I also authorize Chattogram Veterinary and Animal Sciences University (CVASU) to lend this thesis to other institutions or individuals for the purpose of scientific research. I further authorize CVASU to reproduce the thesis in whole or in part by photocopying or in another way for scientific research purposes, at the request of other institutions or individuals. Thesis title: ANTIMICROBIAL ACTIVITY OF PROCESSED HONEY FROM A RECOGNIZED CHATTOGRAM CITY COMPANY AGAINST Staphylococcus Aureus AND Escherichia Coli.
This is to report that according to the audit, 18% of the thesis content is plagiarized and covered/not covered according to the plagiarism policy and institutions issued by CASR, Chattogram Veterinary and Animal Sciences University. All praise is due to Almighty Allah, who has blessed me with the strength, ability and patience and enabled me to pursue higher education and complete the thesis for the degree of Masters of Science (MS) in Food Chemistry and Quality Assurance under complete the Food Chemistry and Quality Assurance department. Goutam Buddha Das, Vice Chancellor, Chattogram Veterinary and Animal Sciences University (CVASU) for giving special opportunities and providing such research facilities.
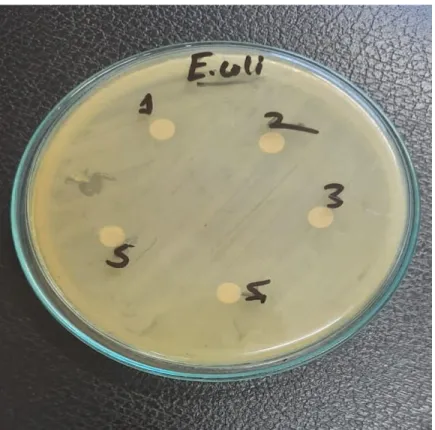
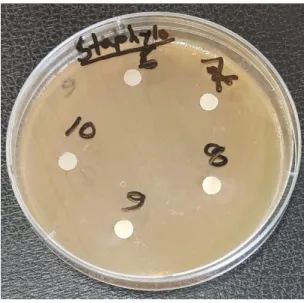
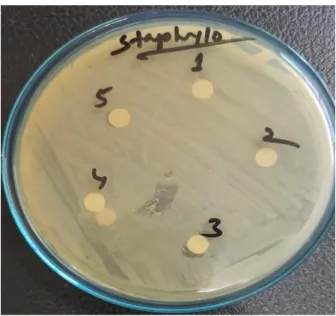
Ashraful Islam, Assistant Professor, Department of Applied Chemistry and Chemical Technology, CVASU for his guidance and moral support. Antimicrobial activity of honey samples was performed individually, whereby 5 discs of individual samples were placed on an agar plate.
Introduction
According to some studies, honey has antibacterial properties that can fight against bacteria such as Escherichia coli, Campylobacter jejuni, Salmonella entercolitis, Shigella dysenteriae (Adebolu, 2005), Mycobacterium (Asadi-Pooya et al., 2003), methicillin-resistant Staphylococcus aureus and vancomimine-resistant Enterococci (Cooper et al., 1999 & 2002 and Al-waili et al., 2005), common gastrointestinal pathogenic bacteria (Lin et al., 2011), Streptococcus pyogenes biofilms (Maddocks et al., 2012 ). In addition, honey has been shown to have antifungal properties against most common dermatophytes (Brady et al., 1997) and some species of yeast, Aspergillus, and Penicillium (Quinn et al., 1994). Depending on the floral source, grazing areas, climate where the bees are reared, and the natural composition of the nectar, the biological properties of honey can vary (Abd-El Aal et al., 2007).
Blood may contain more iron, antioxidants and rare elements when honey is consumed (Theunissen et al., 2001). Compared to regularly used antibacterial treatments, honey showed a more pronounced inhibitory effect (85.7 percent) on gram-negative bacteria (Pseudomonas aeruginosa, Enterobacter spp. and Klebsiella), according to a study by Abd-El Aal et al. 3 Although honey is probably the keeps water activity low enough to inhibit the growth of most bacteria when diluted with water absorbed from wounds.
However, honey contains other important beneficial properties that are less affected by storage conditions (Cooper et al., 2002). The use of natural compounds was considered as one of the possible solutions.
Review of literature
- Honey
- Chemical composition
- Water content and water activity (a w )
- Sugar content
- Proteins and Amino acids
- Vitamins and Minerals
- Volatile compounds
- Hydroxymethylfurfuraldehyde (HMF)
- Enzymes
- Phenolic compounds
- Pigments
- Pollen, propolis and royal jelly
- Hydrogen peroxide
- Bee derived antimicrobial peptides
- Plant derived antimicrobial phytochemicals
- Antimicrobial efficiency of Honey
- Antibacterial activity of honey
- Antifungal activity of honey
- Antiviral activity of honey
- Antioxidant activity of Honey
- Methods of measurements of antibacterial activity
If there are fewer yeasts that ferment honey, the moisture content of the honey will be higher (Piana et al., 1989). Glucose content and the glucose/fructose ratio, honey crystallization and environmental factors all affect water activity (Gleiter et al., 2006). Blossom honey had lower water activity in the liquid form than honeydew honey with the same water content (Gleiter et al., 2006).
Some researchers have discovered strong correlations between honey moisture content and water activity (Cavia et al., 2004). Furthermore, an important antibacterial honey component is the osmotic pressure caused by high sugar concentration (Jeddar et al., 1985). It was suggested that ratios of some of these components, as well as sugar amounts, could be good markers to determine the authenticity of honey (Nozal et al., 2005).
