PARTIAL PURIFICATION AND CHARACTERIZATION OF
Brevibacterium
sp. AMYLASE
WIDYO TRI PUTRANTO
DEPARTEMENT OF BIOLOGY
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
ABSTRACT
WIDYO TRI PUTRANTO. Purification and characterization of Brevibacterium sp. amylase. Under supervision of ANJA MERYANDINI and YOPI.
Amylase is an enzyme that used widely in various industrial field. This enzyme specifically catalyze the hydrolysis of -1,4-glycosidic bond on starch. To fulfill industrial need, the search for a new promising source of amylase is important. One of amylase source that has not been well explored yet is marine microbe. The purpose of this research was to purify and characterize the amylase from Brevibacterium sp. isolated from Jakarta Bay. The Brevibacterium sp. was cultured in Artificial Sea Water (ASW) medium. Measurement of specific amylase activity was performed by modified Miller method. The highest crude enzyme activity was produced 96 hours after the cultivation which is reaching 1,2 U/mL. After precipitation using ammonium sulphate, the crude enzyme solution were purified by gel filtration chromatography. SDS-polyacrylamide analysis result showed that the molecular weight of the enzymes were 79 kDa, 55,7 kDa, and 33,6 kDa. The partial purified enzyme has optimum activity on temperature 50oC and pH 6,4.
Key words: amylase, purification, characterization, Brevibacterium.
ABSTRAK
WIDYO TRI PUTRANTO. Purifikasi dan karakterisasi enzim amilase dari Brevibacterium sp. Dibimbing oleh ANJA MERYANDINI dan YOPI.
Amilase adalah enzim yang digunakan di berbagai bidang industri. Enzim ini mengkatalisis hidrolisis dari ikatan 1,4-glikosidik pada pati. Untuk memenuhi kebutuhan industri, pencarian terhadap sumber amilase baru yang menjanjikan sangat penting. Salah satu sumber amilase yang belum banyak dieksplorasi adalah mikroba laut. Penelitian ini bertujuan mempurifikasi dan mengkarakterisasi enzim amilase dari Brevibacterium
sp., yang diisolasi dari
perairan Teluk Jakarta. Isolat Brevibacterium sp. dikulturkan di media Artificial Sea Water (ASW). Pengukuran aktivitas amilase dilakukan menggunakan metode Miller yang dimodifikasi. Aktivitas amilase tertinggi pada enzim ekstrak kasar diproduksi pada jam ke-96 setelah pengkulturan, yaitu mencapai 1,2 U/mL. Setelah presipitasi dengan menggunakan ammonium sulfat, larutan enzim ekstrak kasar dimurnikan dengan kromatografi gel filtrasi. Hasil analisis SDS-PAGE menunjukkan berat molekul protein adalah 79 kDa, 55,7 kDa, dan 33,6 kDa. Berdasarkan karakterisasi, enzim bekerja optimum pada suhu 50oC dan pH 6,4.PARTIAL PURIFICATION AND CHARACTERIZATION OF
Brevibacterium
sp. AMYLASE
WIDYO TRI PUTRANTO
An Undergraduate Thesis
Intended to Pursue Bachelor Degree of Science
In Faculty of Mathematics and Natural Sciences
Bogor Agricultural University
DEPARTEMENT OF BIOLOGY
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
Title
: Partial Purification and Characterization of
Brevibacterium
sp.
Amylase
Name
: Widyo Tri Putranto
NIM
: G34080116
Approved by,
Supervisor I
Supervisor II
(Prof. Dr. Anja Meryandini, M.Si)
(Dr. Yopi)
NIP. 196203271987032001
NIP. 196912201989011001
Endorsed by :
Chief of Department
Department of Biology
Faculty of Mathematics and Natural Sciences
Bogor Agricultural University
(Dr. Ir. Ence Darmo Jaya Supena, M.Si)
NIP. 19641002 198903 1 002
PREFACE
Praise to Almighty God for His blessing so this thesis can be done. The title of this thesis is
“Partial Purification and Characterization of Brevibacterium sp. Amylase. This research was taken place at Biotechnology Research and Development Center, Indonesian Institute of Science, Cibinong. This research was conducted from February until June 2012.
