82
Mustafa and Samudro Med J IndonesExtracorporeal
Membrane Oxygenation (ECMO)
:
New Technology
or
Just
A New Tool
for
Developing Countries
?
Iqbal Mustafa,
Heru
SamudroAbstrak
Manajemen pasien dengan ventil.asi melcanik pada gagal napas akut dan
/
atau aù;Jt respiratory dishess syndrome di negara-negara berkembang biasanya dilalalkan oleh ahli anestesiologi.Di
negara maju pun, gagal napas akut, terutama adult respiratory distress syndromemempunyaimortalitasyang masihtinggi. Extracorporeal membrane oxygenator (ECMO) merupakaninovasi teknologi tinggi dalam bidang inænsive care medicine yang dimulai sejak 20 tahun lampau. Pada beberapa unit perawatan intensif di negara majA ECMO digunalcan pada gagal napas akut sebagai rescue therapy atau sèbagai terapi alternartf pada predil<si mortalitas tertentu.Di USA,
ECMO telah merupakan terapi standar pada gagal napas neonatus. Hasil terapi ECMO berbeda-beda pada kelompok umur yang berbeda. Hasil terbaik didapat pada neonatus, yaitu dengan 70-907o berhasil dengan selamat, sedangkan pada anak dan dewasa, didapatitnortalitas 45-55Vo untukpasienyang diprediksi mempunyai mortalins sekinr 807o denganvenrtbsimekanik Apakahmungkin dilakukan ECMO di negaraberkembang? ECMO tidak dapat disangkal sangat efektifuntuk terapi pada neonatus dengan gagal napas, tetapi ECMO sangat membutuhkan tenaga. Biaya ECMO juga sangat tinggi, kira-kira 2 kali terapi perawatan intensif standar. Dengan mempertimbangkan cost benefit analysis, ECMO tampalotya lebih baik dilakukan di negara-negara berkembang hanya pada rumah sakit tertentu yang mempunyai cukup pengalaman operasi jantung terbuka, dan hanya dilakul<nn pada gagal napas neonatus.Abstract
The management of patient's mechanical ventilation, in acute respiratory failure and / or adult respiratory distress syndrome in developing countries is generally done by anesthesiologist. Even in developed counties, patients with acute respiratory failure and
particularly adult respiratory distress syndrome have a very high mortality rate. Extracorporeal membrane orygenation ( ECMO) is an innovation of high technology in the intensive care medicine which emerged nvo decades ago, In certain centers in several develctped countries, ECMO
for
acute respiratory failure is used as a rescue therapy or as an altemative therapy at a certain predicted mortality rate. In fact, in neonatal respiratory failure in the United States, ECMO is considered as a standard therapy. IJnfortunately, the result of ECMO isdffirent
atdffirent
age groups. The best results is in neonates, i.e, 70-90Vo surtival rote, whilefor
older ckiWren and adults the mortality rate is 45-55Vofor
patients with predicted mortality rate around 80Vo with mechanical ventilation. Wouldit be
possible 1o start ECMO therapy in developing countries? ECMO has been unquestionably swccessful in treating a large nurnber af term infantswith
respiratory failure, but ECMO is very labor intensiye. The cost for ECMO is very high, it is about tv,ice as kiglt as standard intensive care treatment. Taking into considerations the cost benefit analysis and cost effictive analysis ECMO would be better carried out in developing countries only at certain hospitals with enough bypass or open heart surgery expertence (tr-2 selected centers), and is best done ontry in neonatal respiratory failure.Keyword.s: ECMO, Developing countries, Cost, Neonates
HISTORY
Extracorporeal membrane oxygenation
(ECMO) is a
form
of
invasive cardiopulmonary support that
canprovide temporary
physiologic
stabilization in
revers-ible circulatory
and/or-respiratory
failure.
Thehistory
of
ECMO application
in
clinical
situation has
beencontroversial.
In
essence,ECMO
is
an innovative
Intensive
Care Unit,
National
CardiacCentre
Hospital,Jalarta,
Indonesiaintensive
careunit
application
of operating room
car-diac technology. The
use ofan artificial lung for
ex-tended applications
was
not
considered
a
serious
possibility
until
Kolf
and Clowes, demonstrated that
the interposition
of
a gas permeable membrane
be-tween the blood
and gas greatly reduced
both blood
trauma
andembolic
accidents dueto direct
gasblood
exposurein
the heartlung
machines thenin uJe.l Work
with
new fabrication and
membrane
materials
andimproved
design concepts
contributed
to
the
steadyevolution
of
membrane lungs
through the
tr950s andVol 6, No 2, April - June 1997
oxygenator, oxygenation
on
cardiopulmonary
bypassfor heart surgery had been
achieved
with a
bubble
oxygenator
which
although
providing
anefficient
gasexchange,
causedinjury to
the
cells at
the
blood/gasinterface. This
injury
resulted inhemolysis
andplatelet
destruction when cardiopulmonary
wasprolonged.
In
1972
Hill reported the first
successfulECMO
case; a 24- yearold
man
with
severe bluntthoracic
traumawho
was treated
for four
days
with
support
using
amembrane
lung and veno-arterial bypass
via the
femoral vein
and artery. Followingthis
initial report
of
success,ECMO
support
becomemore
widespreadfor
patients
with
acute
respiratory failure. At that
time
there were
no
clear
indicators
of the limits to which
mechanical
ventilation
support
could
be
usedbefore
ECMO
was considered.a
Also
the
optimal ventilator
setting, especially
the setting
for positive end
ex-piratory
pressure (PEEP), was
significantly different
from
thesetting that we
accepttoday.)
