs (e.g. 2’FL)
Non-fucosylated neutral HMOs (e.g. LNnT, LNT)
Sialylated or “with sialic acid” (= acidic) HMOs (e.g. 3’SL)
2’FL
The proportion of fucosylated, non-fucosylated neutral and sialylated HMOs in term breast milk was recently reported as 35–50%, 42–55%, and 12–14%, respectively.
6The neutral oligosaccharides, to which 2´FL and
LNnT belong, account for more than 75% of the total HMOs.
6Proportion of HMO categories in human milk:
2’-Fucosyllactose
ß1-4 α1-2
LNnT
ß1-4 ß1-3Lacto-N-neotetraose
ß1-4
LNT Lacto-N-tetraose
ß1-4 ß1-3
3’SL
ß2-33-Sialyllactose
ß1-4 ß1-3
Smilowitz et al. Annu Rev Nutr 2014
FUCOSYLATED HMOs DETERMINED BY GENETIC FACTORS
The ability to secrete the enzyme fucosyltransferase is genetically determined. 1,3 Women with active Secretor gene = Secretors (Se+), 80% of all women. 5
Oligosaccharide amount and composition in human milk vary between individuals and
diurnally, by infant gestational age, over the course of lactation as well as with the mother’s nutritional status. 1,2,4
The most extreme interpersonal variations are based on women’s secretor status. 1,2
1 Bode et al. Adv Nutr 2012; 2 Goehring et al. Plos One 2014; 3 Blank et al. Adv Nutr 2012; 4 Morrow et al. J Nutr 2005; 5 Urashima et al. Adv. Nutr. 2012; 6 Jantscher-Krenn et al. Minerva Pediatr 2012
Levels of HMO also fluctuate over time with less being produced with time
THE MOST COMMON HMOs IN NATURE AND RESEARCH
2´FL and LNnT belong to the neutral oligosaccharide groups that account for a total of more than 75% of the total HMOs. 1
2’FL is by far the most abundant HMO in the milk of Se+ women (80% of women worldwide): average 3 g/L (20% of total HMOs). 1,2
LNnT constitutes ~ 2-3 % of the total HMO fraction, which qualifies it as one of the 10 most important HMOs by abundancy out of a pool of more than 200. 1
Individually or together, 2’FL and LNnT have so far been the main focus of pre-clinical and clinical research on the effects of HMOs in infants.
2’FL
ß1-4
2-Fucosyl-Lactose (trisaccharides)
LNnT
Lacto-N-neotetraose (tetrasaccharides)
LNT
ß1-3
Lacto-N-tetraose (tetrasaccharides)
α1-2
ß1-4
ß1-3 ß1-4
ß1-4
ß1-3
1 Roehrig, Glycom A/S, Denmark, Kgs. Lyngby 3rd of June 2015; 2 Urashima et al. Adv Nutr 2012
MAJORITY OF HMO REMAIN IN GUT LUMEN AND PASS INTO STOOL
1 Ruhaak et al. Anal Bioanal Chem 2014; 2 Rudloff et al. Acta Paediatr 1996; 3 Rudloff et al. Glycobiology 2006; 4 Rudloff et al. Br J Nutr 2012; 5 Obermeier et al. Environ Health Stud 1999; 6 Rudloff et al.
