IDENTIFICATION O CONTENT, AND SL FAT FROM CHOCOL
FACULTY OF AGRICUL BOGO
MANUSCRIPT
N OF TRIACYLGLYCEROL PROFILE, SOLI SLIP MELTING POINT OF COCOA BUTTER
LATE BAR PRODUCTS IN INDONESIAN M
By:
TERESIA TANDEAN F 24051218
2009
RICULTUR AL ENGINEERING AND TECHNO OR AGRICULTURAL UNIVERSITY
BOGOR
LID FAT ER LIKE
MARKET
IDENTIFICATION O CONTENT, AND SL FAT FROM CHOCOL
This Manuscript is in the Fulfillment of th Engineering and Tech
Faculty o
FACULTY OF AGRICUL BOGO
N OF TRIACYLGLYCEROL PROFILE, SOLI SLIP MELTING POINT OF COCOA BUTTER
LATE BAR PRODUCTS IN INDONESIAN M
By:
TERESIA TANDEAN F 24051218
pt is Submitted to the School of Undergraduate Studi the Requirement for the Degree Bachelor of Agri chnology in Department of Food Science and Technol
of Agricultural Engineering and Technology Bogor Agricultural University
2009
RICULTURAL ENGINEERING AND TECHNO OR AGRICULTURAL UNIVERSITY
BOGOR
LID FAT ER LIKE
MARKET
tudies Agricultural
Technology
BOGOR AGRICULTURAL UNIVERSITY
FACULTY OF AGRICULTURAL ENGINEERING AND TECHNOLOGY
IDENTIFICATION OF TRIACYLGLYCEROL PROFILE, SOLID FAT CONTENT, AND SLIP MELTING POINT OF COCOA BUTTER LIKE FAT FROM CHOCOLATE BAR PRODUCTS IN INDONESIAN MARKET
By :
TERESIA TANDEAN F 24051218
This Manuscript is Submitted to the School of Undergraduate Studies in the Fulfillment of the Requirement for the Degree Bachelor of Agricultural Engineering and Technology in Department of Food Science and Technology
Faculty of Agricultural Engineering and Technology Bogor Agricultural University
Born on March 29, 1987 in Medan Graduate on September 4, 2009
Approved by,
Dr. Ir. Purwiyatno Hariyadi, MSc Academic Advisor I
Ir. Soenar Soekopitojo, MSi Academic Advisor II
Acknowledged by:
on behalf of Head of Food Science and Technology Department Secretary,
Teresia Tandean. F24051218. Identification of Triacylglycerol Profile, Solid Fat Content, and Slip Melting Point of Cocoa Butter Like Fat from Chocolate Bar Products In Indonesian Market
Supervised by: Purwiyatno Hariyadi and Soenar Soekopitojo
ABSTRACT
Chocolate is one of the main product from cocoa bean which vary depends on its quality and price. Chocolate is well -known with its brittle and good snap in room temperature and could melt quickly and completely in temperature of mouth. Fat that plays the big role in the melting properties of chocolate is cocoa butter.
In view of the fact that cocoa butter is the most expensive chocolate component whose price and availability often change, there have been long term efforts to replace it, fully or in p art, with other vegetable fats, so-called cocoa butter alternatives, which would be cheaper . Furthermore, the absence of regulation about CBA addition has caused to the variety of chocolate confectionery products quality in Indonesia. The cocoa butter-like fat or CBA profile needs to be analyzed to know the variety of chocolate bar quality in market. Cocoa butter profile s were identified by analyzing the triacylglycerol profile, solid fat content, and the slip melting point.
There are 13 chocolates that have been chosen vary in type and prices (Chocolate A to M). Based upon their characteristic, the chocolates can be divided into three main groups: CB dark chocolates (Chocolate A, B, and I), CB milk or white chocolates (Chocolate C, D, F, G, and H), non CB milk chocolates (Chocolate E, J, K, L, and M). Similar triacylglycerol component to CB was found in dark chocolates meanwhile m ilk and white chocolate s are almost similar but have small peaks, which imply the presence of milk fat. Quite different chromatograms were found in non CB chocolates. It also can be concluded that the non CB chocolates are made from CBS or lauric type -fat. This study found that StUSt, StU2, and St3 contribute to the higher SMP in chocolates. Also, the higher POP and POS, or lower SOS cou ld give lower SMP.
In room temperature, the presence of milk fat has lead to the higher SFC of dark chocolates than milk chocolates. It is supported by their higher SMP than milk chocolates. Non CB chocolates have higher SFC or hardness tha n dark and milk chocolates. Since the SMP of non CB chocolate were above oral temperature, range from 37.3 to 41.40C, it will give a waxy feel sensation after eating chocolate.
CURRICULLUM VITAE
The author was born in Medan, North Sumatra on March 29th,1987. She is the eldest child from Mr. Hendri and Mrs. Fifi in her family with one sister and one brother. She had her kindergarten and elementary sch ool in Sutomo 2, Medan (1990-1998). In 1998, she and her family moved to Jakarta and she continued her elementary, junior high scho ol and senior high school in SDK Penabur Modernland (1998-1999), SLTPK Penabur Modernland (1999 -2002) and SMAK Penabur Gading Serpong, Tangerang (2002 -2005).
In 2005, the author continued her further study in IPB with SPMB (Seleksi Penerimaan Mahasiswa Baru) way and followed the TPB (Tingkat Persiapan Bersama) for one year. Thus, she was accepted as a food science and technology student in the faculty of agriculture technology, IPB in 2006.
ACKNOWLEDGMENTS
First, I would like to thank God for His blessings, guidance and protection upon me throughout the whole research work and manuscript preparation.
In this opportunity, I would also like to thank Dr. Ir. Purwiyatno Hariyadi, MSc. and Ir. Soenar Soekopitojo, MSi., my advisors of this manuscript work, who have guided me throughout my manuscript with their patience and knowledge, for their ideas and inputs that played a big part in the completion of this manuscript.
I am as ever, especially indebted to my family, big thanks for my lovely Mom, Dad, Nini and Awi, who have always support me, for their love and their understanding in my life.
I would also like to give a special thanks to best friends, Vidy, Nene Vero, Mpin, Bobo, Edo JH, Willy, Iboy, Nene Pauline, Vera, Marsella, and Gacci, for the sharing stories, endless support, and unforgettable experiences together especially in these four years.
I am grateful for the kopelkhu friends, Sarah, Dina, Efrat, Dion, Ester, Twi, Meiyu, Bang Benardo, Bang Agus, Bang Maryo, and others that have taught me much about life value and given me a solid community, which can grow together and solve problems together.