In view of
the large number
of anecdotal
reports that
had appeared
in the medical literature, the
National
Heart
Lung and Blood Institute
supported
a
multi-center
trial in severely
ill
patients
with
acute
respiratory
failure (ARF),'comparing
the efficacy ofcombined extracorporeal veno-arterial bypass
andconventional continuous
positive pressure
ventilation
(CPPV)
with CPPV alone. The trial involved
nine
centerswhich together enrolled
90 pa^tientsduring
2.5year period The entry criteria were:' The fast
entry
criteria, which included patients with
a
POzof <50
mmHg for
more than
two hours
when ventilated
with
afraction of inspired oxygen
(FIOz)of
1,0 andpositive
endexpiratory
pressure (PEEP) of 5 cm HzO or greater;the slow entry criteria which include,
patients
with
a PaOzof 50
mmHg
for
more than
12 hours when venti-lated with a FIOz > 0,6 and PEEP >5 cm H20, andintra-pulmonary shunt 30%
of cardiac
output when
measured at
FIOz of 1,0.Eligible
patients
hadto
be>12
years and<65
years andpatients
wereexcluded
if
thepulmonary
insult
waslonger
than2l
daysduration,
if the wedge pressure was
>25 mmHg, or
if there was
chronic or irreversible
disease inother organ
system.In both
groups
of
patients the
mortality
rate was
ap-proximately
90Vo and there were nodifference
between the groups.The
observedmortality
of
90Vo wasdisap-pointing
and unexpected.This finding led to loss of enthusiasm
for the use of
ECMO support
in
acuterespiratory failure
(ARF). But
this trial did
demonstrate that extracorporeal support
can
be providedsafely
for
prolonged
period
of
time.
The
main rationale behind the ECMO
srudy was to
correct hypoxemia and "buy time" for
spontaneousE.C.M.O 83
healing of lungs.
Veno-arterial
bypass was chosenover
veno-venous bypass
because
of the better arterial
oxygenation and the iower intrapulmonary
shunt
achievedthrough veno arterial
bypass.zHowever the
validity of
the result
has beencriticized
becauseof the
study
design. Reading thedetails
of the
study
in the 1990s,
most people
would
consider that
the limits of
conventional treatment defined
in
this
study, especially
the level of PEEPthat
was used, arevery different from those acceptable
today when
it is
not
unusualto
uselevels
of PEEP up to
20-25 cmHz0
in the
most
severe cases.Also in
the
last 20
years, there have been many
sig-nificant
advances in thetechnology
of
extracorporeal
circulation.
Consequently,
the complications
as-sociatedwith
untoward
events such astubing rupture,
bleeding,
hemolysis have been considerably
reduced.)'6
Also,
the deleterious
effects
of CppV
onhypoperfused
lung tissue were probably
underes-timated
and theconcept
of
"buying
time" was
applied
in
alung
environment that was
not optimum
for
pul-monary repair
and recovery. ''oIn
1976,
Gattinonni
from Milan Italy
tested
in their
laboratory
a new artificial lung,especially
designed forcarbon dioxide removal,
for possible clinical
use
in
hypercapneic
patients.They
havefound
thatextracor-poreal
COzremoval (ECCOzR)
is an effective
techni-que
for control
of ventilation
in animals. Also they
found
thatit
was possibleto
decreasemechanical
ven-tilation to
zero
while providing a
continuous oxygen
flow
(apneic
oxygenation) equal to the oxygen
con-sumed and
that the use of low frequency
positive
pressure ventilation
of 3-4 breaths
per minute at
limited peak pressure
avoid
theprogressive
atelectasisformation observed during
apnea.)With
thatfinding
an eraof application
of ECCOzRwith
continuous low frequency positive
pressure ventilation(LFPPV)
hascome;
the hypothesisis that
this
tech-nique could promote healing of
the lungs by
avoiding
the
deleterious
effects of high volume high pressure
ventilation.6'9
TERMINOLOGY
84 Mustafa and Samudro
an
extracorporeal
blood flow
ranging
between
ZAVoand 30Vo
of
the cardiac output.
Partial
extracorporeal
COz
removal
(PECOzR) indicates avery
low flow vein
to vein
circulation with
only partial removal of
the C02produced QA-6AVo). Japanese
clinicians
have used theterm
extracorporeal
lung
assist(ECLA) to
describe
atechnique
simiiar
to ECCOzR.
The
ternn
ECLA
or
ECCRS (extracorporeal respiratory
support)generally
refer to the useof
a membranelung
without
eflnphasiz-ing
any specific objective
(oxygenation,
or
C02removal).tu The above confusion
in
terrninoiogy
originated
from the initial
atternpts
at
emphasizing
oxygenation as the main goal
in
the use
of the
membrane
lung. High
flow a-v
bypass
(ECMO)
not
only
improves oxygenation,
but
also removesall
COzproduced, and provides
fuli
cardiac output.
Veno-venous bypass (ECCOzR) removes COzefficiently
andprovides oxygen
in
an amountroughly proportional to
the
ratio of
extracorporeal
blood flow
to
the cardiac
output, but offers no support
of
the heart"Whatever terminology
is -used, acorrect description
of
the system must include:11
tr.
The type
of
bypass(V-A
orV-V)
2.
Theratio of
the extracorporeal bloodflow
(ECBF)
to
the cardiac
output, which is
essentialto
under-stand the contribution
of
the system
to the
oxygenation
3. The
ventilatory
managementof the natural lungs.
PRINCIPLES
OF
GAS EXCHANGE,
AD-VANTAGES
/
DISADVANTAGES OF DIF.
FERENT TECHNIQUES
Oxygenation
with ECMO
is accomplished
via
themembrane
lung. Oxygen
diffuses
from
the
fresh
gassupplied
across the membrane to the lung. The amountof oxygen
that can belransferred
across the membranelung is
limited
by:"
a.
The
permeability of
the membraneb. Surface
of
the membranec.
Blood flow
through
the membrane.Varying
the
fraction
of
0z
in
the
flow
gasto
attain
apost
oxygenator
POzof
100-200mmHg provides
ade-quate
arterial oxygenation.
To
increase oxygen
delivery
and raise
POzblood
flow
through
the
oxygenator must be increased.
This both increases the
amount
of blood
oxygenated
via the
membrane
and decreasesblood
flow
to the patients poorlyfunctioning
lung. The
silicone
msmbranelung is
highly
permeableto COz
and
is
very efficient at
COzremoval.