HMOs are generally regarded to be non-
digestible by the infant’s gut due to lack of human production of the
necessary enzymes. 1
Approximately 1%- 2% of the HMOs are absorbed in the small intestine or colon and reach the systemic circulation. 1-6
From Rudloff and Kunz, Adv Nutrition, 2012
Anal Bioanal Chem 2014
MAIN EFFECTS OF HMOS THAT WE CURRENTLY KNOW ABOUT
Luminal & mucosal effects
Microbiota establishment
& function
Protection from infection Mucosal barrier function
Systemic effects
Immunity - Allergy Brain
Metabolic health
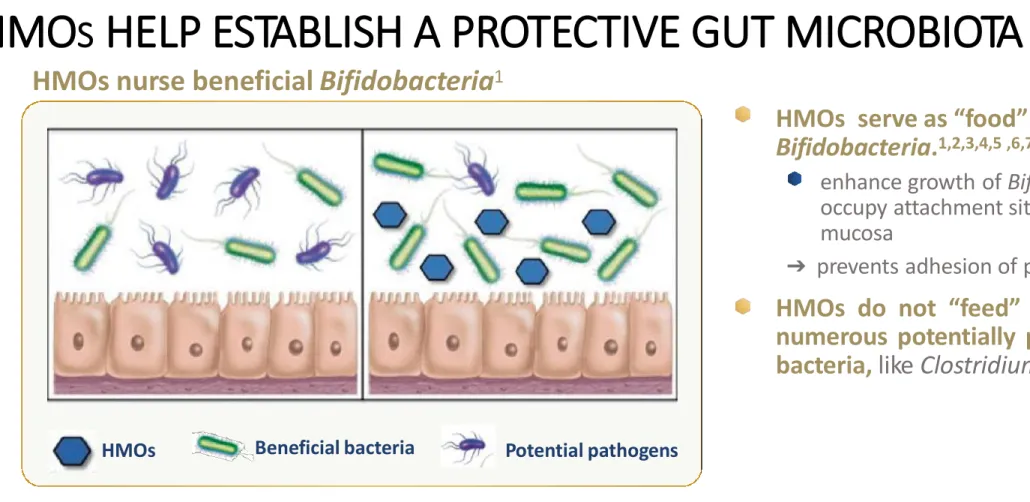
HMOs shape the early-life gut microbiota by promoting the growth of Bifidobacteria, which may be involved in the programming of a healthy immune system. 1,2,3
HMOs nurse beneficial Bifidobacteria 1
HMOs serve as “food” for specific Bifidobacteria. 1,2,3,4,5 ,6,7
enhance growth of Bifidobacteria that occupy attachment sites on the gut mucosa
➔ prevents adhesion of pathogens.
7HMOs do not “feed” growth of numerous potentially pathogenic bacteria, like Clostridium. 7,8,9
fig. adapted from Bode, Glycobiology 2012
HMOs Beneficial bacteria Potential pathogens
HMO S HELP ESTABLISH A PROTECTIVE GUT MICROBIOTA
1. Sela et al. Trends in Microbiology 2010; 2 Zivkovic et al. PNAS 2011; 3 Oozer et al. Am J Clin Nutr 2013; 4 Smilovotz et al. Annu Rev Nutr 2014; 5 Zivkovic et al. Functional Food Reviews 2013; 6 Yatsunenko et al. Nature 2012 ; 7 Sela et al. PNAS 2008; 8 Yu Glycobiology 2013; 9 Hoeflinger J. Agric. Food Chem. 2015;
HMOs function as soluble receptor analogs (decoys) that compete for bacterial binding against the intestinal mucosa 1
Many pathogens need to attach to the mucosal surface to cause infectious disease. 1
HMOs (especially fucosylated structures) resemble the host epithelial cell surface glycans (= receptors) to which pathogens adhere. 2,3
Pathogens (bacteria, viruses and their toxins) are deceived by the structural similarity and attach to the HMOs. 4 HMOs thus serve as soluble decoy receptors to prevent pathogen binding to the host cell and thereby reduce the risk of infectious disease. 1
HMOs serve as decoys for pathogens. 1
1 Bode et al. Adv Nutr 2012; 2 Smilovic et al. Annu Rev Nutr 2014; 3 Sprenger et al. Eur J Nutr 2016; 4 Jantscher-Krenn et al. Minerva Pediatr 2012
HMO S BLOCK PATHOGENS FROM DOING HARM
The surface of intestinal epithelial cells, like that of any living cell, is covered in glycans. They
represent the first and crucial interface to the intestinal epithelial cell’s environment. 1
HMOs can directly interact with intestinal epithelial cells and modulate their glycan expression. 2,3
Modulating the glycans of the host cell can be an alternative mechanism to prevent pathogen
attachment to the host cell, increasing resistance to infection. 3
HMOs improve host defense by strengthening intestinal barrier function. 1
1 Angeloni et al. Glycobiology 2005; 2 Bode, Glycobiology 2012; 3 Jantscher-Krenn et al. Minerva Pediatr 2012
HMO S STRENGTHEN THE GUT BARRIER FUNCTION
SUMMARY : MAIN KNOWN IMMUNE FUNCTIONS OF HMO
HMOs help establish a potentially protective gut microbiota with predominance of Bifidobacteria.