This thesis would not have been possible without the support of several thoughtful and generous individuals. My acknowledgements to Prof. Dr. Anja Meryandini as my first supervisor and Dr. Yopi as my second supervisor for the guidance along the process of this thesis. I also wish to acknowledge Dr. Achmad Farajallah as examiner and representative of Department of Biology, FMIPA, IPB for the advices and discussion for the completion of this thesis. I would like to thank my beloved family, especially my parents for their supports and prayers. I would like to say thank you to Mba Nanik, Mba Ade, Mas Ashif, Mba Lia, Mas Alex and Mas Dicky for every advices they gave to me. I would like to say thank you as well to my comrades, Dewi, Viska, Yana, Rohana, Yufi, Riza, Lady and Titi for their cooperation on laboratory, and also for all Biology 45 friends for their supports, prayers and motivations.
This research was supported and funded by bio catalyst and fermentation laboratory, Bioproses division, Research Center Biotechnology Indonesian Institue of Science. I hope this thesis will be usefull for all readers.
Bogor, September 2012
vi
CURRICULUM VITAE
Writer was born in Bogor at 8 August 1990 as the third child of three from the parents FX Mugihardjo and Triasih Rudiatun.
On 2008, writer graduated from SMA Regina Pacis Bogor. On the same year, writer successfully continued his education on Bogor Agricultural University through Seleksi Nasional Mahasiswa Perguruan Tinggi Negeri (SNMPTN). Writer chose Biology major, Faculty of Mathematics and Natural Sciences.
On 2010, writer join field study on Pangandaran Beach, Ciamis, West Java, and wrote a
TABLE OF CONTENTS
Page
LIST OF TABLES ... viii
LIST OF FIGURES ... viii
LIST OF APPENDIX ... viii
INTRODUCTION ... 1
Background ... 1
Objective ... 1
Place and Time ... 1
MATERIALS AND METHODS ... 1
Preparation of Brevibacterium sp. culture ... 1
Production of enzyme. ... 2
Bioassay of Amylase ... 2
Ammonium Sulphate (NH4)2SO4 Precipitation ... 2
Gel Filtration Chromatography ... 2
Characterization of purified enzyme ... 2
Molecular Weight Determination ... 2
RESULTS ... 2
DISCUSSION ... 4
CONCLUSION ... 6
BIBLIOGRAPHY ... 6
LIST OF TABLES
Page
1 Purification of Brevibacterium sp. amylase ... 4
LIST OF FIGURES
Page 1 Curve of amylase activity from Brevibacterium sp. on ASW medium with pH 8 at room temperature ... 22 Protein precipitation using various concentration of ammonium sulphate ... 3
3 Amylase activity and protein profile of fraction from gel filtration chromatography ... 3
4 Temperature effects on Brevibacterium sp. amylase activity using 0,5% substrate at pH 6,6. ... 3
5 pH effects on Brevibacterium sp. amylase activity using 0,5% substrate at room temperature ... 3
6 SDS PAGE profile ... 4
7 Protein marker linear curve ... 4
LIST OF APPENDIX
Page 1 Composition of bacteria growth medium ... 92 Composition of DNS solution ... 9
3 Amylase activity on ASW medium with pH 8 on room temperature ... 9
4 Precipitation using ammonium sulphate ... 9
5 Temperature effects on amylase activity ... 9
6 pH effects on amylase activity ... 10
7 Protein marker standard data ... 10
1
INTRODUCTION
Background
Enzymes are catalysts that help to convert other molecules called substrates into products. An enzyme able to catalyze chemical reaction at least until 1 million times faster than reaction without catalizer (Yuwono 2005). Enzymes are giant molecules with molecular weight varies from 5 kDa to 5000 kDa, with typical values in the range 20 kDa– 100 kDa (Bugg 2004). Enzymes work by lowering the activation energy of a chemical reaction, and thus dramatically accelerating the rate of the reaction. Most enzyme catalyze just one kind of reaction or a group of reactions with high similarity. The specificity was the consequence of the uniqueness of
each enzyme’s active site. Active site is part
of an enzyme where the reaction takes place. The enzyme commission (EC) numbers divided enzymes into six main groups according to the type of reaction of catalysed: oxidoreductases, transferases, hydrolases, lyases, isomerases and ligases. Among the groups, hydrolases are presently the most commonly encountered class of enzymes within the field of enzyme technology. One of the hydrolases groups used widely in various industries is amylase.