COzremoval is
doneby increasing the
rateof
gas sweeping across the membraneor by
decreasing theconcentra-Med J Indones
tion of
Cûzin
the sweep gas (some centers add
COzto
the
inflow
gas)to increase the gradient betweerr
blood
and oxygenator
inflow
gas.ECI\{O
can be usedfor
2 basicphysiologic
functions:
a. To process
blood, rernoval
of
COz and saturation itwith
oxygen, replacing
the lungs.b. To bypass the heart, by taking venous
bTood atlow
pressure and
reiilftlsing
it
into
the aorta at high
pressure.
During
veno-venous
bypass or ECC0zR
(extracor-poreal
COzremoval) about
7A-\AVo of oxygenation
occurs through the natural iung" The arnount of oxygen
provided
by
the
rnernbranelung
depends
on the
ex-tracorporeal blood flow,
hernoglobin
(concentration
and saturation)
andthe
arnount
of
oxygen
of the
gasmixture. During routine
perfusion, the extracorporeal
biood
flow
rangesbetween2tVo
and3OVo of thecardiac
output,
and
the fraction
of
oxygen consurnption
provided by
the membrane is about the same.Extracor-poreal
blood
flow
and
oxygen
can be increased to
provide
total respiratory
support.
A
high blood flow
and 0z 1007o
from
the gasmixture
can maintainedlife
for days without
gas exchange
in
the natural
lung.
Clearanceof
thetotal
COzminute production (200-400
ml/min)
requires
ablood flow of
1,5to 2,5l/min
and 15 to 20l/min of
ventilation. The
COz ciearance is lessdependent on extracorporeal
blood
flow;
the keyfactor
for
COz clearanceis the surface
areaof
the membranelung
(a 9m2
surface
is
usually required
!!
provide
adequate COzremoval in
an adult
patient)."
It is
oc-casionally impossible
to institute "complete"
veno-arterial
bypass because
of other
mechanical factors.
However the
advantages
of
complete
veno-arterial
bypass are reduced cardiacwork load and
assuranceof
circulatory
adequacy, goodarterial oxygenation,
com-plete dissociation
of lung function
and gas exchange,good estimation
of
oxygen
delivery
and consumptionbased
on known pump
flow
and delivered
oxygen
contentof
the arterial blood. Alsoit
allows
theinspired
oxygen tension to be
reduced,
largely withdraw
theu"ntlluto.y
support and minimizing
barotrau-u.13'9
PartialECMO allows
some of the venousreturn
to passthrough the lungs.
This blood
remains
desaturated aslong
as the
diseasepersists.
When partial ECMO
isachieved using
femoral arterial
access,coronary
andbrain oxygen
delivery
areuncertain.
Some pulmonary gas mustbe
maintained when
ECMO
support
is not
complete.
Veno-venous bypass
is
generally presumed to
mini-mize
the incidenceof
embolic central nervous system
Vol 6, No 2, April - June 1997
captured
by the
venous cannula
bypass.The pump output is direc
circulation
allowing
some
gasn (passive
dif-uptake
by
theuired
if
veno-venousor partial
veno arterial
bypassis
used to clear carbondioxide
at the rateit
is producedby
thebody.l1
Again with
this technique barotrauma is reduced. The
limitation
of
this
approachis
that reduced
ventilation
and
pulmonary distending
pressuremay
allow
thedis-easedl
scapableof
apneic
y overcomeby
mai
pressure.TECHNIQUES
Veno-arterial
bypassThis is
a standardtechnique currently
usedin neonatal
respiratory
failure,
andit is
also
increasingly
usedin
pediatric population
wirh
circulatory
faiiuie.la The
first
step is to prepare theECMO
circuit,
which is done
by removing air from
thecircuit,
and thenprimed
thecircuit with red blood
cell and
albumin (tô
decreaseplatelet adhesion).
Normal
acid base status
in
thecir-cuit
is attained
by buffering the circuit
with sodium
cannula
(8-10F) is
placed
into
the
right
common
carotid artery
and advanced to the ascending aorta. 1 50u/kg
ofheparin
is given as aninitial
intravenousbolus.
The ECMOcircuit
and cannula arecarefully
connectedto ensure
that no
air has been introduced
into
thei
amps
andg
0
mUmin,
c
80Voof its
estimated 140-150 mUkg
averagein
neonatus.12Veno-venous bypass
vein. Extracorporeal
circulation
wasobtained
entirely
via single double lumen catheter,
with
the outerlumen
usedfor
drainage and the innerfor
return
of
theblood
to
the patient.A
modified
andsimple technique is the
sapheno-saphenous
vein technique.
The blood
is
drained
via
a catheter advancedinto
the
femoral vein
from
the saphenous
vein on
one side and
is
returned
through the saphenous
vein
on
the other side. Oozingfrom
the surgical cutdowns was
aconstant
sourceof
bleeding in the anticoagulated
patient
(5OVoof
thetotal
blood
loss).o Percutaneuscannulation
of
the
femoral
orjugular vein is
now popular,
andcatheters up 29
F
or even 34 F
areavailable
allowing total
COzremoval
(blood
flow
up to 3 to 4l/min).
The catheters areeasily
inserted and therisk of bleeding
isnegligible.
Difficul-ty in maintaining adequateflow
andoxygenation,
com-bined
with
venous drainage problems in the distallimb,
havelimited
the useof
veno venous bypass
in
infants
although some centers have had
success
in
other
pediatric patients.
A
double lumen
catheterproviding
both
venous drainage
and
oxygenated
return
to
theinternal
.jugular
vein,
is being used
in some
cent".r.12'15"
ENTRY
CRITERIA
Neonates
The original criteria for the selection
of babies
for
ECMO
treatment were based on
institutional review
that identified infants
with
a
greater than
g\Vopredicted
mortality
if treated
with
mechanical ventila_
tion. A
retrospective
review by
those institutions
i:'J
i,:iïrâ
teria
ry,ra ^l.