HMOs block pathogens from doing harm by functioning as soluble receptor analogs to which pathogens adhere.
HMOs strengthen the gut barrier function.
HMO S AND GUT MICROBIOTA – PRECLINICAL MODELS
*Formerly germfree mice that are inoculated with bacteria commonly present in the infant gut microbiota
1 Sela et al. Trends Microbiol 2010; 2 Li et al. J Nutr 2012; Yu et al. Glycobiology 2013; 3 Marcobal et al. Cell Host Microbe 2011; 4 Sprenger et al. unpublished; 5 Yu et al. 2013; 6 Hoeflinger et al. JAFC 2015
HMOs promote specific Bifidobacteria growth and metabolic activity in vitro and establishment in humanized gnotobiotic mice.* 1-4
HMOs do not allow for growth of numerous potentially pathogenic bacteria, like
Clostridium, Enterobacter, Klebsiella, Escherichia. 5-6
.
HMO S AND GUT MICROBIOTA – PRECLINICAL DATA
LNnT stimulates Bifidobacterium spp. growth and promotes short-chain fatty acids in vitro.
LNnT provides selective advantage to B. infantis over Bacteroides species in vivo.
2´FL boost growth of specific Bifidobacteria, and production of short-chain fatty acids in vitro.
Enterobacteriaceae strains do not grow on 2´FL, 6´SL, or LNnT in vitro.
HMO S AND PROTECTION FROM INFECTIONS
What do we know ?
HMO S AND PROTECTION FROM INFECTIONS – PRECLINICAL MODELS
1 Brassart et al. Infect Immun 1991; 2 Cravioto et al. J Infect Dis 1991;3 Weichert et al. Nutr Res 2013; 4 Barthelson et al. Infect Immun 1998; 5 Duska-McEwan et al. FNS 2014; 6 Idänpään-Heikkilä et
HMOs block numerous pathogens, like Campylobacter jejuni, Candida, E. coli, Salmonella, Streptococcus
pneumonia, Pseudomonas aeruginosa, Influenza in vitro. 1,2,3,4,5
HMOs reduce respiratory and gastrointestinal
pathogen load in animal models and, in some cases, protect from weight loss. 6,7,8,9,10
al. JID 1997; 7 Palacios et al. J Biol Chem 2003; 8 Hester et al. Br J Nutr 2013; 9 Li et al. The IMSE Journal 2014; 10 He et al. Gut 2016
Does it protect against diarrhoea
PROTECTION FROM GASTROINTESTINAL INFECTION – IN VIVO
Method
One of the most common causes of bacterial diarrhea is Campylobacter jejuni.
Suckling mice from transgenic dams (=
transfected with a plasmid containing the human FUT1 gene) and control pups from nontransgenetic dams were challenged with inocula of Campylobacter jejuni.
Every third day, one pup from each litter was tested for campylobacter intestinal
colonization.
Results
The neutral, fucosylated oligosaccharide fraction in milk of transgenic mice protects their nursing pups from intestinal
colonization by Campylobacter.
Fucosylated HMOs (2’FL) inhibit Campylobacter binding to intestinal host cells.
Shaded areas indicate the period during which an appreciable number of mice are infected.
Campylobacter
Ruiz-Palacios et al. J Biol Chem 2003
2´FL prevent adherent-invasive E. coli (AIEC) infection and reduce intestinal inflammation in a mouse model.
2´-FL pretreatment
prevented body weight loss due to prevention of AIEC infection
reduced colon inflammation.