Amylase is an enzyme that specifically catalyze the hydrolysis of glucosidic linkages in polysaccharide. It catalizes the hydrolysis of -1,4-glycosidic bond on starch (Liu et al. 2011). The amylases (α-amylases, β-amylases, glucoamylases) are one of the most important families of enzymes in the field of biotechnology (Rodriguez et al. 2006). The α -amylase belongs to a family of endo--amylases that catalyses the initial hydrolysis of starch into shorter oligosaccharides (van der Maarel et al. 2002).This hydrolysis results in the production of maltose and oligosaccharides. Amylase also helps the carbohydrates in a food break down more quickly and easily. This makes amylase as popular enzyme for food industry. Amylase is a highly demanded industrial enzyme in various fields such as pharmaceuticals, textiles, detergents, etc (Sivaramakrishnan et al. 2006). The most common industry using amylase is starch industry. This kind of industry using amylase for hidrolizing starch to produce fructose and glucose syrup (Nielsen & Borchert 2000).
In human, alpha amylase is synthesized in the acinar cells of the saliva glands and stored in secretory granules inside the cells. Amylase not only produced by human, but also by
animals, plants, and microorganisms (Kandra 2003). Although there are many sources of amylase, producing amylase from bacteria provides more advantages. Producing amylase from bacteria requires less time and space for production, easiness to modify, consistency of the enzyme, and cheaper.
Marine bacteria is one of amylase source that still has not been well explored yet. In other words, there might be abundant of promising amylase sources from marine. Many industrial applications need enzymes that stable at extreme temperature, pH, and salt concentration. In this case, marine microbial enzymes may offer advantages. The optimum activity of marine bacterial enzymes usually occurs at high salinity, making these enzymes utilisable in many harsh industrial processes. In addition, most marine bacterial enzymes are considerably thermotolerant, remaining stable at room temperature over long periods (Mohapatra e al. 2003).
Sea is a large saline water environment, for consequence, in order to survive, marine bacterias must have salt tolerance characteristic. The example of bacteria with that kind of characteristic is Brevibacterium genus, gram positive bacterias from Actinomycetales family. Brevibacteria are short shaped, nonbranched, asporogenous, obligately aerobic, rods which may exhibit a marked rod-coccus cycle when cells become older (Gruner et al. 1993). Brevibacterium is known as soil bacteria which causing odor on human feet. One of this genus member, Brevibacterium linens , is known as the most important surface bacteria in the cheese-making process due its role in the colouring surface and its typical flavouring activity (Motta & Brandelli 2002).
Objective
The objective of this thesis was to purify and characterize amylase from Brevibacterium sp., isolated from Jakarta Bay.
Place and Time
This research conducted from February 2012 until July 2012 in Research Center for Biotechnology, Bioproses division, Indonesian Institute of Science, Cibinong.
MATERIALS AND METHODS
Preparation of Brevibacterium sp.
2
Production of enzyme. The production of enzyme was using preculture method in ASW liquid medium. Preculture medium volume is 50 mL and the culture medium is 450 mL. Brevibacterium sp. cultured in solid ASW medium was inoculated to the preculture medium. Both the preculture and culture medium were incubated in shaker incubator with speed 150 rpm and temperature 26oC. The preculture medium was poured into the culture medium after three days, and then incubated again with the same condition for 8 days. The crude enzyme from the media was separated every 24 hours from cell by cold centrifuge with speed of 11000 rpm for 15 minutes. The amylase activity from each day isolated crude enzyme was measured using modified Miller method. The production then performed once again to harvest the enzyme with the highest activity.
Bioassay of Amylase. First, 250 µL of substrate solution was added to all tube. Substrate solution was made by mixing starch with 0,2 M of buffer phosphate pH 6,6. The substrate solution concentration was 0,5%. Then 250 µL of enzyme was added into the tubes. The addition of enzyme from one tube to another was given some interval. This interval was meant to synchronize the reaction time to be exactly 30 minutes for each tube. After the addition of enzyme, all tubes were set aside for 30 minutes to react. The 3,5-Dinitrosalicylic acid (DNS) solution then added with the same interval and order. All of the tube then heated in a boiling water for 20 minutes. All of tubes were then measured on 540 nm wavelength. The result in form of absorban then converted to U/mL. Unit protein defined as amount of enzyme that catalyzes the transformation of 1 micromole of substrate per minute.
Ammonium Sulphate (NH4)2SO4
Precipitation. The crude enzyme was
precipitated using ammonium sulphate. This step is to find out the best ammonium sulphate concentration to precipitate the enzyme. Different concentrations of ammonium sulphate tested to enzyme. The analyzed concentrations were 30%-70% with 10% interval. Ammonium sulphate poured slowly into crude enzyme. The solution stirred for 1 hour then centrifugated on 11000 rpm for 15 minutes to obtain its deposit. Finally the protein deposit was diluted with 0,2 M of buffer phosphate pH 6,6.