Lots of thank is also addressed to all my friends in IPB, Ella, Cath, Chacha, Beli, Eping, , Wiwi, Tata, Suhendri, Sisi, Irene Susan, Midun, Galih, Kamlit, an d others, which I cannot mention them one by one for the wonderful memories for the last four years and their supports during my study at IPB
LIST OF CONTENTS
Page
ACKNOWLEDGEMENTS……….i
LIST OF CONTENTS………. ii
LIST OF TABLES………..……. iv
LIST OF FIGURES……….……… v
LIST OF APPENDIXES………. vi
I. INTRODUCTION A. BACKGROUND………...1
B. OBJECTIVES...………... 2
II. LITERATURE REVIEW A. CHOCOLATE....………... 3
B. COCOA BUTTER………... 5
C. COCOA BUTTER ALTERNAT IVES....………... 9
1. Cocoa Butter Equivalent (CBE) ... ...10
2. Cocoa Butter Replacer (CBR) ... ... 14
3. Cocoa Butter Substitute (CBS)... 15
D. DETECTION OF COCOA BUTTER EQUIVALENT………… ……....16
III. MATERIALS AND METHODS A. MATERIALS AND INSTRUMENTS.……… …..….19
B. METHODS 1. Sampling Method... ... 19
2. Total Fat Content... ... 20
3. Cocoa Butter-like Fat Extraction... ... 21
4. Cocoa Butter-like Fat Profil Analyses a. Slip Melting Point Determination ... 21
b.Triacylglycerol Composition…... 22
c. Solid Fat Content (SFC) ... ...22
5. Detection of Cocoa Butter Equivalent... ... 22
IV. RESULTS AND DISCUSSION A. PRODUCT DESCRIPTION………..…………..24
C. TRIACYLGLYCEROL COMPOSITION .……….31 D. SOLID FAT CONTENT ……….39
E. SLIP MELTING POINT ……… ……….41
LIST OF TABLES
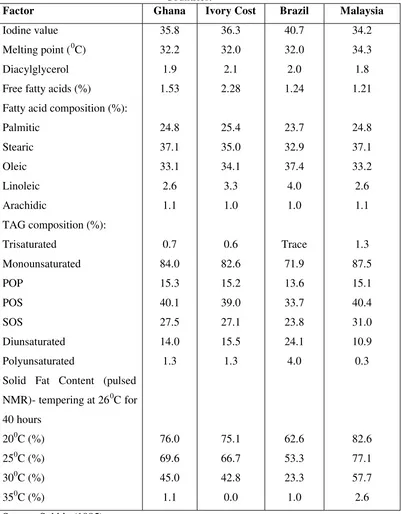
Table 1. Characteristic and Composition of C B from Countries………..7
Table 2. Solid Fat Content (SFC) of Cocoa Butter………...…8
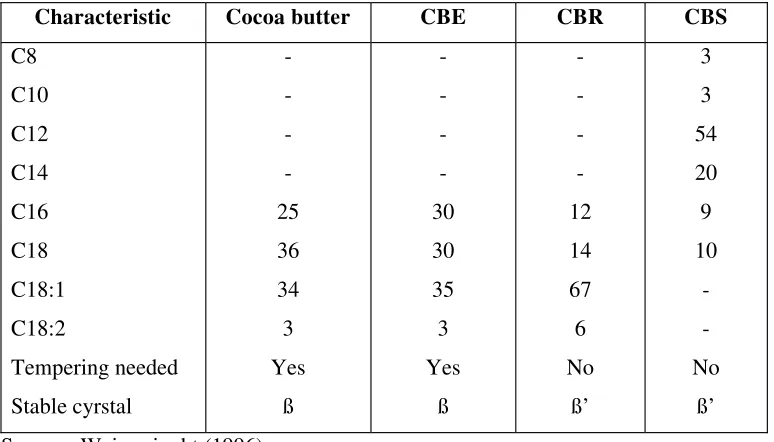
Table 3. Characteristic of CB and CBAs………...10
Tabel 4. Fatty Acid and Triacylglycerol Composition of Exotic Fats…………..12
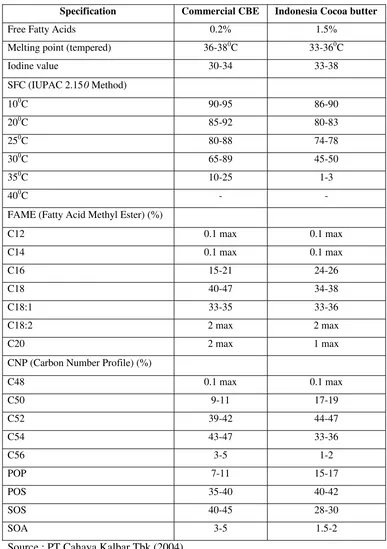
Table 5. Chemical and Physical Properties of Commercial CBE and Indonesia Cocoa Butter………..… 13
Table 6. Triacylglycerol Composition of Some Commercial CBR s……….15
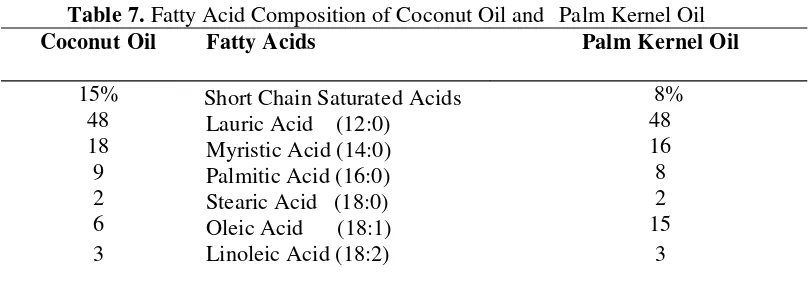
Table 7. Fatty Acid Composition of Coconut Oil and Palm Kernel Oil…….…..16
Table 8. Chocolate Bar Products Label Information……….25
Table 9. Retention Time of Triacylglycerol Standard……….. 35
Table 10. Triacylglycerol Composition of non CB Chocolates…………...38
Table 11. Slip Melting Point Profile of Chocolate Bar Products…………...42
Table 12. Triacylglycerol Fractions and SMP of Chocolate Products..…………43
Table 13. The TAGs Fractions and SMP o f non-CB Chocolates……….47
LIST OF FIGURES
Figure 1. Cocoa butter SFC Curve Depending on Temperature………... 8
Figure 2. The Total Fat Content of The Chocolate Bar Products…………...27
Figure 3. The Cocoa Butter-like Fat Content of Chocolate Products……...29
Figure 4. Cocoa Butter-like Fat Content in Fat Based………...29
Figure 5. The Chromatogram of Cocoa Butter………..31
Figure 6. The Chromatogram of Dark Chocolate, Chocolate A………...31
Figure 7. The Chromatogram of Milk Chocolate, Chocolate C……….…...32
Figure 8. The chromatogram of White Chocolate, Chocolate D……….…..32
Figure 9. The Chromatogram of Non-CB Chocolate……….……...…33
Figure 10. The % Area of TAGs: POP, POS, and SOS of CB -chocolates……...36
Figure 11. The % StUSt of CB-chocolates………... 37
Figure 12. The % StStSt, StU2, and U3 of CB Chocolates…..……….…37
Figure 13. The SFC Curve of Chocolate Products……….……...40
Figure 14. The %StUSt and Slip Melting Point of CB Chocolates………..……. 44
Figure 15. The %StStSt and Slip Melting Point of CB Chocolates………...… 44
LIST OF APPENDIXES
APPENDIX I Total Fat Content of Chocolate Samples…………...59 APPENDIX II Statistical Analysis of Total Fat Content Data…… ………….60 APPENDIX III Cocoa Butter-like Fat Content in Chocolate Samples……….62 APPENDIX IV Statistical Analysis of Cocoa Butter Content in
ChocolateSamples……… ....…...63 APPENDIX V Statistical Analysis of Cocoa Butter Content in
Total Fat………...65 APPENDIX VI Slip Melting Point of Chocolate Samples……….…… ..…….67 APPENDIX VII % Solid Fat Content Data of Chocolates in
Different Temperature……… ...…..……70 APPENDIX VIII Detection and Quantification of Cocoa Butter
Equivalent………...… …….…71
APPENDIX IX Triacylglycerol Composition (% area) of the
Cocoa Butter-like Fat from Chocolate Product….…….….…72 APPENDIX X Chromatogram of Triacylglycerol Standard…….……..…….73 APPENDIX XI Slip Melting Point and Degree of Unsaturated
I. INTRODUCTION
A. BACKGROUND
Chocolate is the generic name for the homogenous products that obtained by an adequate manufacturing process from cocoa materials which may be combined with milk products, sugars and/or sweeteners, and other additives (CODEX STAN 87-1981). There are many varieties of chocolate products in the market such as chocolate bar, baking chocolate, chocolate stick, chocolate dipping, chocolate rice, and others (Trianawati, 1996). According to Jeffery (1991), fat is the compound that holds the whole ingredients together and it is the properties of its solid phase which determines the physical, rheological, and acceptance properties of the chocolate.
The uniqueness of chocolate is due to the cocoa butter, the important component in chocolate, which has char acteristic of solid in room temperature but melt quickly at temperature of mouth (Muchtadi, 1997). Cocoa butter is the most expensive constituent of the chocolate products. It is composed predominantly of (>75%) symmetrical triglycerides with oleic acid in the 2-position. Approximately 20% of tri acylglycerols are liquid at room temperature and cocoa butter has a melting range of 32 to 35°C and softens at around 30 to 32°C. This is essential to the functionality of cocoa butter in its applications (Shukla, 2006). Cocoa butter consists of mainly 2-oleyl-1-palmitoyl-3-stearoylglycerol (POS), 2-oleyl - 1,3 –distearoylglycerol (SOS), and 2-oleyl-1,3–dipalmitoylglycerol (POP) that have big role in determining the characteristic and slip melting point of chocolate ( Torey, 1983).
In general, CBAs were divided into three main following groups based upon their physical and chemical characteristics and their compatibility with cocoa butter: cocoa butter equivalent ( CBE), cocoa butter substitutes (CBS), and cocoa butter replacer (CBR) (Lipp dan Anklam, 1998). A proper CBA mixture fats of chocolate is a key determinant in a successful chocolate product which is largely responsible for general consumer satisfaction of t he chocolate (Hanneman, 2000).
The using of CBA as a cocoa butter -like fat has been regulated by European Economic Community (E EC). The addition up to 5 % in a chocolate formulation or up to 15% in a fat phase for product labeled chocolate (Soon, 1991). Fats that allowed for chocolate are palm, Illipe, Sal, Shea, Kokum gorgi, and Mango kernel oil. However, there is no such regulation about CBE addition in chocolate that has been stated in Indonesia.