PROTECTION FROM GASTROINTESTINAL INFECTION – IN VIVO
Method
Mice were inoculated via gavage with adherent-invasive E. coli (AIEC) which are able to invade epithelial cells of the colon. Half of them received 2´FL for 4 days before they were infected.
Results
AIEC infection manifested as body weight loss (∼10%).
He et al. Gut 2016
HMO S AND INFECTIOUS DIARRHEA – OBSERVATIONAL STUDY
2’Fucosyl-HMOs related to protection from infectious diarrhea
Campylobacter diarrhea occurred less often in infants whose mother’s milk contained high levels of 2´FL
(measured as % of HMOs). 1
Moderate-to-severe diarrhea of all causes occurred less often in infants whose milk contained high levels of total 2-linked fucosyl-oligosaccharide (measured as % of HMOs). 1
This study provides evidence suggesting that HMOs are clinically relevant to protection against infant diarrhea, with high 2‘FL levels associated with greater protection. 1
1 Morrow et al. J Pediatrics 2004
HMO S AND INFECTIOUS DIARRHEA – OBSERVATIONAL STUDY
2’Fucosyl-HMOs related to protection from infectious diarrhea
A high ratio of 2-linked (FUT2-dependent) to non-2-linked fucosyloligosaccharides (FUT3-dependent) in human milk was associated with lower risk of symptomatic infection with heat stable toxin (ST) of E. coli in breast-fed infants.
Enterotoxigenic Escherichia coli (ETEC) is the leading bacterial cause of diarrhea in the developing world.
FUT2 = α1-2-fucoslyltransferase FUT3 = α1-3/4-fucosyltransferase
Milk with higher 2´-linked fucosyloligosaccharide ratios affords greater protection against infant diarrhea.
Newburg et al. Glycobiology 2004
For Nestlé Internal Use Only
HMO S AND IMMUNE COMPETENCE /
ALLERGY
IMMUNE EDUCATION / COMPETENCE – IN VIVO
Method
Mice were sensitized with ovalbumin (OVA).
Sensitized mice were orally challenged with OVA and 2´FL, 6´SL or lactose.
Results
Treatment with either 2´FL or 6´SL led to a significant attenuation of oral OVA-
challenge-induced intestinal allergy as assessed by diarrhea score.
2´FL and 6´SL reduced the severity of food allergy-induced diarrhea.
Castillo-Courtade et al. Allergy 2015
HMOs may reduce the risk of IgE-associated Eczema
• Higher levels of 2′FL in human breast milk were associated with a lower incidence of IgE-
associated eczema (P=.0065) among
C-section-born infants, with a family risk for allergies, at 2 years of age
Association between 2′FL in
human breast milk and IgE
eczema in C-section–born infants
CLINICAL INTERVENTION TRIAL WITH HMO 2´FL + GOS (Abbott)
ß1-4
α1-2 ß1-3
ß1-6 ß1-4
n n
GOS
galactose glucose fucose
Marriage et al. JPGN 2015
2’FL
RCT WITH 2´FL (ABBOTT): STUDY OBJECTIVES AND METHODS
Objective
To assess growth and tolerance in healthy term infants who received infant formulae with a caloric density closer to human milk supplemented with 2´FL + GOS from 0-4 months and to study uptake of 2´FL.
1To investigate effects of feeding formulae supplemented with 2´FL on biomarkers of immune function.
2Methods
Randomized, placebo-controlled, double-blinded, multicenter intervention trial at 28 sites throughout the United States
Feeding groups (from ≤ 5 day to 119 days of life)
Exclusively formula-fed infants randomly assigned to 1 of 3 low-caloric (64.3 kcal/100 ml) formulae:
enrolled in blood collection subgroup (immune markers)2
1 Marriage et al. JPGN 2015; 2 Goehring et al. J Nutr 2016
Exclusively breastfed infants
• HM n = 107 n = 51
• Experimental Formula 1 (EF1) : test formula 1 (0.2 g/L 2´FL + 2.2 g/L GOS) n = 105 n = 54
• Experimental Formula 2 (EF 2): test formula 2 (1 g/L 2´FL + 1.4 g/L GOS)
• CF: control formula (2.4 g/L GOS)
n = 111 n = 101
n = 48
n = 48
RCT WITH 2´FL (ABBOTT):
GROWTH AND 2’FL ABSORPTION/ EXCRETION
Methods
Randomized, placebo-controlled, double-blinded, multicenter intervention trial at 28 sites
throughout the United States
Results
No significant differences among any groups for growth parameters (weight, length or head circumference) over the 4-month study period.