Birthweight >
2kg;
Gestarion
>
35 weeks
2. Absence
of intraventricular
hemorrhage (outside
germinal
matrix, >
GradeI).
3. Absence
of
severe anduncorrectable coagulopathy
4.
Absence
of
congenital anomalies incompàtiblê
with life.
This infant must
be
without lethal
congenital
anomalies,
central nervous system abnormalities
or
coagulation disturbances that precluded the use of
heparin.
Because
of the
high
incidence
of
in_traventricular hemorrhage
in
the pre-term
neonate,ECMO is
used
only for infants more than 35
weeksgestational
age and more than 2kg birth weight. This
effectively
excludes
most pre-term infants
with the
infant
respiratory distress syndrome. Usual criteria
usedto predict which
patients are
likely
to die from
their respiratory
failure
are.tg'zo86 Mustafa and Satnudro
a.
Oxygenation
Index
(Mean
Airway
Pressure x
Fi02x 100Æa02)
0I
> 40 indicates
agreater
than 807omortality
0I
>
25< 40 indicates
amortality 0f
50-80%.
b.
A-aD02 (Alveolar afterial
difference
of
partial
pressureof
oxygen).
A-aD02 >610 mmHg
for 8
consecutive hours or
605
mmHg for
4 consecutive hours at a peakairway
pressure
of 38
cmH20.
c.
UnresponsivenessPa02 < 55 mmHg and
pH
< 7.40
x
3hr
d.
Acute
Deterioration
Pa02
<
40 mmHgfor
>2
hours
andor
pH<7.15for>2hours.
Pa027 < 55 mmHg and pH
<7.4
for
3 hours.Older Children
(l
month - 18
years)
Little is
known
about factors predicting death in
pediatric respiratory
failure.
Indications
for
institutin
gECMO
were derived
from the
neonatal
experienceor
using the NIH ECMO study
criteria combined
with
aintra pulmonary
shunt >307o when measured at an FIOzof
I .0 andpositive
endexpiratory
pressure of 5 cmHz0.Adult
ECC0zR
(Extracorporeal
C0 z, Removal)
The
entry
criteria for ECCOzR in
adult
also aremainly
based
on
gas exchange values
during
standard
ven-tilatory conditions, and the NIH ECMO study
criteria
is
still used.
But nowadays additional
parameters such aslung
mechanic, pulmonary hemodynamic
and evenlung imaging
are used.
Gattinoni
introduced
criteria
based
on
gas
exchange,
poor
CT
scan response to
increasing levels
of
PEEP
(asevidenced on
CT
Scanwith overinflation of
ventilated
lung
areasrather
thanrecruitment
ofnew
alveoli)
andcompliance lower
than30 ml/cmHz0
(0,5
mg/kg,
cm-H20)
for
patients to
beselected
to
undergo-ex-tra corporeal
life
support.sRecently Anderson also use the NIH-ECMO
studycriteria, compliance criteria,
and usedpulmonary
shuntmore than 3OVo or carbon dioxide tension
greater than44
mmH.g
after and despite optimal ventilator
andpharmacologic therapy
as an indicator forseverity
of
disease.
Thirty
percent shunt correspondence
to
arterial
oxygen
saturation
less than 0,95 at aninspired
oxygenfraction of 1,0
andavenous oxygen
saturation< 0,65. Potential
for
reversible
disease wasdefined
asage
less than 60 years, daysof mechanical
ventilation
less than
6
days,
andno
pharmacologic
immunosup-.6
presron.Med J Indones
Absolute contraindications were terminal disease
or
severe
neurologic impairment, active
bleeding
prohibiting
acuteanticoagulation,
and mechanicalven-tilation
for
more
than
10 days. Optimal ventilator
therapy
was defined
as the lowestinspired
oxygen
fractionsufficient
to
maintain
arterial saturation
greater than 0,95,optimal positive
endexpiratory
pres-sure
andpenk
inspiratory
pressure based onhighest
mixed venous
oxygen saturation,
andrate
adjustedto
maintain
carbon
dioxide
tension at
40 mmHg or
lesswithout_exceeding
apeak
inspiratory
pressure
0f 50
cmHz0.6'"
Other
authors also use theold
NIH criteria
but
put
additional
criteria like Murray
score>
2,5 and
failure
to improve respiratory
parameters
with
dif-ferent modes mechanical ventilation (Brunet),
andPaOzlFIOz
<
150 mmHg, PEEP> 10 cmHzO,
(fast
entry):
maximum
medical therapy
for
24-l2O
h PaOz/FIOzûmmHg,
PEEPl0cmHzO,
Qs/Qt
>
30Vo atFIOz
10,
Ctsat <
30ml/cmHzO
or
recurrent
baro-.22
trauma.
PaOz
=
partial
pressureif
arterial oxygen
FIOz
= fraction of
inspired oxygen
Qs/Qt
=
intrapulmonary
shuntPEEP
= positive end-expiratory
pressureCtslat
=
staticcompliance
onCT
scanGENERAL
MANAGEMENT
At
thebeginning
of the bypass the FIOz and
the
meanairway pressure are
maintained
atpre
bypasslevel
to
prevent
suddenlung
collapse
or edema.
When blood
gasesstart
to
improve, oxygen ventilating the
lungs
and the membrane
lungs
are decreasedprogressively.
The whole
systemis monitored,
and inadult
ECCOzRwith catheter
sizes up to 29 F theblood
flow
of 3-4
l/m
can bedelivered.
PEEPlevel
should be reduced hourlycautiously.
Thelungs
areventilated
three tofour times
per
minute in limited
pressuremode (34-45
cmHzO), the lowfrequency is maintained
until
lung
improves.-With
further improvement,
anesthesiaand paralysis
are
suspended
and the patient
is
allowed to
breathspontaneously
(in pressure
support,
intermitent
man-datory
ventilation
or continuous
positive
airway
pres-sure).