The prospect of chocolate confectionery market in Indones ia is encouraging. The market of chocolates is wide open with relatively few producers. In the past five years exports have increased 23.16% annually on the average. In 2007, exports were valued at US$ 13.54 million or around Rp121.8 billion. (Anonim, 2006 ). The absence of regulation about CBA addition has caused to the variety of chocolate confectionery products quality in Indonesia. The cocoa butter-like fat or CBA profile in chocolates needs to be analyzed to know the variety of chocolate bar quality in market. Cocoa butter profile can be identified by analyzing the triacylglycerol profile, solid fat content, and the slip melting point.
B. OBJECTIVES
The objectives of this research are :
1. To identify the triacylglycerol profile, determine the solid fat content, and slip melting point of cocoa butter or cocoa butter like -fat from chocolate bar products in Indonesian market
II. LITERATURE REVIEW
A. CHOCOLATE
Cacao trees (Theobroma cacao) is originated in the Americas and have been cultivated for at least an estimated 4,000 years where they thrive under conditions of semishade, warmth, and high humidity (Tanabe and Hofgerber, 2006). Cocoa beans are the seeds of the fruit of Theobroma cacao L. Part of cacao fruit used as food is cocoa bean, which covered by placenta when it was harvested. The placenta is used as fermentation medium for microbial growth. In the fermentation and drying process, the cocoa shell is removed and results in nibs. The nib is the main material for chocolate production (Minifie, 1999).
The terminology of cacao is defined for ra w material related to fruit or cocoa bean or trees. Cocoa is name that preferable used in trading, especially in chocolate processing used for produce chocolate drink, for example cocoa powder. Cocoa powders (cocoas) are defined in the Codex Standard 105 -1981 as products obtained by mechanical transformation into powder of cocoa press cake produced by partial removal of the fat from cocoa nibs or cocoa by mechanical means. Cocoa butter is also produced during this operation and is defined in the Codex Stand ard 86-1981. Chocolate is defined in the Codex Standard 87-1981 as the homogeneous product obtained by an adequate process of manufacture from a mixture of one or more of the following: cocoa nibs, cocoa mass, cocoa press cake, cocoa powder including fat -reduced powder, with or without permitted optional ingredients and/or flavoring agents (Codex Alimentarius, 1981).
Chocolate and chocolate typed products are varies in market such as chocolate bar, baking chocolate, chocolate stick, chocolate dipping, chocolate rice, and others. Chocolate bar is chocolate product that formed in a bar and directly consumed. Baking chocolate usually used for bakery products or melted and used as cake decoration. Chocolate dipping is used for ice cream coating and in liquid form. Chocolate coating is used for extr usion coating products, cake, and bakery. Chocolate chip is round and used for cookies making, and chocolate stick usually used for filling in sweet bread (Trianawati, 1996).
According to Beckett (1994), the basic ingredients for chocolate manufacturing are similar generally, including chocolate liquor, sugar, cocoa butter, milk products (for milk chocolate), emulsifiers, and flavors, which are blended together. The result is a paste with a rough texture and plastic consistency. Dark, milk, and white chocol ates involve certain basic operations: ingredient mixing, refining, conching, standardization of viscosity, and tempering.
The ingredient mixing is needed to produce a blended mixture. Refining serves to reduce the particle size of the mass, and thus incre ase the surface area, resulting in a smooth texture. The actual size will depend upon the product desired and type of chocolate, with dark chocolate generally having a smaller finished particle size. After refining, the mass is transferred to large shear mixers called conches. This is the last manufacturing process where texture and flavor are affected (Minifie, 1999). Some of the benefits of conching are the improvement of the rheology/reduction in viscosity - less cocoa butter needed, elimination of harsh volatiles for a mellower taste, removal of moisture (reduce lumping and graining), and improved mouth feel (smoothes sharp particle edges) (Tanabe and Hofgerber, 2006). However, conching tied and covered the chocolate particles, sugar, and milk by fat laye r so it can gives smooth feel in mouth (Mulatoet. al., 2005).
which its viscosity (internal friction of fluids) is affected by the presence of solids in suspension, as well as by temperature. Viscosity and rheology can be affected by several factors. Smaller particle size in a constant formula will give a higher viscosity.
Tempering is the controlled cooling melted chocolate with agitation that will promote the formation of small stable fat crystals throughout the chocolate. Besides agitation, time and temperature play an impor tant role in the tempering process. Tempering can be done manually or in an automatic tempering unit. In general, It involves heating process of the chocolate to approximately 110 to115°F (43–46°C) to melt the fat crystals. It is then cooled down with agitation to between 80 to 84°F (27–29°C), and subsequently reheated to about 86 to 88°F (30–31°C) before moulding or coating. In general, dark chocolates are tempered about 1 to 2°F (0.5–1.0°C) higher than milk chocolate (11). However, exact temperatures and procedures will depend upon the tempering equipment and type of chocolate used. Tempering process will result in stable cocoa butter crystals which provide snap, good gloss, proper texture, bloom resistance, contraction for demoulding, and less permeable barrier (Tanabe and Hofgerber, 2006).
B. COCOA BUTTER
when eaten, it let completely in mouth with s oft and creamy texture, also gives a cooling sensation and there is no waxy feel sensation after eating (Gunstone, 2002).
CB is composed of 98-99% simple lipids and 1 -2% complex lipids. The simple lipid fraction comprises 96.0% triacylglycerols (TAGs), 2 .2% diacylglycerols (DAGs), 0.2% monoacylglycerols (MAGs), 0.6% free fatty acids (FFAs), and 0.9% other simple lipids (D‘ Alonzo et al., 1982). Chemical and physical properties of CB depend on its composition and distribution of TAGs and the FFAs. Unsatura ted fatty acids in TAGs mainly consist of oleic acid (83%) which in position sn -2.
The physical properties of cocoa butter is caused by the composition of TAGs, which is 70-80%, dominated by three symmetric triacylglycerols, saturated-unsaturated-saturated (StUSt), which are palmitic -oleic-stearic (POS, 36-42%), stearic- oleic-stearic (SOS, 23- 29%), palmitic-oleic-palmitic (POP, 13-19%) and with trace amounts of asymmetrical tri acylglycerols (PPO, PSO, and SSO) (Wainwright, 1996). The unique triacylglycerols composition together with the extremely low level of di acylglycerols gives cocoa butter its desirable physical properties and ability to recrystallize during processing in a stable crystal modifica tion. The complexity of the crystallization of cocoa butter is because triacylglycerols can crystallize in a number of different crystal modifications, depend s on triacylglycerols composition and on crystallizing and tempering conditions during manufacturing and storage (Shukla, 1995). In addition, the triacylglycerol composition is affected by the origin of the cocoa beans. The fatty acid composition, various analytical constants, and triacylglycerol composition of different cocoa butters are given in Table 1.
tempering stage is necessary to ensure that all the CB crystallises in form V, thereby making the product stable and giving it a high level of gloss and snap.
Table 1. Characteristic and Composition of Cocoa Butter from Different Countries.
Factor Ghana Ivory Cost Brazil Malaysia
Iodine value
The percentage of solid fat in a typical CB at different temperatures is shown in Table 2. The level of liquid fat present in a product is significant , not only in determining the sensory (particularly textural) quality but also in influencing the shelf-life of chocolate products. The fast-melting characteristic of CB between 30 ºC and 35ºC is responsible for fast meltdown of chocolate in the mouth. A high solid fat content at body temperature would be perceived as an unpleasant waxy mouthfeel (Subramaniam, 2000).
Table 2. Solid Fat Content (SFC) of Cocoa Butter Temperature (0C) Solid Fat (%)
20 75.5
25 70.5
30 41.0
32.5 15.0
35 0.6
Source : Soon (1991)
Cocoa butter SFC values calculated at temperatures below 250C characterize its hardness, while the values calculated at temperatures between 25 and 300C indicate the resistance of cocoa butter to heating. In the range of 27 to 330C, intensive melting of cocoa butter occurs, bring s about the cooling sensation in the mouth and flavour release (Torbica, et. al., 200 6).
C. COCOA BUTTER ALTERNATIVES
Traditionally, cocoa butter is the only main source of vegetable fat that has been used for chocolate product. Cocoa butter that determine and provide the characteristic of chocolate , such as crunchiness at room temperature, sharp melting point, long shelf-life, high gloss, and good flavor. Nevertheless, as a natural fat cocoa butter also has some drawbacks:
CB is a relatively costly raw material and ha s fluctuating price
A tempering process is needed to take into consideration the polymorphism of fats.
The melting attributes of cocoa butter may be inadequate for usage for certain purposes and climate conditions
The variability of its quality depend on its origin (cocoa bean seed
varieties).