All formulae were well tolerated.
Infants fed 2´FL-supplemented formulae with a caloric density similar to human milk had a growth from birth to 4 months of age similar to that of human milk-fed infants.
Weight growth plotted on WHO growth charts
Female infants Male infants
CF=control formula; EF=experimental formula (EF1=0.2g/L, EF2=1 g/L 2´FL
HM= human milk
Marriage et al. JPGN 2015
RSV
stimulated
n = 48 n = 51 n = 54 n = 48
In ex vivo RSV-stimulated cell (PBMC*) cultures, breastfed infants were not different than either of the groups fed the formulae with 2´FL+GOS, but they had lower concentrations of inflammatory cytokines TNF-α and IFN-Ƴ (p ≤0.05) than infants fed the control formula. Similar trends were observed for IL-1ra, IL-6, and IL-1β. (RSV stimulated. Fig.
A+B).
*Peripheral blood mononuclear cells = immune cells including lymphocytes, monocytes, and dendritic cells
Labeled means withou a common letter differ (p ≤ 0.05)
RCT WITH 2´FL (ABBOTT):
RESULTS - BLOOD IMMUNE MARKERS
Plasma cytokine concentrations (A) in 6-wk-old infants and ex vivo cytokine production by RSV-stimulated PBMCs*
Infants fed formulae with 2´FL + GOS have blood immune markers (baseline and RSV stimulated) similar to breastfed infants, infants fed formula with GOS do not.
Goehring J Nutr. 2016
For Nestlé Internal Use Only
FIRST CLINICAL INTERVENTION TRIAL WITH 2 HMO S :
2´FL + LNnT (NESTLÉ)
ß1-4 α1-2
ß1-4 ß1-3
ß1-4
2’FL LNnT
Puccio et al. JPGN 2017
86
STUDY OBJECTIVES
Overall purpose:
Assess growth, tolerance, morbidity and stool microbiota in healthy term infants who received infant formula supplemented with HMOs from 0-6 months
Primary objective:
Weight gain of infants fed an intact protein experimental formula (EF) with two human milk identical
oligosaccharides – 2´FL (1.0 g/L) and LNnT (0.5 g/L) - compared to infants fed an identical control formula without HMOs (CF).
Hypothesis: Weight gain (g/d) from enrollment to age 4 mo in infants fed EF will be non inferior to that of infants CF (non-inferiority margin ∆= -3g/day)
Secondary objectives:
Growth
Digestive tolerance
Morbidity [parental reported adverse events (AEs) / medication use]
Stool microbiota profile and metabolic signature
Puccio et al. Abstract at Nutr Growth Conference 2016; Steenhout et al. Abstract at Exp Biol Conference 2016; Puccio et al. Publication in progress
STUDY DESIGN AND STUDY POPULATION
Prospective, randomized, controlled, multi-center interventional clinical trial of 2 parallel formula-fed groups conducted in Italy and Belgium
Study population
Healthy, full-term (≥ 37 weeks, ≤ 42 weeks) infants, birth weight 2500-4500g, age ≤ 14 days at enrollment, exclusively formula-fed at the time of enrollment.
Reference group: exclusively breastfed infants, enrolled at 3 months of age.