There is a
tendency
to
use
pressure
support
ventilation
(supported
spontaneousrespiration) at
amuch
earlier stage and
therefore
arather
long
period
(more
than 3 weeks)is spent in partial
extra corporeal
COz
removal.
During
this time variable proportions
of
the
COzload
areeliminated
by the
membrane
and thenatural lung.
During
these long
bypasses
(days
or
VoI 6, No 2,
April
- June 1997lung may
be so diseased as toreachzero
gas exchangefor
weeks andstill
achievetotal recovery.
Theg"neàl
management of ECCOzRpatient (antibiotics,
nutrition,
water balance) is
no different from
other
critically
ill
patients.
The patient is
disconnected when
his
blood
gasesremain
normal for
4-12 hours(with FIOz
of
0,4)
without
extracorporeal
gas exchange
(gas
lines
clamped).'
In
neonatesonce
the canulla
arein
place
flow
is
in-itiated
at about 50cclmin
and increasedin increments
in 50- 100 cc to
approximately
100-l20 cclkglmin.
This
correspondence
to
about
80Zoof
the
cardiac output.
The
ventilator
settingsgradually
are reduced to an FIOzof 0.2
I
, a peakinspiratory
pressureof
15 cmH20, anda positive end expiratory
pressure
of
5
cmHzO.
In-otropic drugs
such as dopamine and
vasod.ilator
gradually
are discontinued.
Hemoglobin,
hematocrit,
platelets,
WBC,
electrolytes
andcalcium are
monitor-ed
every
8 hours.24Typically
aninfant
on
ECMO
will need
4 mEq/kg of
sodium
and50-70
daily. Due
to the sequestration
of
platelet
into
the membraneavoid transfusing
degrada-tion products
from
the plasma supernatant.Ultrasound
y
to
detect
hemorrhage.
aphy
studies
to provide in_
chamber
vo
rdial function
and
central
shunts.Lung
restfor
48 hours can be done andusually allows
an
ru
(which
c
air
leak)
flation
is
ith
mild
cumulate
ral
minutes
of
hand ventilation and then returned
thepatient
tolow ventilator
settings.Modifying ventilator
settings
haslittle
effect
on
blood
gas tensionsor
acidbase
balance. Oxygenation
is
improved
by
increasing
flow
from
the pump, which
in
turn
clependson the
amount
of
geto
the
circuit,
the
interna
cannula, proper
cannula
po
over
the
ECMO
e
ned
in the
in
ly
requiresb
4
kinked
cannula,
pericardial effusion,
or increased
in_trathoracic pressuie
secondary
to
pneumothorax
or
pneumocardium may
alsoimpede
venousreturn.
are avoided.
Ifthere
is
a needfor central lines
it
should
OUTCOME
Adults
As
it
haspreviously
discussedthe
high mortality
of
NIH ECMO
study
(907o) has changed sinceGartinoni
1986
they
atients.
To
European
the
s
,,
".""rt1?;iig
Also
enplace again
as awaY
support. Anderson
describes succesfull treatment
of
50Voof l0
patients
with severe
adult respiratory
distresssyndrome.
Children
(1
month-l8
years)
In
the
'70s
experiences
of ECMO
in
non-neonatal
pediatric respiratory failure
are
limited. However in
the last
5
years,
following
successin the
neonatalpopulation,
thenumbers of ECMOÆCCOzR
interven_tion in pediatric
patients increaseddramatically. In
USalone
asof August
1991,220 pediatric patients
meet_ing
entry
criteria
were reported
having
recieved
ECMOÆCCOzR
for
severepulmonary failure, with
asurvival
rate
of
46Voin
the
cummulative
population.
However,
astatistically
significant
difference
between erabefore
andafter January
1989 wasobserved
with
therecently
treated grouphaving
a greatersurvival
rate(52Vo
vs.
30Vo).
An
analysis
of
this
220 pediatric
patients has established
variables
measuredbefore
theinitiation
of extracorporeal
life
support that are predic_tive
of survival
for
pediatric respiratory failure for
whom conventional mechanical ventilation
isbelieved
to have
failed.
Thesevariables indicate that
age, dura_tion of
mechanical
ventilation
before
ECMO,
the
de_gree
of
oxygenation impairment and ventilator
pressure are
important predictors
of survival.
An
optimistic view is that
the present
technology
of
anage-diatric
steady
88 Mustafa and Samudro
Neonates
The use
of
extracorporeal
life
support
for
neonatalrespiratory
failure
has
progressed
from
laboratory
studies
to
initial clinical trials
to routine
clinical
prac-tices. Thefirst
succesful case wastreated in
1975 andreported
in
1976 and
clinical
experience gradually
increased
over the
next
severalyears.
By
1985, therewere
18 centersusing
andevaluating
ECMO for
new-born infants.
A
central registry was
established
andexperience
with
715
"ur"r-*"r"
reported
in
1988.28Two
prospective randomized
studieswere carried out
using
adaptive designs,
both
of
which
demonstrated thatextracorporeal
supportoffered
bettersurvival
thanconventional
treatment
of
the time.
In
1988, the
Ex-tracorporeal
Life
Support Organization
(ELSO)
wasorganized
to
standardize the treatment,
maintain
thecentral
data
registry, and
document and
control
thegrowth of
thetechnology
in
andorganized fashion.
In
7979,theNational
Institute of Health held
aworkshop
on the diffusion
of
high-tech medicine
into
generalclinical
practice using neonatal ECMO
as
the
prototypic example.
