lv{alm,sian Joumal of ivlicroscopl' Vol 6 (20 ]0) pg. 69-74
LOW-TEMPERATURE COMBUSTION SYNTHESIS
OF
Bi,rTi:OrePLATE.LIKE
STRUCTURE AND SOME
OFITS CHARACTERIZATION
A. Umar Al-Amani, S. Srimala-, M.N. Ahmad Fauzi and A.R. Khairunisak
School of Material and Mineral Resow'ces Engineering, Universiti Sains Malaysia, 14300 Seberang Perai Selatan, Nibong Tebal, Penang
Bismuth titanate, BiaTiiOp was prepared at different hydrolysis temperatules ond characterized with XRD, Rietveld analysis, TG-DTA, FESEM and TEM. The results
indicated that the pure bismuth titanate was obtained after combtrstion at lo*et temperature of about 260 "C. Interestingly, no calcination step involved to obtain bisrnuth-strtrctured layered. The effect of hydrolysis temperatttre at 4A ''C, 50 ''C'
and 60 oC on phase.fotmation, lattice parameter, crystallite size, thennal behavior
as well as grain morphologies was investigated.
Keywords: Bi+Tr:Or:; Low-temperature combustion; Chmacterization
INTRODUCTION
Bismuth titanate, (Bi4TirOr2) has been
used for ferroelectric tandort access memory
(FRAM)
devicesdue
to
its
outstandingfenoelectricity [1]. This compound has been prepared using a rvet chemicai technique such
as
sol-gel, hydrothamal, precipitation audmolten-salt synthesis
l2l.
Wet
chemical technique is preferred because a single phase Bi4Ti3Or2 can be obtained at low temperature[3]. In early stage, Bi.rTirOrz has been prepared
using conventional solid state which often results
in
high
aggiomerationand
low sinterabilityof
powders becauseof
high calcination beyond 1000 oC and repeatedgrinding [4].
Several studies have emerged as the leading techniques to lorm BiaTi3Or2 have been
reported.
Han and
Ko t5l
producednanocrystalline Bi4Ti3Or2 powder using an
intense meehanical activation of a rnixtr::'e of
o-BizO: and TiO2 after a milling time
of
9hours. Gu et
al. [6]
reported that BiaTi3O12nanopiates were synthesized by polyethylme
glycol-assisted hydrothermal rnethod
at
200oC. However, there are some lirritations in
both
processes suchas the
difficulty
to understand the phase evolution and rnechanicalactivation condition and the courplexity of hydrothermal process in terms of experimentai
set up.
Therefore,
in
thiswort
we use thecombustion method to produce Bi+Ti:Oi:. The
current approach offers great advantages such
as simple experimental set up, capability to
produce high purity powder
with
excellent homogeneity, eliminate calcination step andrelatively
low
fonnation ternperatureof
asingle phase [7-9]. However, comprehensive
investigation
on
the
processing parameter needs to be conducted in order to r.mderstandthe relation between processing parameter with properties of the BiqTiror2. In this paper, the
effect
of
hydrolysis temperahreon
theformation of BirTi:Orz is reported.
i - r ----. i -i-::r: iJ \, -4hmad Faci and A.R. Khoinnisak
\II
TH OD S .1.\D \I-A,TERL{LS3,s:.-.-:: :t:::=r: oe;rrlahldrale, citric
::.::::
:':=1.--:-f\',
riop:oporide iiere used:: :=-::t:
::ia.:IrarS.Inlialll',
bismuth nitrate:=-:r11i::.