Feeding groups
Control Formula (whey-predominant, with LC-PUFAs) (Control/CF)
Test Formula (control formula + 2 HMOs ; 1 g 2’FL/L and 0.5 g LNnT/L) (Test/EF) Breastfed group (BF)
n = 87 n = 88 n = 38
Puccio et al. Abstract at Nutr Growth Conference 2016; Steenhout et al. Abstract at Exp Biol Conference 2016; Puccio et al. Publication in progress
Mean weight in girls and boys from 0-12 months of age, compared with the WHO growth curve (ITT population)
GROWTH
Mean weight from enrollment to age 12 months tracked closely with the WHO growth standard and was not significantly different between groups at any study visit.
Weight development between birth and the age of 4 months was not significantly
different between infants receiving formula with HMOs (Test) and those receiving
formula without HMOs (Control).
Mean weight gain in the test group was non-inferior to the mean weight gain in the control group.
Puccio et al. Abstract at Nutr Growth Conference 2016; Puccio et al. Publication in progress
Mean weight gain (g/d)
Control Test Difference between groups p-value
ITT population 29.52 29.39 -0.13 0.864
PP population 30.15 29.84 -0.30 0.715
DIGESTIVE TOLERANCE
1Stooling and other GI symptoms, as well as behavioral pattern results were analyzed by Cochran-Mantel-Haenszel test, *p<0.05. 2Bristol scale score from 1 (hardest stool) to 7 (most liquid stool).
3Infant flatulence, spitting-up and vomiting.4Infant restlessness/irritability, colic and waking-up at night
No significant difference in stool consistency between the groups, except at 2 months (softer in the formula with HMOs (EF) vs. control formula (CF))
No significant difference in stool frequency between the groups
Gastrointestinal symptoms 3 and behavioral pattern 4 were similar between the groups
Puccio et al. Abstract at Nutr Growth Conference 2016; Puccio et al. Publication in progress
MORBIDITY
The occurrences of total AEs, GI-related AEs, SAE, and AEs related to study formulae were similar between the formula with HMOs and control formula groups.
AEs: adverse events
SAEs: serious adverse events SOC: system, organ and class
Puccio et al. Abstract at Nutr Growth Conference 2016; Puccio et al. Publication in progress
AE S IDENTIFIED A PRIORI AND MEDICATION USE
Infants receiving formula with HMOs (vs. CF) had significantly fewer reports of bronchitis through 4, 6 and 12 mo, lower respiratory tract infections (AE cluster) through 12 mo, antipyretics use through 4 mo, and antibiotics use through 6 and 12 mo. The protective
effects continued after the 6 months intervention period.
Puccio et al. Abstract at Nutr Growth Conference 2016; Puccio et al. Publication in progress
CAESAREAN-BORN INFANTS BENEFIT EVEN MORE FROM FORMULA SUPPLEMENTED WITH HMO S
Caesarean-born infants receiving formula with HMOs (vs. CF) had significantly fewer reports of bronchitis and lower respiratory tract infections (AE cluster) through 12 months.
Puccio et al. Abstract at Nutr Growth Conference 2016; Puccio et al. Publication in progress
CONCLUSIONS 1: CLINICAL TRIAL WITH 2’FL AND LNnT
Infant formula supplemented with 2’FL and LNnT is safe, well-tolerated, and supports normal, age- appropriate infant growth.
Formula supplemented with 2’FL and LNnT reduced the likelihood of parental reported morbidity (particularly bronchitis and lower respiratory tract infections) and medication use (particularly antibiotics, antipyretics).
Findings suggest the addition of 2´FL and LNnT may provide immune benefits. However, these findings warrant confirmation in future studies.
Caesarean-born infants benefit even more from formula supplemented with HMOs. However, these findings warrant confirmation in future studies.
Puccio et al. Abstract at Nutr Growth Conference 2016; Puccio et al. Publication in progress
RDA Genus
The 3 groups - BF, Test, Control - significantly separated at genus level (p<0.001);
Centroids of Control & BF clearly separated whereas Test & BF partially overlapped.
BF Control Test
BACTERIAL DIVERSITY
Beta diversity: Inter-individual diversity at 3 months of age
Formula with HMOs shifted global gut microbiota composition closer
to that of breastfed infants.