Quercetin, chrysin, pinocembrin, pinobanksin, kaempferol, galangin and luteolin are the main flavonoids contained in honey (Kaskonien and Venskutonis, 2010; Dong et al., 2013). In addition, honey often contains pollen from trees and plants that have been contaminated by the wind (Bruni et al., 2015). Propolis contains more than 300 different chemicals, including flavonoids, phenolic compounds, terpenes, esters and anthraquinones (Kalogeropoulos et al., 2009;).
During the oxidation of honey glucose in oxygen, hydrogen peroxide is naturally formed (Brudzynski et al., 2011). According to some research, the effects of hydrogen peroxide can be influenced by other components of honey (Brudzynski et al., 2011). 34;bee-derived protectors." These peptides play a role in both individual and social immunity (Klaudiny et al., 2005).
This is also the cause of American Foulbrood (AFB), a serious bee disease (Katarina et al., 2002). According to research (Kwakman et al., 2010), the number of plant components that contribute to the antibacterial activity of honey is too small to detect. Proteus mirabilis and Bacillus cereus were the two species most vulnerable (Escuredo et al., 2012).
A combination of peroxide and non-peroxide compounds may also be suppressing bacterial growth (Loh et al., 2011). The main antibacterial component in honey, hydrogen peroxide, was found to be inhibin (White et al.1963).
Materials and Methods
- Site and period of experiment
- Collection of Samples
- Preparation of samples
- Test microorganisms
- Reagents and apparatus
- Reagents
- Media
- Apparatus
- McFarland standard preparation
- Culture suspension preparation
- Media preparation
- Antimicrobial effect of samples
All commercial honey samples were used in their raw form as it was in the jar that the companies process commercially. All samples were first collected in sterilized small plastic bottles in small quantity for easy handling. Pure isolated cultures of Escherichia coli and Staphylococcus aureus were collected from PRTC (Poultry Research and Training Centre), Chattogram Veterinary and Animal Sciences University, Chattogram using nutrient agar aseptically.
Then, the collected isolates were subcultured with selective agar media to obtain more pure isolates. For the 0.5 McFarland standard 9.95 ml of sulfuric acid and 0.05 ml of barium chloride solution were mixed in a sterile screw-capped test tube. 4-5 colonies of each isolate were picked up in the sterilized screw cap tube containing 2 ml of sterilized saline.
The bacterial culture was then emulsified in sterile normal saline and the turbidity adjusted to 1.5*10^8 (CFU/ml equivalent to 0.5 McFarland standard). Mueller Hinton agar powder 38 g was weighed and mixed with 1 L of distilled water as described on the label. After mixing, the medium was sterilized in an autoclave and kept in a water bath for cooling.
To test the efficacy of the extracts, the disk diffusion method was used and its effect was assessed by measuring the zone of inhibition around the disk. A sterile cotton swab was dipped into the standardized bacterial suspension and used to uniformly inoculate the Mueller Hinton agar plates. Then all disks were placed on the plates and pressed gently to ensure full contact with agar.
A distance of at least 15 mm was kept from the edges of the plates to demonstrate overlap of inhibition zones. After incubation, the plates were examined and the diameter of the zone of inhibition was measured for each isolate.
Results
Antimicrobial activity against Escherichia coli
Antimicrobial activity against Staphylococcus aureus
Discussion
27 was not diluted in the current study, there was no antibacterial effect to speak of. Enzymes are often adversely affected by heating above physiological temperatures, and a previous study of glucose oxidase in honey indicated that heating at 50°C for 20 minutes drastically reduced enzyme activity (Schepartz and Subers, 1964). A honey that loses its ability to produce H2O2 after undergoing standard heat treatment may lose useful therapeutic activity.
The honey industry would greatly benefit from a test to predict antibacterial activity based on H2O2 stability, but H2O2 levels alone appear to be a poor predictor of final activity levels, so more research is needed. The condition of the bees and the caliber of their diet can affect the amount of glucose oxidase in the honey. However, honey may also contain catalase, peroxidases, and antioxidants such as gallic acid and caffeic acid, which can break down or prevent H2O2 from damaging microbial organisms, so glucose oxidase alone cannot tell how much H2O2 is produced in a given honey sample.
Furthermore, it has just been revealed that MGO directly alters several proteinaceous chemicals in honey, and if this happens it could also impact the activity of glucose oxidase. As a result, the final concentration of H2O2 in a given honey sample depends on a number of factors that may be present and active to a variable extent. It is not surprising that the different honey samples behaved quite differently during heat treatment, as each of them could be affected by honey processing.
It is common practice to heat commercial table honey to a temperature of 45 °C in order to speed up the filtration process and remove particulate material. However, it is essential to understand that even very low heat processing can reduce the antibacterial action. The viscosity of honey does not change significantly above 30°C, so processing honey at lower temperatures should be feasible without causing a significant increase in difficulty.
These samples should be tested before the antimicrobial test to see if they are authentic or counterfeit duplicates. Therefore, for honey produced for medicinal purposes, minimal processing is advised and samples should be evaluated after processing to verify that the antibacterial activity has not been dramatically reduced.
Conclusions
Additive potential of ginger starch on antifungal potency of ginger starch on antifungal effect of honey against Candida albicans. Mechanism of honey's bacteriostatic action against MRSA and VRE involves hydroxyl radicals generated from honey's hydrogen peroxide. Inhibitory and bactericidal activity of selected South African honeys and their solvent extracts against Helicobacter pylori.
T Bactericidal activity of different honey types obtain clinical and environmental isolates of Pseudomonas aeruginosa. Carbohydrate composition of honey from different floral sources and their influence on growth of selected gut bacteria: An in vitro comparison.