A blooming tendency Limited gloss retention
For these reasons replacing part of it with alternative fats in chocolate is a great interest for economical reasons. Many researches have been developed to create a confectionery fat/ specialty fat that can replace cocoa butter or substitute part of it, which are often called cocoa butter alternatives (CBAs) (Fuji Oil Europe, 2004).
The CBAs can be divided into the following three main groups based upon their characteristic and the raw materials used to produce them:
2. Cocoa butter replacers (CBRs). These are fats that are partly compatible with cocoa butter. CBRs have the same fatty acids distribution to cocoa butter but different in triglycerides structure
3. Cocoa butter equivalents (CBEs) or extenders. These are fats that are fully compatible with cocoa butter (chemical and physical properties similar to those of cocoa butter) (Lipp dan Anklam, 1998). The cocoa butter and CBA’sdifference characteristic are listed in Table 3.
Table 3.The Characteristic of Cocoa Butter and Cocoa Butter Alternatives
Characteristic Cocoa butter CBE CBR CBS
C8
1. Cocoa Butter Equivalents (CBEs)
resultant vegetable fat will behave as 100% cocoa butter equivalents (Shukhla, 2006).
Although CBEs are not produced by mixing individual triacylglycerols, as they are very expensive to produce, this is the logic behind the whole procedure of producing CBEs. CBEs can be made by blending, fractionation, and interesterification (PORIM, 1997). Careful preparation and blending of these materials result in a tailor -made fat equivalent to cocoa butter in physical properties. Therefore, these fats are called CBEs
Europe Economy Community (EEC) defines CBEs as (Minifie, 1999) : 1. The StOSt triacylglycerols > 65 % (St =Saturated, O =Ole ic)
2. Triacylglycerol with sn-2 position contain unsaturated fatty acids > 85%. 3. Total unsaturated fatty acids content < 45 %.
4. Di–or tri unsaturated fatty acids < 5 %. 5. Lauric acids content < 1 %.
6. Trans fatty acids < 2 %.
Raw materials that can be used for CBEs production are material that rich in POP, POS, and SOS, to increase the saturated unsaturated saturated (StUSt) content in product such as Illipe butt er ( 86% StUSt), shea nut oil (39 wt%), sal fat (56 wt%), and palm oil (38 wt%). The fatty acids and triacylglycerols composition of exotic fats is given in Table 4. Palm oil is fractionated to produce middle -melting fraction rich in POP; and exotic fats, such as shea, sal, and illipe (Borneo tallow), are fractionated to get triacylglycerols cuts rich in POS and SOS.
cocoa butter will have higher price. It is because of more cost should be used to get the pure simetric triglycerides fraction for CBEs production.
The higher POP content in CBEs will result in longer tempering time process needed and the crystallization product will soften. CBEs are usually softer than cocoa butter but cocoa butter is less tolerance with milk fat (Wainwright, 1999). Table 5. provides the chemical and physical properties of one commercial CBE and Indonesia n cocoa butter.
Tabel 4.Fatty Acid and Triacylglycerol Composition of Exotic Fats
No Fatty acids / TAGs (%) Cocoa butter Palm Illipe Shea
1 Palmitic (P) 25 45 16 4
2 Stearic (S) 36 5 46 43
3 Oleic (O) 34 38 35 45
4 Linoleic (L) 2 10 - 7
5 Arachidic (Ar) 1 - 2
-6 PPP - 5 -
-7 POS 39 3 35 5
8 SOS 26 - 45 46
9 POP 16 26 7
-10 SOAr 2 - 4 2
11 SLP 4 2 -
-12 PLP 2 7 -
-13 SLP 1 2 -
-14 PPO - 5 -
-15 SOO 4 3 3 27
16 POO 4 19 - 2
17 OOO - 3 - 5
Table 5. Chemical and Physical Properties of Commercial CBE and Indonesia Cocoa Butter
Specification Commercial CBE Indonesia Cocoa butter
Free Fatty Acids 0.2% 1.5%
Melting point (tempered) 36-380C 33-360C
Iodine value 30-34 33-38
SFC (IUPAC 2.150Method)
100C 90-95 86-90
200C 85-92 80-83
250C 80-88 74-78
300C 65-89 45-50
350C 10-25 1-3
400C -
-FAME (Fatty Acid Methyl Ester) (%)
C12 0.1 max 0.1 max
C14 0.1 max 0.1 max
C16 15-21 24-26
C18 40-47 34-38
C18:1 33-35 33-36
C18:2 2 max 2 max
C20 2 max 1 max
CNP (Carbon Number Profile) (%)
C48 0.1 max 0.1 max
C50 9-11 17-19
C52 39-42 44-47
C54 43-47 33-36
C56 3-5 1-2
POP 7-11 15-17
POS 35-40 40-42
SOS 40-45 28-30
SOA 3-5 1.5-2
2. Cocoa Butter Replacers (CBRs)
CBRs are produced from partial hydrogenated non -lauric fats, consist of fractions hydrogenated oils: soybean, cotton, corn, peanut, safflower, and sunflower oils. CBRs are mainly used in enrobed and coated biscuits and fillings. The improved heat stability and bloom stability achieved by CBRs have proved to be particularly useful in warmer climates.
CBRs has the same fatty acid distribution to cocoa butter but different triacylglycerols structure. CBR s only compatible with small ratio to cocoa butter (Lipp dan Anklam, 1998). The hydrogenation parameters can be manipulated (increase the reaction temperature, reduce the hydrogen pressure, inactivate part of reaction or use sulfure catalyst promotor) in maximizing the trans-octodecanoic formation to reach the desired melting profile in product. However, CBRs melting curve have an inferior tendency to cocoa butter and have lower eating quality. CBRs are suitable for bakery products which the texture and mass of substrate dominates the taste of products. CBRs’ quality can be improved with fractination by removing the undesired triacylglycerol component. Consequently, the desired melting profile and the eating quality can be impr oved. CBRs using has some advantages according to Wainwrig ht (1999):
-Tempering is not needed because CBRs can crystallize spontaneously in ß‘ polymorph form
-Have a good gloss and high gloss retention -Compatible with other non -lauric fats
Table 6.Triacylglycerols Composition of Some Commercial CBRs
CBR Triacylglycerol
POP SOP SOS SOA
A 58.1 14.8 26.8 0.0
B 73.8 9.0 17.2 0.0
C 52.3 18.3 26.2 0.0
D 24.7 26.7 41.2 7.3
Source : Nikolova-Damyanova dan Amidzhir (1992)
3. Cocoa Butter Substitutes (CBSs)
CBSs are produced from lauric fats, which are obtained from various species of palm-tree, the main varieties being palm that produces palm-kernel oil and coconut. The excellent melting properties and quick crystallization make CBS suitable for moulded products and where a thin coating is required. These fats differ from nonlaurics in that they contain 47 to 48% l auric acid, together with smaller amounts of other medium - and short-chain fatty acids. CBSs are made by hydrogenation, interesterification, and fractination (Wainwright, 1999).
CBSs have totally different chemical composition but have the same physical properties with cocoa butter. Consequently, CBSs only can be used for substituting 100 % cocoa butter (Lipp dan Anklam, 1998). The principal advantages and disadvantages of the lauric CBSs are as follows: good oxidative stability; long shelf life, excellent eating quality and flavor release; no waxy aftertaste, texture very similar to that of cocoa butter (i.e., excellent hardness and snap and not greasy to the touch), solidify quickly tempered or untempered, excellent gloss and gloss retention, and availabl e at a cost far less than cocoa butter.
and fat splitting enzymes (lipase), there is a danger of fat hydrolysis and the liberated lauric acid has a distinct soapy flavor, which can be detected even at low concentrations. Besides, CBSs relatively have a low tolerance to milk fat.