u'as added rnto a conical tlask::a:
r.rl2neii
drstilled wata-. The conical flaskn:s -:i:r
placedin a
water bath and the:e:ce:a:r:e
\las
confolled at40 0c
under;lrjia:i
sILnng. Simultaneously, citric acid+ai
di.solr.ed in distilled rvater and stirred atrcc:n :arnperarure. After that, citric aqueous
u.ere added to bismuth aqueous and constantly
sired
to
iorm bismuth mixture. Separately,titanium (IV) isopropoxide rvas dissolved in 2-)Iethaoxyethanol (Sigma-Aldnch, ?_99 %) to
torm aqueous solution of Tia- ion. Then, the
.lqueous
of
Ti'-
ion rvas slowly added intobismuth mxflue. The obtained mixture (which
aiso knou.ri as precursor soiution) was adjusted
to pH 7 by usLng NFIaOH (29 % solution). The precusor solution
with milky
color
lvashldrolyzed at 40 oC, 50 'C, and 60
'C
andstirred for 24 h to obtain more homogeneous
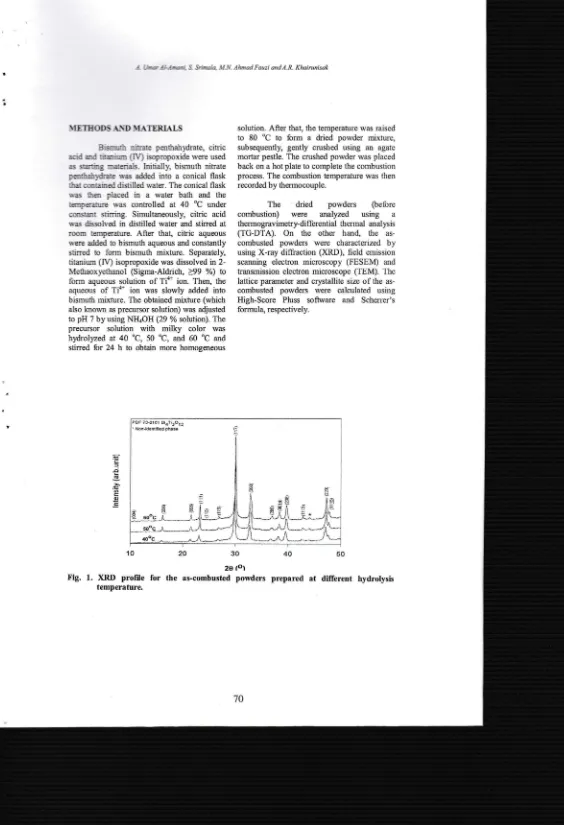
Fig.
1.10
XRD
profile temperature.solutron. After that, the temperuture rvas raised
to
80'C to
forma
dried powder rnixture,subsequently, gently crushed using an agate
mortar pestle. 'Ihe crushed powder was placed
back on a hot plate to cornplete the cornbustion process. The combustion temperatue was then recorded by thermocouple.
The dried
powders
(belbrecombustion)
were
anaiyzed
using
ilthermogravimetry-differential thennal analysis
(TG-DTA).
On the
other hand,the
as-combusted powders were characterized by using X-ray diffraction (XRD), fie1d emission
scanning electron microscopy (FESEN{) aad
transmission electron microscope (TEM). The
lattice parameter aad crystallite size of the
as-combusted powders were calculated using High-Score Pluss softrvare
and
Schener's formula, respectively.20 (o!
for
the
as-combusted powders preparedat
different hydrolvsisN c o
50
40
30
t3,?141 Bi4Ti3Ol
[image:3.595.19.583.1.826.2]LOIY-TEMPERATURE COMBUST]ON SYNTHESIS OF Bi'Ti30II PLATE-I}KE S?RUCTURE AND SOME OF ITS CHARACTENZATION
RESULTS AND DISCUSSIONS
XRD arulysis
The XRD profile for the as-combusted
porvders prepared
at
different hydrol;sis temperatue is shown in Fig. 1. The result showthat all ttre difliaction peaks are corresponding
to BiaTi3O12 phase and matched well with PDF
#
73-2181. The absenceof
any other peakconfinaing
a
single phaseof
BiqTir0rz wassuccessfully obtained
at
lorv-temperaturecombustion synthesis. Besides that, it was also
noticed that the hydrolysis temperature had
significimt influence
on
diftaction
peakcharacieristic.