The workshop
washeld
in
1990and
published
in
1993. Theconclusions
from
the 1990workshop
were that neonatal
ECMO
was
establishedas
standard
treatment
for
severe
respiratory failure
unresponsive to other
methodsof
management.Presently over 4.700
neonates have been treatedwith
extracorporeal
life
support since
1984,with
anoverall
cumulative survival
rate
for
all
respiratory
diagnoses83Vo as
of
1991.In
10- yeartime
(1979-1989)
aspira-tion
syndrome was shown tobenefit
mostfrom
ECMO,
risk
of
mortality was
reduced
from
80Vo(based
onhistorical control) to
about 'l.5Vo-9Vo.Respiratory
distress syndrome was showntohave83Vo
survival
rate,
while in
pulmonary hypertension
of
thenewborn
and airleak
syndrome the rateswere877o
and60Vo,
respectively. Interestingly
sepsis/pneumonia
also
could
benefit from
this
kind of intervention with
survival
rate 77Vobut ECMO
has madeonly
modestimprovements
in
the survival rate
of
neonates
with
congenital
diaphragmatic hernea;
from
neonatal
patients
with CDH of
theExtracorporeal
Life
Support
organization registry (1980-1992), the survival
ratewith ECMO
is
62Vo.There
hasbeen concern
that carotid ligation
may
beassociated
with late
secondary cerebral ischemia.
In-fants should
befollowed with routine
neuro-develop-mental
andmedical evaluation.
Thefollow-up
studiesto
datereport
anextremely encouraging
7O7oto
8O7oof children with normal development.''
Resultsfrom
Med
I
Indones(Bartlett) were reported
in
1994
recounting the
ex-perience
with
460 newborn
casesover 20
years
with
overall
87%
survival. The worldwide
experience
recorded in the neonatal
ECMO registry
in1994
should
aSlVo
survival of
9258 caseslater
and documentedin
Table 2. The registry report
appearsannually
in
themeeting edition
of
theJournal
of ASAIO.
Table
l.
Outcome in neonatalrespiratory
failure
treatedwith ECLS. University of Michigan data
No of Cases 7o Survival
MAS RDS PFC CDH
Sepsis
Other
t'70 91
38 68 78 24
97 88 92 66
83
75
Total
MAS
= meconium aspiration syndrome.RDS
= infant respiratory distress syndrome.PFC
= persistent fetal circulation.CDH
= congenital diaphragmatic hemia.Sepsis
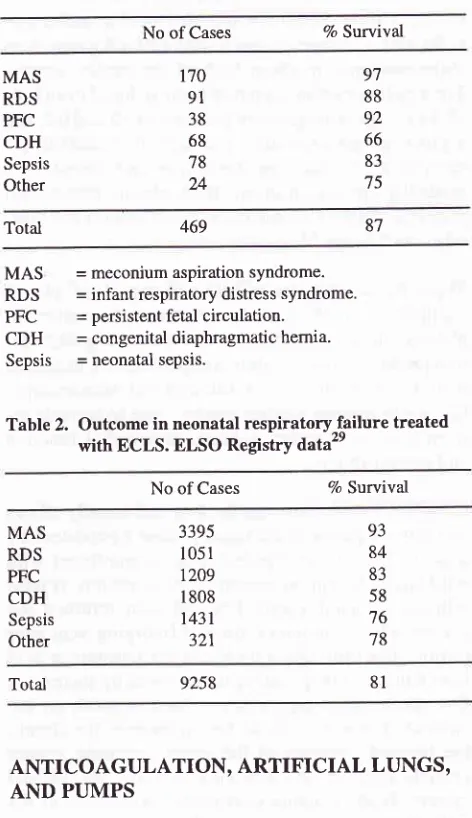
= neonatal sepsis.Table
2.
Outcome in neonatal respiratoryTailure treatedwith ECLS. ELSO Registry
data"
No of Cases % Survival
87
469
MAS RDS PFC CDH
Sepsis
Other
3395 1051
t209
I 808
1431 321
93 84
83
58
76
78
Total 9258
ANTICOAGULATION,
ARTIFICIAL
LUNGS,
AND PUMPS
Due to
continuous heparinization,
bleeding
has been themajor problem
with ECMO.In
adult
theintroduc-tion ofpercutaneus cannulaintroduc-tion
has lessened thebleed-ing
from
thecannulation
site.Anticoagulation
In
neonates andpediatric population
thereis
atenden-cy to
maintain
lower
activated clotting
time
(from
200-240 msec
to
180- 200 msec), and ahigher platelet
[image:7.595.308.544.203.612.2]Vol 6, No 2,
April
- June 1997count
(from
>50.000-600/pl
before
1988to
more than100.000/pl recently).
Surfaceheparinized circuits that
could
berun
without systemic anticoagulation and
atnormal
clotting
times
arebeing
tested and arerecently
become
available.
Onereport
has described the useof
this
technique
in
a patient whounderwent succesfull
extracorporeal
lung assist for
35
days despitediscon-tinuing intravenous
hegarin becauseofsevere bleeding
after the
first
14 days.There are
two
typesof
pumpswhich
are being usedfor
extracorporeal
life
support, i.e.,
centrifugal
pump
androller pump. Most
centers
areusing
roller pump but
centrifugal pump become increasingly popular.
Al-most
all
centers
exclusively used Scimed Kolobow
spiral
silicon
rubber
membranelungs.
Theselungs
aregood
for long term use, some have
lasted
uninterrup-tedly
for
more than
3
weeks
with
satisfactory
gasexchange
performances. They
routinely
last wellover
a week.Membrane lung
leakage isextremely
rarewith
this
device.Microporous fiber
lungs
with
heparin
surfacecoating
are now
available,
but
the
major disadvantage
of
this
device is plasma leakage. The
hydrophobic
pores,after
a
certain
time
of
contact
with
blood,
become
hydrophilic
andstart
to
leak plasma at variable
rates.A
very new microporous
fiber
lungs
with
less plasma leakageis
now
available.
A
new
protease
inhibitor,
nafamostat mesylate
hasbeen
developed
as ashort
life
anticoagulation
and hasbeen
widely
used
to control blood
coagulatin during
treatment
with
artificial
organs, however
further
ex-perimental
trials
will
be needed todetermine whether
anticoagulation
with
nafamostal mesylate
is
superior
to
standardanticoagulation using
heparinin
ECMO.3l
COMPLICATIONS
AND COST
As with
every
technology
advance, thebenefits
of
aninvasive intervention
must beweighed
against therisk.