Table 7.Fatty Acid Composition of Coconut Oil and Palm Kernel Oil
Coconut Oil Fatty Acids Palm Kernel Oil
15% Source : Goh and Berhad (2002)
D. DETECTION OF COCOA BUTTER EQUIVALENT
Former Council Directive 73/241/ EEC concerning cocoa and chocolate products did not contain any legislative requirements concerning type and level of addition to chocolate of vegetable fats other than cocoa butter. In the EU Member States, various nationa l regulations existed, permitting addition of 5-10% other fats in the production of chocolate (in Austria, Denmark, Finland, Ireland, Portugal, Sweden, and The United Kingdom). Different regulations caused problems in the trade of chocolate and chocolate product which led to the unification of legislation applicable to that alimentary commodity. That was why Directive 2000/36/EC (so -called Chocolate Directive) was issued, laying down the maximum permissible content of 5% CBEs in the final product and forbid ding any addition of CBRs and CBSs (Bohacenko et.al., 2005).
including their critical evaluation, is given in reviews (Lipp and Anklam 1998b; Ulberth and Buchgraber, 2003)
From the review, it follows that there are numerous methods available for the purpose, based particularly on gas chromatography (GC) and high -performance liquid chromatography (HPLC). As to the analysed substances in question, special attention is paid to fatty acids (F As), triacylglycerols (TAGs), and minor fat constituents (sterols, sterol degradation product and terpens). In HPLC and GC non polar columns, The TAG fractions separation was studied by Barcarolo and Anklam (2001) according to their acyl-C-number, i. e. the total number of carbon atoms in the FA chain. Three major TAG fractions, identified as C50, C52, and C54, were found in CBs and CBEs. Moreover, the TAGs separation using medium polar capillary columns coated with phenyl -methyl silicone stationery phase are able to separate TAGs based on their acyl -C-number and the number of double bonds in the molecule (Geeraert and Sandra, 1987).
The issue becomes more complex since the analytical data obtained are to be interpreted and t he determination based on the TAG composition profile, independent on CBEs having been added or not to chocolate. An equation has been developed to calculate the demonstrate CBEs presence by Fincke (1980) and Padley and Timms (1980) in Bohacenko et al. (2005). The equation is proven by the fact that supported by the experimental results, that the contents of C50 and C54 found in genuine cocoa butter samples will show a linear relationship, after the contents of three major TAG fractions have benn normalised (C50 + C52 + C54 = 100%). This can be expressed by the following equations:
% C50 = 43.8 - 0.737 x % C54 (Padley and Timms) % C50 = 44.9–0.768 x %C54 (Fincke)
Despite of the error determination of CBEs addition into account, Padley and Timms (1980) proposed the following relationship for the qualitative detection of CBE presence in chocolate:
III. MATERIALS AND METHOD
A. MATERIALS AND INSTRUMENTS
Samples were chocolate bar product s purchased from commercial store in Bogor area. Cocoa butter were obtained from PT Karya Putrakreasi Nusantara, Wilmar Group, Medan, Indonesia. Materials used for analyses are acetone PA, acetonitril PA form Merck, aquades, and hexane, which are obtained from CV Fisconina, Bogor. SFC standard 0%, 31.5%, dan 72.9% were obtained from Bruker Minispec, Rheinstetten, Germany.
Instruments that have been used for this research are High Performance Liquid Chromatography (HPLC Hewlett Packard series 1100) equipped with a refractive index (RI) detector; A Zorbax Eclipse XDB C -18 (250 x 4.6 mm, Agilent Technologies Inc., USA) column in series with Microsorb MV (250 x 4.6 mm, Rainin Instrument Co. Inc., USA) column was used for the analysis of TAG , capillary tube, Bruker Minispec PC 100 Nuclear Magnetic Resonance Analyzer (Rheinstetten, Germany), Soxhlet extractor, heater, refrigerator, thermometer, magnetic stirrer, NMR tube, filter paper, and glasswares.
B. METHODS
1. Sampling method
As the matter of fact that this research objectives are to characterize the quality of cocoa butter from chocolate product in market, writer chose samples which are well -known already in Indonesia. Assuming to well -known chocolate bar products are distributed well and wholy same in big department store s, so writer chose to make a small survey of chocolate products in big retail stores such as Hypermart, Giant, and Carefour.
chocolate bar products which will be divided into three class es based upon their prices:
1. Low price chocolate bar : < Rp. 10.000 / 100 g 2. Middle-price chocolate bar : Rp 10.000–Rp. 20.000 / 100 g 3. High-price chocolate bar : > Rp. 20.000 / 100 g And their types:
1. Cocoa Butter (CB) Dark chocolate 2. CB Milk chocolate
3. Non-cocoa butter(CB) chocolate
A quota sampling method is applied for this research. Quota sampling is the non probability equivalent of stratified sampling. Like stratified sampling, the researcher first identifies the stratums and their proportions as they are represented in the population. Then convenience sampling is used to select the required number of subjects from each stratum. 13 samples have been chosen to represent the quality of cocoa butter from chocolate bar product in market.
2. Total Fat Content (AOAC, 1995)
Total fat content was analyzed by Soxhlet method (AOAC, 1995). All the glassware (round bottom flask) was rinsed with hexane and dried in an oven at 102ºC for 30 min and cooled in a desiccator. Accurately 1-2 g of sample was weighed and covered with filtered paper. Sample was put into soxhlet extractor and condenser was set above the round bottom flask. Hexane solvent was poured into round bottom flask adequately. Sample and solvent were heated or refluxed above 5 hours or until solvent have dropped clearly to the flask. Sample was taken out of extractor then the solvent was destilated until there was almost no solvent in flask. The round flask with extracted oil was stored in oven at 105oC, cooled to desiccator and weighed until it has constant weight.
X = Weigh of empty flask and extracted fat Y = Weigh of empty flask
W = Weigh of sample
3. Cocoa Butter or Cocoa Butter -Like Fat Extraction
Before triacylglycerol profile, solid fat content , and slip melting point were analyzed, cocoa butter or cocoa butter -like fat from chocolate bar pruducts should be extracted. The cocoa butter like -fat extraction is done for removing traces or other compounds in chocolate that can be a barrier for cocoa b utter analyses.
Cocoa butter-like fat extraction method was modified from isolation of cocoa butter like -fat procedures according to Abigor et. al. (2003). Acetone (7 ml/ g substrate) was added to the fat mixture. Sample in acetone was heated and mixed thoroughly until become a chocolate solution. The warm acetone solution was quickly filtered through filter paper to remove the undesired compounds. The filtrate was cooled to room temperature (±220C), and any precipitated solids were removed by filtratio n. The filtrate then cooled to 40C for 4 hour and the precipitated TAG crystals filtered at 40C to give the cocoa butter-like fat.
4. Cocoa Butter-Like Fat Profile Analysis
a. Slip Melting Point Determination (AOCS Cc 3 -25, 2003)
the capillary tube when about 5% solid fat is present. The SMP was measured by the average of triplo samples.
b. Triacylglycerol Composition (AOCS, Ce 5c -93, 1997)
The triacylglycerol composition of cocoa butter-like fat were obtained by HPLC in a Hewlett Packard Series 1100 HPLC System equipped with a refractive index (RI) detector. A Zorbax Eclipse XDB C-18 (250 x 4.6 mm, Agilent Technologies Inc., USA) column in series with Microsorb MV (250 x 4.6 mm, Ra inin Instrument Co. Inc., USA) column was used for the analysis with a mobile phase of 85 : 15 (v/v) acetone and acetonitrile at a flow rate of 0.8 mL/min. Each sample was dissolved in acetone to make 5 % concentration. The injection volume was 20 µL. All TAG contents were given in percentage area.
c. Solid Fat Content (SFC) (IUPAC 2.150 ex 2.323, 1987)
The solid fat contents of the samples were measured using a Bruker Minispec PC 100 NMR Analyzer (Rheinstetten, Germany). The procedure was applied accord ing to the IUPAC 2.150 method (1987). Before analyzing, NMR were calibrated using SFC standard 0%, 31.5%, and 72.9%. The samples were filled into NMR tubes and melted at 800C, held at 600C for 30 min using heater. The samples then were cooled to 00C for 90 min After cooling, the samples were kept at 260C for 40 hours, and then cooled at 00C for 90 min. The samples were incubated for 35 minutes at each temperature (10, 20, 25, 30, 35, and 400C) before measuring the liquid signal.
5. Detection of Cocoa Butter Equivalent
and C54 in genuine CB. The qualitative dete rmination were based on the compliance with the relationship proposed by Padley and Timms (1980):
% C50 < 44.095–0.737 x %C54
IV. RESULTS AND DISCUSSIONS
A. PRODUCT DESCRIPTION
Ever-increasing demand of chocolate and chocolate type products in the world also influence the increasing consumption of chocolate product s in Indonesia. Indonesia have produced 96.000 tons of cocoa processing products and 7.000 tons confectionery products each year. Internatio nal market is also still wide open for Indonesian products of chocolates. Indonesian producers have exported chocolates to more than 100 countries. In the past five years exports have increased 23.16% annually on the average. In 2007, exports were valued at US$ 13.54 million or around Rp121.8 billion (Anonim, 2008).
The uncertainty supply of cocoa butter and its fluctuating price have forced the confectioners to seek alternatives (Shukla, 2006). The present of CBA have influenced to the chocolate manufatur ers in Indonesia. Since there is no regulation about CBA addition to chocolate products, the potential of CBA using has increased and thus lead to the variety quality and prices of chocolate products in Indonesia. Chocolate bar products in Indonesia are varies in quality and prices. 13 chocolate bar products have been chosen by quota sampling to represent the variability quality of chocolate bar products in market.