It
was noted that the broadnessof
the
peak reducedwith
increasing ofhydrolysis
temperature,indicating
high crystallinity degree. Basedor
Rietveldarralysis, the variation of lattice parameter was
clearly affected by hydrolysis temperanre. We
also found that a- and b-parameters rvere not always close; thereiore
it
confirmed thatBi4Ti3Orz compounds matched
well
with pseudo- orlhorhombic strucnrre.With
thehcrease
of
this parameter, the volumeof
orthorhombic sfiuctwe
of
BiaTi3012 alsosiightly increased and the result obtained at 60
oC was considered very close to the standard
file. As mentioned in the experimental section,
the crystallite size at the peak position (i17) was calculated using Scherrer's equation [10] and the result is shown
il
Table 1. The resultshowed that the crystallite size was strongly
dependent on the hydrolysis temperature. This was attribute.d by the increase
of
grain size.'I'able 1. Lattice parameter and crystallite size of the as-combusted powder at different hydrolysis temperature calculated fiom XRD data
Lattice pararneters 400c
50nc
600cal A.
b/A
cl
A
V/
i06 pm3Space group (No.)
R (expected)/ % R (profile)/ %
R (weighted proftle)/ o/o
Density (calculated)/ g/cm Crystallite size (nm)
5.4i50(1)
5.4400(1) 32.7730(6) 96s.3 804
Fmmm(69)
4.62321 10.458s 1 t2.3113
8.06 24.1
Fmmm(69)
Fmmm(69)
5.4140( 1)
s.4440( 1)
32.7840(6) 956.2748 4.32741
lt.86494
14.20051 8.052s 26.4 5,420(2) 5.447(2) 32;/75(8) 967.s442 4.92416 11.96359 14.25008 8.0419 26.5TG-DTA analysis
The thermal behavior
of
the dried porvder rvith dilferent hydrolysis temperatures is shorvn in Fig. 2. As seen the decompositionprocess can be divided into hvo distinct stages
in
theTG
plot (Fig. 2(a)). This trend wasA. Umar Al-Amani, S. Srimala, M.N. Ahmad Fauzi and A.R. Khairunisak
temperatures. The first weight loss took place
at temperature rangc at temperatrue range of50
-
181 "C. This causes to the vaporization of theresidual water, organic solvent such as
2-methaoxyethanol, isopropoxide and hy&oxyl groups.
It
lvas
correlatedwith
thg
small endothermic peak at 121 "C and 151 "C (notshown
here) The
secondrveight
ioss accompanied by a sharp exothermic peak tookpiace at different temperahrres i.e, 242 'C,244
'C
ard 246 "C for 40 oC, 50 oC and 60 'C, respectively. Large exothermic peak was due tovigorous oxidation-reduction
reaction
or cornbustiq! between dgqoryposed citric acidand nikate
ions
which then
led
tocrystallization of bismuth tiianate.
This frrding
confirmedthat
theoombustion
reaction
dependenton
thehydrolysis temperatures
of
the
precursorsolution.
It
was wor& to note that no further weight changes and heat effects were observedin the TG-DTA cun/e up to 500 nC, indicating
{a}
50"c
40'c
the temperature ofbelow 260 "C represents a
srtfficient temperature for the formation r,rf a
crystalline Bi+Ti:Or:
from the
con-rbuslion reaction.Micro stru cture analysis
The FESEM (Fig.
3(a)) microstructuresof
the cornbusted powdershydrolyzed at 60 "C are shown in Fig. 3, The images for 40 "C and 50 "C rvere not shorvn as
the change in terms of size and shape were not so obvious. The Bi+TirOrz povvder was mostly composed
of
plate-likegrains
and
theapproximate diameter of grains was about 200
nrn. Since the size was quite fine, the particles
formed
with
high agglomeration as clezrlyobserved by TEM (Fie. 3(b)).