Gel Filtration Chromatography. The
result of ammonium sulphate precipitation then applied to gel filtration chromatography. First, the gel was washed with 0,2 M of buffer phosphate pH 6,6 before applying the enzyme. 1 ml of enzyme was applied and the fractions of 1 ml were collected. The purpose of this process was to gain the protein gradually based on their size. Enzyme in each fraction then measured using spectrophotometer on 280 nm wavelength. The amylase activity was also measured using modified Miller method on 540 nm of wavelength.
Characterization of purified enzyme.
The characterization of purified enzyme includes optimum pH and temperature. The temperatures analyzed were 30oC - 80 oC with 10 oC interval. The pH optimization was also performed with a modification. The modification was a change on the buffer that used to dissolve the substrate and to dilute the enzyme. The pHs analyzed were pH 6 until 7 with 0,2 interval.
Molecular Weight Determination. The
molecular weight determination was performed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) method with 12,5% of polyacrilamide gel. SDS-PAGE on this research was stained with silver staining method.
RESULTS
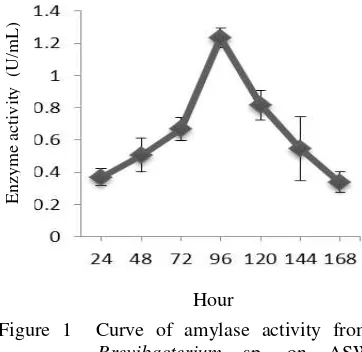
Crude enzyme with the highest activity was produced by Brevibacterium sp. 96 hours after cultivation (Figure 1).
Figure 1 Curve of amylase activity from Brevibacterium sp. on ASW
3
E n zy m e ac tiv ity ( U/m L ) E n zy m e ac tiv ity ( U/m L )medium with pH 8 at room temperature.
The enzyme activity in a variety of ammonium sulphate saturation can be seen in Figure 2. It showed amylase that posses the highest specific activity was in fraction of 50%. The value of enzyme recovery concentrated by ammonium sulphate 50% reached to 41%, the highest among the others.
Figure 2 Protein precipitation using various concentration of ammonium sulphate.
The protein pattern (measured on 280 nm of wavelength) and activity of amylase obtained from gel filtration chromatography can be seen on Figure 4. The result showed the absorban on 280 nm and 540 nm of wavelength shared a same pattern. The pattern showed that protein and amylase activity were at peak on fraction number 48-52. The enzyme on fraction number 48-52 were collected to be characterized.
Figure 3 Amylase activity and protein profile of fraction from gel filtration chromatography.
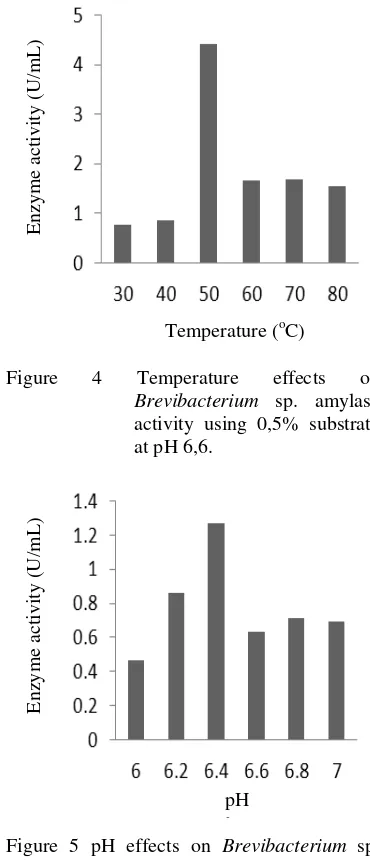
The temperature and pH effects on Brevibacterium sp. graphs (Figure 4 & 5) showed similar pattern. Amylase Activity keep increasing until reached its peak and then get lowering. Brevibacterium amylase showed its activity on temperature 30oC - 80 oC and high amylase activity only appear on temperature 50oC. Analysis of pH optimum showed Brevibacterium sp. has pH optimum on pH 6,4.
Figure 4 Temperature effects on Brevibacterium sp. amylase activity using 0,5% substrate at pH 6,6.
Figure 5 pH effects on Brevibacterium sp. amylase activity using 0,5% substrate at room temperature.
Purification process using ammonium sulphate precipitation and gel filtration chromatography method (Table 1) indicated the decreasing of crude enzyme total activity.