Table 8.Chocolate Bar Products Label Information
No Chocolate Chocolate Type Cocoa Mass Cocoa butter Vegetable fat Milk powder Others Price (IDR) / 100 g Reg #
1 A dark chocolate + + - - maltitol, soya lecithin,
vanilla.
40.000–45.000 ML
2 B dark chocolate + + - + sugar, vanilli, soy lecithin 35.000–40.000 ML
3 C milk chocolate + + - + Sugar, hazelnut paste, soy
lecithin, natural flavor
19.000–20.000 ML
4 D white chocolate - + - + Sugar, honey (3%), almond
nut, soy lecithin, albumin, vanilli flavor
18.000–19.000 ML
5 E milk chocolate + - + + Sugar, malt, whey powder,
palm oil, soy lecithin, mineral, vitamin.
15.000–17.000 ML
6 F milk chocolate + + + + Sugar, soy lecithin flavor 13.000–14. 000 ML
7 G milk chocolate + + - + sugar 12.000–13.000 MD
8 H white chocolate - + + + Sugar, almond nut, soy
lecithin, salt, vanilli flavor
11.000–12.000 MD
9 I dark chocolate + + + + Sugar, almond nut, soy
lecithin, salt, vanilli flavor
10.000–11.000 MD
10 J milk chocolate + - + + Sugar, almond nut, soy
lecithin, vanilli
8.000–9.000 MD
11 K milk chocolate + - + + Sugar, cashew nut,
maltodextrin, whey
6.000–7.000 MD
12 L milk chocolate + - + + starch, whey powder, soy
lecithin, salt, vanilli
3.000–4.000 MD
59 If such CBEs are added during manufacturing of confectionery products, consumers have to be informed about their presence by appropriate labeling According to the Directive 2000/36/EC (“Chocolate Directive”), a number of specified vegetable fats other than CB may be added to a maximum of 5 % of the total weight of the finished product. (Ulberth et. al., 2003). Implisitly, it inform that a product can not be claimed as chocolate if the vegetable fat addition are more than 5% in a product or if there is no cocoa butter contained in a product. However, it has different regulation in Indonesia.
There are two regulations about chocolate products in Indones ia: milk chocolate and chocolate vermicelli. Milk chocolate is defined as a food product that obtained from one of or mixture of (cacao nib, cacao mass, cocoa powder, or cocoa powder without fat) with or without cocoa butter, milk solid, and permitted food additives (SNI 01-4293-1996). Chocolate is defined as a food product in a vermicelli shape obtained from one or mixture of (cacao nib, cacao mass, cocoa powder, or cocoa powder without fat), with or without cocoa butter, and other permitted food additives (SNI-01-4293-1996). In fact, these standards are arranged based on CODEX standard but there is a miss where Indonesian regulation do not mention about the permitted vegetable fat or the maximum addition limit. Hence, it caused the big variability of choco late quality in Indonesia.
The ingredient’sinformation label of the products show s that there are some chocolates contain cocoa butter without any CBA or vegetable fats addition such as Chocolate A, B, C, D, and G. Some chocolates were informed of vegetable fat addition such as Chocolate F, H, and I. Moreover, all of the low-price chocolate , chocolate J, K, L, and M were informed for no cocoa butter contained but vegetable fat as the main fat based ingredient and so-called non cocoa butter (CB) chocolate s. In contrast, chocolate E which included as middle-price chocolate was also informed as non -cocoa butter chocolate in its ingredient label.
B. TOTAL FAT AND COCOA BUTTER CONTENT
mixed in milk choc states that fat acco considerable import processing condition also makes a great d on the eating quali duration in high fat
44.94a
hocolate (Kumara, 2003). Norberg and Karlsham counts for one -third of the content of chocolate portance for the qualit y of the chocolate as it
ions such as tempering and cooling. The type of t deal of difference to the consumer. It has a maj qualities of the final product, including melting be
nd consistency. Finally, the choice of fats in conf l for their shelf life. The total fat content of the
en in Figure 2.
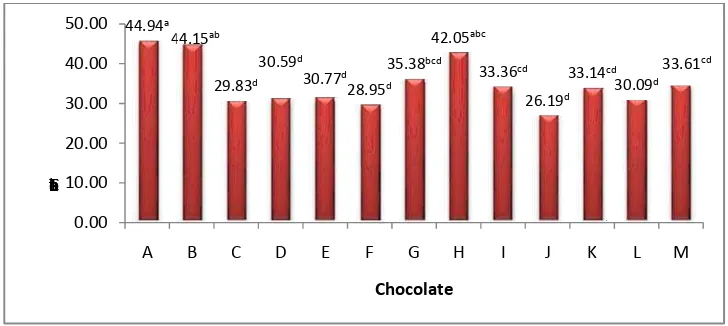
The Total Fat Content of The Chocolate Bar Produc
to US Patent (2001) t he fat content of chocolate to about 40% by weight depending on whether i hocolate but is usually from about 30% to 34% by
l weight of the chocolate. The fat content in c ure influences the degree of crystallinity and cr
) of their corresponding tempered products. Low eted melting at higher temperatures than those wit explain that lower fat chocolates required longe products with higher fat contents, again with a like during consumption (Afoakwa et al., 2008). Lowe
at chocolates can be attributed to reductions in inte
61 interactions and increased free -moving plastic flow, possibly related to yield value of products (Afoakwa et al., in press; Beckett, 2000; Do et al., 2007).
The total fat content table shows t hat Chocolate A, B, and H have high total fat content, 44.94%, 44.15%, and 42.05% respectively. According to the Afoakwa (2008), Chocolate A, B, and H tend to have lower melting duration than others. Chocolate C, D, E, G, I, K, L, and M have total fat content about one third of the content of chocolate. Chocolate F and J h ave lower total fat content than others, which are 28,95% and 26,19% respectively, means that they might have higher melting duration than others. All of the total fat content of chocolates was higher than SNI 01 -4293-1996. According to the SNI, a milk chocolate must have total fat higher than 25%. However, melting properties were affected by many factors such as the cocoa butter type that differentiate the chemical composition and the chocolate processing that affecting to the physical property. Therefore , the high total fat content does not imply the high cocoa butter content in a chocolate.
Cocoa butter (CB) is a major component of the fat phase of chocolate. CB contributes 30 to 40 percent by weight to finished chocolate, and it is responsible for the physical and sensory properties of chocolate. Consequently, cocoa butter or cocoa butter -like fat should be extracted from the chocolate s before they were analyzed. The CB or cocoa butter -like fat is extracted by acetone.
to get the triglyceride chocolates, contain o another important co basis) is used in milk to dark chocolate to
ride crystals. Figure 3 shows the cocoa butter-like fat
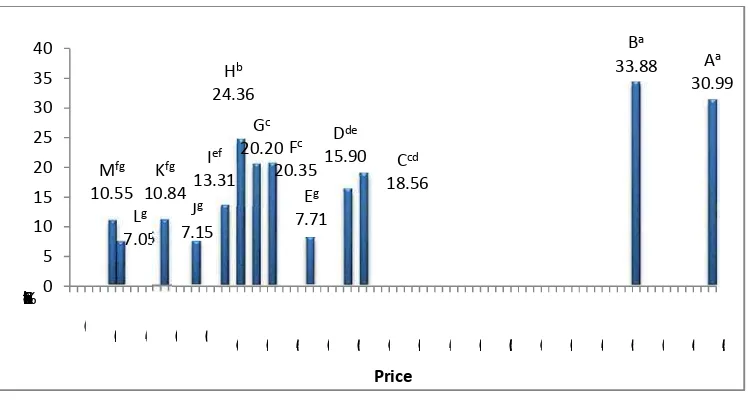
re 3.Cocoa Butter Like Fat Content of Chocolates how the cocoa butter like fat content from Chocol
ilk chocolate, and it is often added at lower levels ( to control hardness (Metin and Hartel, 1998). Gene r content in a product will gives more expensiv
xpensive ingredient in the chocolate is cocoa butter. rity between price and the amount of cocoa butt ng of vegetable fats will increase the cocoa butt does not increase the price significantly. From their
, G, H, I are mentioned as chocolates that have veg products.
than others, which 10.55%. This findi unsaturated saturated amount and other chocolates. These ch vegetable fats and fi chocolate should be contributes more in t content in the fat ba and others. Generally lead to the higher co G, and H have cocoa and M have low coco K, L, and M, which a
h are respectively 7.71%, 7.15%, 10.84%, 7. nding implies that the cocoa butter -like fat or
ted (StUSt) triacylglycerol in these products only r fat or any compounds may have been added chocolates are labelled for not containing cocoa nd filler such a s starch, maltodextrin, or malt. The a
is made to increase the chocolate mass, so reduce ent.