Due to the systemic
heparinization,
therisk for
hemor-rhage
is
very
great. Intracranial
bleeding
in
the
oxygenator
failure,
raceway ruptu
tion,
clots
and-air in circuit, and
c"un
o""ur.24'32
At
ou. institution
deaths
was attributed to
araceway rupture.
The cost ofhealth care has become a pressing concern,
high technology intensive
care interventions areoften
singled
out
t factor contributing to
es_calating
cos
into this
category
andrep-resenting
o
st
exciting
and effective
technologies
developedin the past 25
yearsespecially
in
newborn intensive care. pearson and
Sliort
ex-amined
hospital charges
for
infants who met criteria
for
ECMO
in
the
years
before and after an ECMO
program began. When
they
considered
all
infants,
ECMO
was 2 percent less expensive thanconventional
treatment. When
only
survivors were
considered,
ECMO
was43
percent
less costly. per diemhospital
charges
for
conventionally treated
infants
were 57
percent
of
per diem
charges
for
infants
treated
with
ECMO. So,
the
overall cost differences
were
at-tributable
to
a reductionin
the average
length
of
thehospital
stay in rhegroup
thatreceivei
BCniO.33
Added daily cost
of
ECCO2R
(adult) ro the
patient
amount
to
approximately 4500
USD
for
the
initation
of
bypass and
approximately
2500
USD a
day
while
being maintained on
bypass.
In
Milan,
Italy
addeddaily
cost
is
around
1000
USD
and
in
Melbourne,
Australia
1000
Australian
dollars.34
Oo.
recent
ex-periences
with
a 3year-old
child who
was on
bypass(ECMO)
for
5
days,
the
total cost was
10.500
USD
(1500 USD/day
for
disposable
and laborarory tests,
3000USD
the initiationof
bypass).Care
of
the patient on
ECMO
requires
attendanceof
ECMO
personnel. These personnel are doctors/nurses,perfusionist,
who
are
specially trained
in
the class
room
and in thelaboratory. They
work in
conjunction
with
the patients
nurse
to
carry out
all
the
patients
needs. Team
work
from intensivist, neonatologist,
sur-geons,
and technicians as
well
as consultants
(car_diologist, pulmonologist) is essential.3s
The total cost
of
developing
an
ECMO program
averages
125.000USD,
with a range
of
50.000
USD to
200.000 USD.
USD)
and training costs represente
expense
incurred
for
operating
major
expenseis
for the personnel
(salaries
and wages).Fortunately all of
our personnel
arestill working voluntarily for this
program. We
also havecut
ourtraining
cost byjoining
a 2 weeks ECMOcourse
in
Melboume
-
Australi
a
(for
4
personnel
2doctor,
2 nurses, thetotal cost
was 8000 USD).ECMO
as a partof
an extension therapyof mechanical
ventilation
is
a majorchallenge faced
by
developing
countries
such asIndonesia. Apart
from financial
con-straints, experience
showsthat
to institute ECMO for
90 Mustafa and Sarnudro
patients
who
already has anindication,
and convincingtheir
doctorsof
the effectivenessof ECMO
is not easy;until
it
is too
late when the patients have already
hadan
irreversible
organ damage.Like
othersophisticated
and
high
technologies, the
ECMO could only
be
of-fered
atcertain hospitals. For
setting up suchprogram,
lacking
of
experienced
staff
in cardio-pulmonary
bypass (cardiac
surgery ) is oneof
themain
obstacles.The
ECMO
program
in
"Harapan
Kita"
National
Car-diac Center Indonesia
(a
cardiac hospital
with
600open heart surgeries
per
year)
has beenstarted
since 1991.Due
to
the constraints such
asfunds, logistics,
and
difficulties
in
obtaining referral (the patient
is
delivered
late or thepatient
has noindication),
we wereonly
able to handle 9 ECMO cases(circulatory
support
or pulmonary support
whithin 3
yearstime) with only
2 neonateECMO survivor
(decannulated).It
is recomended that aminimum
of
12 patients per yearbe
treated
in order
to
ensure
that clinical
skills
aremaintained
for
this
complicated therapy.
However,
thanks
to
the
development in
technology
(heparin
bonded oxygenator/circuit)
we
are
very
optimistic
(even
in
a
developing countries)
for
the future of
ECMO
aslong
asit is
done
for
neonatal respiratory
failure, at
1-
or 2
selectedcenters and always taking
into
the consideration "the
costbenefit analysis"
and"cost effectiveness analysis"
Although
important questions remain
unanswered,certain indications
for
the use
of
ECMO
are
widely
accepted,
and
areasof
research arewell-defined,
and aswith
otherinnovative
therapy, a desirefor
rigid
rulesand
for
a
clearer
een standard
andexperimental
ther
and unrealistic.32Decisions about innovations
in
clinical
medicine
remains matters
of
practical wisdom,
rather
than of
theoretical
science.REFERENCES
l.
GibbonJH. Artificial
maintenanceof circulation during
experimental occlusionof
pulmonary artery'Arch
Surg1937,34:1105-31.
2. National Heart, Lung and Blood Institute, Division of Lung
Disease. Extracorporeal suPport for respiratory insufficien-cy: a collaborative study in response to RFP-NHHL 73-20. Besthesda
MD
National, Heart Lung and Blood Institute1979:l-395.
3.Zapol
]WM, SniderMT,
Hill
JD
et al. Extracorporeal
membrane oxygenation in severe acute respiratory failure: a
randomized prospective study.
JAMA
l9'l 9 ; 242:2193 -6.4.
Hill
JD, O'Brien TG, MurrayJI,
et al. Acute respiratory insufficiancy. J Thorac Cardiovasc Surg 64:551,1972.Med I Indones
5. Gattinoni L, Pesenti
A,
Damin G. Extracorporeal carbondioxide removal.