Steenhout et al. Abstract at Exp Biol Conference 2016; Puccio et al. Publication in progress
SPECIFIC BACTERIAL ABUNDANCE
Median interquartile ranges are depicted. Wilcoxon rank sum test at genus level, significant difference indicated by *p<0.05, **p<0.01, and ***p<0.001;
False Discovery Rate (FDR) adjusted P-values between Test and Control groups are 0.16 for Bifidobacterium & Escherichia, and 0.26 for Peptostreptococcaceae uncl.
Test significantly differed from Control on three bacterial genera, being closer to BF.
Formula with HMOs promote the colonization of potentially beneficial Bifidobacterium and reduce potentially pathogenic Escherichia and Peptostreptococcaceae.
Relative composition (% of all sequences) of the 3 groups - BF, Test, Control- for the 3 main taxa that showed significant differences between the Test and Control groups at 3 months of age
Steenhout et al. Abstract at Exp Biol Conference 2016; Puccio et al. Publication in progress
The Good, The Bad and The Ugly
CONCLUSIONS 2: CLINICAL TRIAL WITH 2’FL AND LNnT
Infant formula supplemented with 2’FL and LNnT shifts global gut microbiota composition (bacterial diversity and abundance) closer but not that observed in the breastfed reference group at 3 months.
Infant formula supplemented with 2’FL and LNnT increased potentially beneficial Bifidobacterium and decreased potentially pathogenic Escherichia and Peptostreptococcaceae at 3 months.
Infant formula supplemented with 2’FL and LNnT shifts stool metabolic signature towards breastfed profile and, of note increased lactate.
Further studies are warranted to evaluate whether such a shift in gut ecology of formula-fed infants towards the breastfed standard leads to benefits.
Puccio et al. Abstract at Nutr Growth Conference 2016; Steenhout et al. Abstract at Exp Biol Conference 2016; Puccio et al. Publication in progress
Key Points
• HMOs are the third largest solid components in human breast milk, with more than 200 varieties
• HMOs support immunity in 4 main ways:
― promote the growth of beneficial bacteria
― assist gut barrier function
― prevent pathogen adhesion in the gut
― help modulate the immune system
Key Points
• The safety of 2′FL and LNnT has been approved by the EFSA and the U.S. FDA; furthermore, infant formula supplemented with 2′FL, alone or in combination with LNnT, support normal growth, are safe and
well tolerated.
• HMOs 2’FL and LNnT when added to infant formulae may offer
immune benefits compared to formulae without HMOs.
Thanks for your attention today
• Any questions
CLINICAL INTERVENTION TRIALS
Prospective, randomized, controlled clinical intervention studies (RCTs) with HMOs on:
Safety / growth;
Immune markers (substudy) (2’FL+GOS)
Marriage et al. JPGN 2015 Goehring et al. J Nutr 2016
Safety / growth (2’FL+FOS)
Kajzer et al. The FASEB Journal 2016 (Abstract)
Safety / growth (2’FL+LNnT)
Puccio et al. Abstract at Nutr Growth Conference 2016 Steenhout et al. Abstract at Exp Biol Conference 2016
Puccio et al. JPGN 2017
PROTECTION FROM RESPIRATORY INFECTION – IN VITRO
In vivo, LNnT 1
Method
S. pneumoniae without or with LNnT or sialylated LNnT were administered into the trachea of rabbits. Viable S. pneumonia in bronchoalveolar lavage was determined.
Results
LNnT and sialylated LNnT reduced S.
pneumoniae adhesion.
1 Idänpään-Heikkilä et al. JID 1997; 2 Duska-McEwen et al. FNS 2014
Method
Effects of 2´FL and LNnT on human respiratory epithelial cell lines following viral infection in vitro.
Results
LNnT decreased Influenza (IAV) viral load and 2’FL decreased Respiratory Syncytial Virus (RSV) viral load in airway
epithelial cells.
PROTECTION FROM RESPIRATORY INFECTION – IN VITRO
In vitro, LNnT and 2´FL 2
1 Idänpään-Heikkilä et al. JID 1997; 2 Duska-McEwen et al. FNS 2014