Cocoa Butter Like Fat Content in Total Fat of Chocol ompounds in a chocolate consist of mainly cocoa but ontained in a chocolate such milk fat. Since the fa
be dominated by cocoa butter, so the cocoa butt n the total fat content. Chocolate B has the highest coc
based of chocolate, 76.74%, followed by chocolate lly, it can be sum up that the higher price of a choc cocoa butter content in a c hocolate. Chocolate A, B, ocoa butter content more than 50% but chocolate E,
ocoa butter content in the based fat of product. Choc h are labelled as non -CB based chocolate, seems onl at around 30% of the fat based. Chocolate I which i
ocoa butter content only 39.89%.
C. TRIACYLGLYCER comprising about 2 (C18:1), with mino chocolates were d chocolates. The CB chocolates. The chr shown in Figure 6
butter has a very special triacylglycerol composition al texture and sensory properties. The cocoa but hown by its chromatogram in Figure 5.
Figure 5. The Chromatogram of Cocoa Butter
three dominant triacylglycerols in cocoa butter, P P = palmitic acid, O = oleic acid and S = ste 2002). Cocoa butter has a unique composition, its f
25% palmitic (C16), 36% stearic (C18) and 35% inor amounts of other fatty acids. In this re s
divided into two class: CB chocolates and B chocolates were further divided to dark, milk, hromatogram of Chocolate A which is dark choc 6. This figure i s the representative of dark and H). Also, the chromatogram of chocolate C s shown in Figure 7. that represent the milk c ,and I). The chromatogram of chocolate D that
Chocolate D and G) are shown in Figure 8.
Figure 6.Th
RID1 A, Refractive Index Signal (19-03-09
10 20
RID1 A, Refractive Index Signal (18-03-09
The Chromatogram of Dark Chocolate, Chocolate
The Chromatogram of Milk chocolate, Chocolate
The Chromatogram of White Chocolate, Chocolate
on the chromatograms, it can be characterized imilar triacylglycerol component with cocoa butte three StUSt; POP, POS, and SOS. Milk and white c
F,G, H and I) have the same peaks in their chrom mall peaks before POP peak are seen in the chrom
ies that since the difference between dark chocola
66 and white chocolate is the m ilk fat content, it may concluded that the small peaks are the present of the milk fat which is extracted by the solvent. According to Simoneau and German (1996), the milk fat contained triacylglycerol species ranging from C24 to C54. About 35% of the tota l triacylglycerols were long chain (C> 42). Within these long -chain triacylglycerols, 60% were unsaturated species.
The wide range of triacylglycerols in milk fat results in incomplete miscibility in the solid phase. The solid phase behaviour of milk fat has been explained in terms of three fractions (high -melting, middle-melting, and low-melting) of largely independently -melting solid solutions and is directly related to the molecular composition of milk fat. In this research, the high -melting fraction of milk fat has been included in the triacylglycerol composition of chocolate and the middle -melting fraction of milk fat only partly extracted.
The non-CB based chocolates are milk chocolates. Their extract have been analyzed to their triacylglycerol compos ition and surprisingly, the chromatograms of them were quite different with cocoa butter. The chromatogram of Chocolate J that represent the non -CB chocolates (Chocolate E, J, K, L, and M) is shown in Figure 9.
Figure 9.The Chromatogram of Non-CB Chocolate
Figure 9 shows that the non-CB chocolates have different chemical composition, triacylglycerol components with cocoa butter. Since there are
min
RID1 A, Refractive Index Signal (29 -07-09\FON1.D)
67 three kind of cocoa butter alternatives that in general term applied to confectionery fats, only two kind of CBA, CBR and CBS that have different triacylglycerol component with cocoa butter. CBR, a non lauric fats with a distribution of fatty acids simila r to that of CB but a completely different triacylglycerol structure; due to this different chemical composition they may be added to cocoa butter only in a small amounts. CBS with some physical similarities but chemically totally different from cocoa butt er; due to this they are suitable only for whole replacement of CB (Spangenberg, 2001).
The triacylglycerol component of the non-CB chocolates reflects similar to the palm kernel and coconut oil triacylglycerol components which are dominated by lauric bas ed fats (Noor Lida et. al., 2002). As the matter of fact that CBA, which is made from modification (hydrogenated or fractionation) of palm kernel and coconut oil is CBS, it may be concluded that the non-CB chocolates are wholy consisting of CBS (lauric ba sed fats).
68 Table 9.Retention Time of Triacylglycerol Standard
Peak Retention
Time
Triacylglycerol
Component
Peak Retention
Time
Triacylglycerol
Component
1 11.161 CaLaLa 15 29.418 OOO
2 12.338 CaLaM 16 30.705 POO
3 13.660 LaLaLa 17 31.501 PLS
4 15.535 LaLaM 18 32.097 POP
5 17.247 LaLaO 19 34.073 PPP
6 17.865 LaLaP/LaMM 20 36.642 SOO
7 18.787 MLL 21 37.497 SLS
8 19.853 MML/LaOM 22 38.197 POS
9 20.701 MMM/LaPM 23 40.608 PPS
10 22.165 LMO/LaOO 24 45.766 SOS
11 23.060 MPL/LaOP/MMO 25 48.388 PSS
12 24.212 LaPP/MMP 26 55.186 SOA
13 25.635 PLO 27 58.079 SSS
14 26.731 PLP
Ca = caprilic acid; La = lauric acis; M = mirystic acid; P = palmitic acid; S = stearic acid; O = oleic acid; L = linoleic acid; Ln = linolenic acid; A = arachidic acid.
Figure 10.The % Dark chocola POP, POS, and POS to have higher cont which only contain a its fat based has lea the other hand, Cho lower POS and SOS vegetable fat in it tha
Moreover, ac 3 chocolates that ha are Chocolate F, H, chocolate F, with chocolate POS co respectively. The pr manufacturer are CB Chocolate H and I, 3 triacylglycerol comp This study suits to t the pure and comm olates, chocolate A and B seemed to have the mor OS composition to cocoa butter. T he % POP and ontent than milk chocolates. The fact that dark c
n a little amount of milk powder and mostly cocoa ead to the similarity TAG composition to cocoa but hocolate I, which is also dark chocolate, have hig OS than other dark chocolates. This is due to the a that might have different composition with cocoa but
according to the in formation label of the products have any vegetable fat addition besides cocoa but H, and I. Based on the triacylglycerols component h POS 38.2% has no big difference to the ot composition, Chocolate C and D, 37.7 and products’ label inform that the vegetable fat us CBEs, which are Palm, Illipe, and Shea. On the I, 30.7 % and 24.0% respectively, have significant omponent (POS) to the whole cocoa butter milk
o the Bohacenko et. al.’s(2005) study, which dem mercially produced cocoa butter equivalents show nts than genuine CBs. Surprisingly, Chocolate G th
15.7 15.1
27.9 30.1 29.7 30.5 29.2 31.1
any vegetable fat ad
addition mentioned in the label has lower POS, 35. .
cerols composition such a complex system that POS, and SOS. Cocoa butter also consists of othe OO, SOA, and others that also contribute to the cha
have been summarized to some groups: monouns StUSt) such as POP, POS, SOS, PLO; diuns StU2) such as POO, SOO; and saturated triacy
LP, SSS, PSS, and PSS. The % StUSt of chocolate
Figure 11. The % StUSt of CB-chocolates
12. The % StStSt, StU2, and U3 of CB Chocolates
71 Based on Figure 12, Chocolate B has the highest %StUSt, 88.2% followed by Chocolate A and cocoa butter, 86.3% and 85.1% respectively. Chocolate H has the lowest amount of %StUSt, 78.4%. A study literature from Shukla (1995) implies that the higher monounsaturated triacylglycerol will result in the higher hardness and melting profiles. In addition, although saturated, diunsaturated, and polyunsaturated triacylglycerols only contained in small amount, they also contribut e to the melting properties of chocolates. Higher saturated TAG can give higher melting property because of its saturated and long chain triacylglycerols make them harder to be melt. In contrast, the higher diunsaturated and polyunsaturated could give lower melting properties to the chocolates due to the presence of double bond in the TAGS.
Table 10 gives the triacylglycerol composition of CBS chocolates, Chocolate E, J, K, L, and M. According to Noor Lida et. al. (2002) cocoa butter substitute (CBS) is dominated by palm kernel oil TAGs composition, LaLaLa, LaLaM, CaLaLa, CLaLa, LaLaP/LaMM, and LaLaO.