{b} 50'c 40'c i
60'c
240
femperatun (ocl
Fig.2,
Thermal behavior of as-combusted poxders with different hydrolysis temperatur.e: (a) TC and (b) DTA.teo
:-33-4
200 300
LO'Y.TETIPERAN]RE COJI{BUSTION SYNTHESIS OF BiILOTJ PLATE'I,IKE STRUCTUKE AND SOME OF IT'S CTTARACTENZATION
Fig.
3.
Plate-like grains of as-comtrusted powder hydrolyzed at 60 "C: (a) FESEM and (b) TEM,CONCLUSION
Bi+Tirorz powder rvas successfully obtained by a combustion synthesis at very low temperature.
It
rvas confirmed by XRD andTG-DTA. Siudy
on
hydrolysis temperaure showedthe
variationof
structure lattice parameter that was confirmedby
Rietveld analysis.Dii-ferent exothennic telnperanres were aiso recordedin
DTA,
intlicating tlte hydrolysis ternp emhrre was essenlial paralneterto
produce Bi4TilOt2 powder. Nano-platey stnlchrre of BirTi:Or: rvas clearly observed by f E Slr.lvl ano I -ts.lVI.ACKNOWLEDGMENT
The authors appreciate the technical sripport provided by the School of Materials and lvlineral Resources Engineering, USM. This research was suppofled by the E-science
Fund 305/Pbahx1601,3357, Short term USM 304.PBahan.6035267
and
USM-RU
grant100l,PBahan/8042018.
REFER.ENCES
t1l
Chen,M., Liu,
Z.L.,Wang,Y.,
Wang,C.C., Yang, X.S.,
Yao
K.L.
(2004).Ferroelectric properties of Pr6O11-doped
BiaTi3Ol2, Solid State Commun., \30 735-739.
Lazarevii,
2.,
Stojanovi6, B.D., Varela"J.A. (2005). An Approach to Analyzing Synthesis, Structure and Properties of
Bismuth Tiianate
Ceramics,
Sci.Sintering,3T 199-216.
Pookmanee,
P.,
BoonphaPk,
P.,Phanichphant,
S.
(2004).
Chemicalsynthesis
oF
bismuth
titanatemicroparticles, Ceram.
Int.,
30
1917-1919.
Kan, Y., Jin. X., Wang, P., Li, Y., Cheng,
Y.B., Yan, D. (2003). Anisorropic grain
grorvth of Bi+Tirorz in moiten salt fluxes,
lvlateL Res. Bull.,38 567-576.
Han,
I(,
Ko,T.
(2009). SYnthesis and phase formation of ferroelectric Bi4TiiO12nanoporvder by mechanicai alloying,
J
Allo1, Q6sO6., 473 490495.
Gu, H., Hu,2., Hu, Y., Yuan, Y., You, J.,
Zou,
W.
(2008).The
structure aild photolurninesoenceof
Bi4Ti3Or2nanoplates sl,nthesized by hydrothennal nrethod, Colloid Sutfaces A., 315 294-298.
Patil, K.C., Aruna, S.T., Mimani, T. (2002). Combustion synthesis: an update,
Cun'. Opin. Solid State tVat Sci., 6 50'1
512.
tLl
t3l
t4l
t5l
t6l
A. umar Al-Anani, S. Srinala, lrf.N. Ahnad Fqilzi ctld A.R. Khaintnisak
t8l
Luo, S., Tang, 2., Yao, W., Zhang, Z.(2003).
lrw-temperature combustionsynthesis
and
characterization ofnanosized tetragonal
barium
titanatepowders, Mio'oelectron. Eng.,
66
141-152.[9]
Franco Junior, de Olivera Lima, E.C,Novak,
M.A.,
WellsJr,
PR.
(2007)'Synthesis of nanopadicles of Co,Fe13-'yO.r
by combustion reaction method, J. Iulagn.
Magn. Mater., 308 198--202.
[10] Burton, A.W., Ong, K., Rea, T., Chan,
I.Y. (2009). On the estirnation of average
crystallite
size
of
zeolitesfrom
the Scherrer equation: A critical evaluation ofits
applicationto
zeoliteslvith