In:
Textbookof
critical
care, 2nd ed. Saunders, 1989-656.6. Anderson HL, Delius RE, Sinard IM, et al. Early experience
with
adult extracorporeal membrane oxygenationin
themodern erâ. Ann Thorac Surg 1992;53:553'
7. Egan TM, Duffin J, Glyin MFT, et al. Ten year experience
with extracorporeal membrane
oxygenationfor
severerespiratory failure. Chest 1988;94:681.
8. Muller E, Knoch M, Moltermann, Lennarts. Extracorporeal respiratory support @CCOzR)
in
severe acute respiratory failure (ARDS), Medicine intensive. The6"'
World Con-gresson
Intensive andCritical
CareMedicine.
1993.Abstract 80.521.
9. Hickling KG. Extracorporeal COz removal
in
severe adultrespiratory distress syndrome. Anaesth Intensive Care, 1986;14:46-31.
10. Morioka
T, Terasuki
H, Tsuno
K
et al.
Indication andmethods of extracorporeal
lung
assist (Ecla as ECMO).Presented at the 4th Congress of Western Pacific Association of critical care medicine (WPACCM), Denpasar, September
6-9,1987.
11. Gattinoni
L,
PesentiA, Bombino
M
et al. Extracorporeal carbon dioxide removalin contemporary management in
critical care, mechanical ventilation and assisted respiration.In:
GrenvikA,
Downs JB, Rasaneu J, Smith R eds. New York: Churchill Living Stone, 1991:91-113.12. Fuhrman BP, Dalton HJ. Progress inpediatric extracorporeal
membrane oxygenation Crit Care Clin 1992;8:19l-201. 13. Gattinoni
L, Pesenti
A,
MarcolinR
et al. Extracorporealsupport
in acute respiratory failure. lntensive Care World
1988;5:42-45.14. Bartlett RH, Roloff DW, Comell RG et al. Extracorporeal circulation
in neonatal respiratory
failure.A
prospective randomized study, Pediatri cs 1985;7 6'.47 9.15. Anderson
III
HL,
Attori
RJ, Custer JR, Chapman RA'Barlett RH.
Extracorporeal membrane oxygenation for pediatric cardiopulmonary failure. J Thorac Cardiovasc Surg 1990;99:101l.
16. Kirkpatrick
BV, Krammel
TM,
MucllerDG,
OrmazabalMA,
Greenfield LJ, SalzbergAM.
Useof
extracorporeal membrane oxygenationfor
respiratory failurein term
in-fants. J Pediatr 1983;'12:872.
17. Chevolier JU, Durandy
Y,
BatisseA,
Mathe JC, Lostil J.Preliminary extracorporeal lung support for the neonatal adult respiratory failure. Lancet 1990;3 35:1364.
18. Horton SB, Horton
AM,
Mullaly RJ et al. Extracorporeal membrane oxygenationlife support a new approach.
Per-fusion I 993 ; 8: 239 -247 .
19. OritzRM. Pediatric Clinical 1987:'34:.39.
20. Barlett RH. Cunent status of extracorporeal
life
support inneonatal respiratory failure. Yeas Book
of
Intensive Care and Emergency Medicine. Springer 1995l.209-l'l '21.
Morris
AH, Suchyta. Extracorporeal
COz removalfor
ARDS. Crit Care Res 1990;l:184.22. Lewandawski, Falke KJ. An imperative for scratinizing ex-tracorporeal membrane oxygenation. Curr Opin Crit Care
VoI 6, No 2,
April
- June 199723. Dembitsky WP, Willem DC, Jaski BE. peripheral vascular
access for organ support in extracorporeal cardiopulmonary support
in
critical
care. Editedby
Zwischenberger JB,Bartlett RH.
ExtracorporealLife
Support Organization1995. Ann Arbor 1995;205-19.
24. Dellius RE, Bove EL, Meliones JN et al. Use of
extracor-poreal life support in patients with congenital heart disease.
Crit Care Med 1992;20:1216-22.
25. Redmond CR, Graves ED, Falterman KW, OchnerJL, Arens
RM. Extracorporeal membrane oxygenation for respiratory
and cardiac failure
in
infants and children. J ThoracCar-dovasc Surg 1987 ; 93:199.
26. Kanter KR, Reuning DG, Weber TR, Zambie MA, Braun p,
Martychenkov. Extracorporeal membrane oxygenation for
postoperative cardiac support
in
children.J
ThoracCar-diovasc Surg 1987; 93:27-35.
27. Thomas P. Green, Otwel, James C, et al. The impact
of
extracorporeal membrane oxygenation
on
survival
inpediatric patients
with
acute respiratory failure. Crit CareMed 7996;24:323-9.
28. Toomsian JM, Snedecor SM, Cornell RG, Cilley RE, Bartlett
RH.
National experiencewith
exûacorporeal membraneE.C.M.O
9t
oxygenation for newbom respiratory failure. ASAIO Trans
1988;34:140-7.
29.Tracy TF, De Losh
T,
Bartlett RH. Registry Report.Ex-tracorporeal Life Support Organization 1994. Am Soc
Artif
Intem Organs.
30. Wetterberg T, Steen S. Total extracorporcal lung assist a new
clinical apporoach. Int Care Med l99l;17:73-7.
31. Anderson
HL,
Edmunds Jr. Coagulation, anticoagulationand the interaction
of
blood and artificial surfacesin
ex-tracorporeal cardiopulmonary support in critical care, Edited
by
Zwischenberger JB, Bartlett RH. ExtracorporealLife
Support Organization 1995. Ann Arbor 1995; 53-70.
32.Yagler
C,
Avila C, Lagunoff
D.
Alumunium containingemboli
in
infants treatedwith
extracorporeal membraneoxygenation. N Engl J Med 1988:2:75-9.
33. Lantos
JD,
Frader J. Sounding board extracorporealmembrane oxygenation and the ethics of clinical research in
pediatrics. N Engl J Med 1990; 9:409.
34. Butt W. Personal communication.
35. Ortiz. RM, Cilley RE,
Barlett.
Extracorporeal membraneoxygenation
in
pediatric respiratory failure. pediatr Clin