Table 10.The Triacylglycerol Composition of non CB Chocolates
TAGs (% area) M J E K L
CaLaLa / CLaM 14.4 13.5 12.8 13.9 12.3 LaLaLa 19.8 20.3 18.7 20.5 11.9 LaLaM 16.1 17.8 16.3 16.8 9.1 LaLaO 12.4 14.2 13.4 12.7 11.5 LaLaP/LaMM 8.6 9.7 9.9 8.4 11.4
LMM/LaOM 6.3 6.7 7.9 5.7 11.5
MMM/ LaPM 5.9 5.5 7.4 5.0 13.6
LMO/ LaOO 1.9 1.4 0.5 2.1 1.2
LPM/LaOP/MMO 2.7 2.5 3.5 2.3 5.0
LaPP/MMP 1.6 1.0 0.5 1.9 1.0
Others 10.1 7.5 9.1 10.5 10.8 Ca: Capric; La: Lauric; M: Myristic; L: Linoleic; P: Palmitic; O: Oleic
72 myristic acids. For instance, hydrogenation of palm kernel oil produces a range of lauric-type fats with slip melting point (SMP) varying from 32 to 41°C. This study suits to those chocolates that have higher slip melting point than others. The high SMP of chocolates is due to the presence of saturated acid, lauric acids and myristic acids.
Based on the international regulation about permitted vegetable fa t addition to chocolate, Chocolate E, J, K, L, and M should not be claimed as a chocolate. Surprisingly, Chocolate E that involved in middle -price chocolate also is a non CB chocolate. It should be noted that all the chocolate samples taken were the produc ts renowned manufacturers, expected to observe the directive to give a real chocolate product to the consumer. Chocolate E, J, K, L, and M still could be called milk chocolate according to SNI 01 -4293-1996 since there is no any requirement about cocoa butt er using. Consequently, these products cannot be sold in other countries especially U.K, Canada, Europe, and U.S which have a strict regulation about chocolate.
D. SOLID FAT CONTENT
Texture plays a vital part in consumer’s perception and pleasure of eating food, and confectionery is of no exception; it provides a wide variety of both taste and textural properties. The hardness of a chocolate is a key determinant of a succcessful product and the perfect product should be brittle at room temperatur. The brittleness of the chocolate is directly correlated with the hardness or the SFC/ melting properties of CB (Kattenberg, 2001). According to Peterson (1985), the best way to measure the hardness of CB is to use the low resolution pulse NMR technique in order to define the SFC as a function of temperature.
73 kept at 260C for 40 hours to ensure the cocoa butter or the cocoa butter -like fat are converted to β polimorph (Gunstone, 2002).
Figure 13.The SFC Curve of Chocolate Products.
Cocoa butter SFC values calculated at temperatures below 250C characterize its hardness, while the values calculated at temperatures between 25 and 300C indicate the resistance of cocoa butter to heating. In the range of 27 to 330C, intensive melting of co coa butter occurs bringing about the cooling sensation in the mouth and flavour release (Torbica et. al., 2005).
The results of SFC anaysis using NMR at different temperatures show that dark and milk chocolate s, Chocolate B, C, D, F, G, and H have SFC curve similar to cocoa butter curve. The uniqueness of cocoa butter is define d by its SFC curve where there is no significant decrease below 250C or flat but a dramatic decrease in solid fat content was happened between 25 until 350C. The dramatic decrease in SFC is responsible for the cooling sensation of the chocolate, in which a large amount of heat latent is absorbed by the fat when its changing to the liquid fraction.
Dark chocolate, chocolate B tends to have higher SFC than milk chocolate below 250C which means its hardness is higher tha n the hardness of
0
10°C 20°C 25°C 30°C 35°C 40°C
74 milk chocolate. The higher %StUSt amount in dark chocolates prove the higher hardness than milk chocolate. Moreover, milk fat prevent a fat bloom in chocolate but the soften effect from milk chocolate is undesired effect from a chocolate especially dark chocolate, which is known by its good snap (Minifie, 1999). The milk or anhydrous milk fraction (AMF) addition to milk chocolate is aimed to change the flavor perception, increase the appearance, such as bloom stability, gloss retention, and melting properties (Aguilar et al., 1994 and Full et al 1996). According to Shukla (200 6), the addition of milk fat to cocoa butter results in marked lo wering of the melting point adversely affecting the crystallization behavior and the hardness. There are two reasons for this strong decrease in hardness: liquid oil components of the milk fat soften the cocoa butter due to their fluidity and the solid fat components form eutectics with the triacylglycerols of cocoa butter.
Furthermore, non-CB based chocolates have higher SFC or hardness than dark and milk chocolate s. This finding was supported by the high saturated TAG content, higher than dark and milk c hocolates. It is shown that SFC curve of non CB chocolate, chocolate L different with cocoa butter where there is no significant decrease in its solid fat content between 27 and 330C. The SFC curve of non -CB based chocolate proves that it does not contain cocoa butter because cocoa butter is defined by its steep melting profile between mouth temperature. That non -CB based chocolate has gradually decrease in the increasing temperature implies that it can not give cooling sensation which result from dramatic decrease in SFC that release high heat latent energy. In additon, the SFC of non -CB based chocolate at 400C is remained about 20%; it means that non -CB based chocolate is more difficult to melt than dark and milk chocolate.
E. SLIP MELTING POINT
The melting profile of cocoa butter also can be describe d by its slip
melting point (SMP). Slip melting point is defined as the temperature at which the fats and oils have 4% solid fat. This parameter is used for
75 the fats and oils change with the chain length of FAs, unsaturation ratio, trans
FA content and the position of the FAs in the glycerol backbone (Karabulut et
al., 2004). Slip melting point is the point at which the fat is s often and rise up to the tube. Slip melting point is important to know whether the chocolate product can melt easily between mouth temperature (30 to 35oC). The slip melting point profile of chocolate products are shown in Table 1 1.
Table 11. Slip Melting Point Profile of Chocolate Bar Products No Chocolate Slip Melting point
1 A 32,6 - 33,2
2 B 33,6 -33,9
3 C 29,3 - 29,9
4 D 29,9 - 31,2
5 E 39,7 - 40,3
6 F 28,6 - 29,8
7 G 31,3 - 32,5
8 H 33,4 - 34,2
9 I 27,7 - 28,1
10 J 40,5–41,2
11 K 37,6–38,1
12 L 37,3 - 37,7
13 M 40,8 - 41,4
14 Cocoa Butter 31,8–32,6
Slip melting point is a fast screening analysis for determine the quality of cocoa butter. According to Hanneman (2000), a good chocolate should ha s slip melting point between oral temperature 30 to 350C. 13 chocolates have been analyzed which consist of import chocolate (Chocolate A to F) and local chocolate (Chocolate G to M). According to the table, it can be seen that in general, local chocolates have higher slip melting point tha n import chocolates. For example: the imported milk chocolate, chocolate C, D, and F have lower slip melting point than local milk chocolates, chocolate G and H.
76 the cocoa butter-used type. For example: Indonesia cocoa butter have higher melting point than other cocoa butters (Anonim, 2008). Also, it can be caused by the different chocolate formulations and processing. A special tempering procedure also can increase the melting properties of a chocolate.
This study also found that dark chocolates, chocolate A and chocolate B have higher slip meting point tha n milk chocolate, chocolate C, D, and F. It has been stated it is because of the milk fat addition has increase d the liquid fraction to the fat and lead to the lower melting point. Table 12. shows the relationship among slip melting point, StUSt, and StStSt composition of chocolate products.
Table 12.The Triacylglycerol Fractions and SMP of Chocolate Products.
No Chocolate SMP St2U StStSt StU2 U3
POP +
POS SOS
POP+POS / SOS 1 H 33,4 - 34,2 79.5 2.7 6.9 1.3 46.9 30.7 1.5 2 B 33,6 -33,9 88.2 1.2 8.3 0.9 56.4 30.1 1.9 3 A 32,6 - 33,2 86.3 1.1 9.7 1.5 56.7 27.9 2.0
4 Cocoa Butter 31,8–32,6 85.1 1.5 8.1 1.9 54.8 28.5 1.9
5 G 31,3 - 32,5 81.7 2.1 7.6 1.8 49.0 31.1 1.6 6 D 29,9 - 31,2 83.6 2.1 7.2 1.5 51.3 30.5 1.7 7 C 29,3 - 29,9 81.2 2.4 7.5 2.0 49.8 29.7 1.7 8 F 28,6 - 29,8 82.6 2.1 7.7 1.7 51.6 29.2 1.8 9 I 27,7 - 28,1 78.4 1.6 11.2 2.4 53.1 24 2.2
Figure 14.Th
. The %StUSt and Slip Melting Point of CB Choco
.The % StStSt and Slip Melting Point of CB Choc