Temperature (oC)
pH Ammonium sulphate concentration
4
79 kDa 55,7 kDa 33,6 kDa 25 kDa 35 kDa 140 kDa 260 kDa 100 kDa 70 kDa 40 kDa 260 kDa 238 kDa Table 1 Purification of Brevibacterium sp. amylasePurification Step Total Activity (Unit) Total Protein (mg) Specific Activity (U/mg) Purification (fold) Yield (%)
Crude enzyme 629.5 55 11.4 1 100
Ammonium sulphate
precipitation 257.46 7.28 35.37 3.1 40.9
Sephadex G-75 31.12 0.35 88.91 7.8 4.9
Protein precipitation using ammonium sulphate made specific enzyme activity reduced by 59,1% and caused total protein decreased sharply from 55 mg to 7,28 mg. After all the precipitation and purification process, the overall yield of 4,9% was obtained with the improvement of specific enzyme activity 7,8 times.
(1) (2) (3)
Figure 6 SDS PAGE profile (1) marker, (2) crude enzyme, (3) purified enzyme.
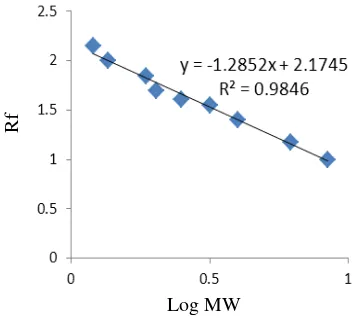
The SDS PAGE with silver staining showed there were four bands of protein. To determine the enzyme molecular weight, the first step was to find the Rf (Retardation factor) value of the protein marker. The migration distance of substance and the migration distance of solven front must be measured in order to find the Rf value.
Rf =
After obtaining Rf and log MW value of protein marker, the standard curve was made to find thelinier equation. The standard curve
was made with Rf as vertical axis and log MW as horizontal axis.
Figure 7 Protein marker linear curve.
According to the analysis of molecular weight and retardation factor of marker, the linear equation was: y = -1.049x + 2.251. Molecular weight of the enzyme was determined with this equation. X is Rf, and Y
is the logaritma value of the enzyme’s
molecular weight. From the calculation using this equation, the molecular weight of the purified proteins were 238 kDa, 79 kDa, 55,7 kDa and 33,6 kDa.
DISCUSSION
Brevibacterium sp. crude enzyme with the highest activity was produced 96 hours after incubation. Different incubation time can be seen on a research conducted by Nouadri (2010). In his research he found that Penicillium camemberti PL21 produced crude enzyme with the highest activity on 168 hours after incubation. Each bacteria produced crude enzyme with the highest activity on different time, depends on many factors, such as the adaptability of bacteria in their growth medium.
Purification was very important to study a particular enzyme because in analysis there
Log MW
Rf
5
might be interference from other enzymes in the extract that use the same substrate or cofactor. Purified enzyme also very important for industrial use, especially in pharmaceutical and clinical sectors. The crude enzyme usually purified using chromatography methods. Among the other precipitation methods, precipitation by ammonium sulphate and aseton was the most common method (Seftiono 2001). Ammonium sulphate used oftenly because of its high solubility, low cost, and the stability of protein in ammonium sulphate solution can last for years. The salt in the solution was then removed by dialysis. Salt precipitation does not usually lead to a highly purified protein, but can assist in eliminating some unwanted proteins in a mixture and concentrating the sample. That is why this precipitation was followed by gel filtration chromatography method.
The enzyme was purified using gel filtration chromatography. Gel filtration chromatography separates biomolecules based on their molecular size differences (Stanton 2004). Gel-filtration chromatography is a popular and versatile technique that permits the effective separation of proteins and other biological molecules in high yield. Gel on gel filtration consisted series of three dimension molecule that crosslinked each other in form of beads (Harsono 2001). In the column, pores that formed inside the gel selected the molecules based on their size. Small molecules diffuse freely into the pores and their movement through the column is retarded, whereas large molecules are unable to enter the pores and are therefore eluted earlier. This selection makes the larger molecule will pass through the gel faster than the small one. The separating media used in this research was Sephadex G-75, which is effectively separate proteins with 3.000-80.000 Da size.
Purification process made total protein decrease because each process eliminates unwanted substances from enzyme solution and this eliminated substances also could be in form of protein. Specific activity is enzyme activity value per milligram protein. Purification factor is the comparation of specific activity value of every purification process with the crude enzyme specific activity. The purification factor indicated the purity of enzyme as the result of purification process compared to crude enzyme.
Yield value is total amount of enzyme after purification compared to total amount of enzyme before purification. Compared to
another bacterias amylase purified with same method, yield value of Brevibacterium sp. amylase is very low. Purification of Penicillium camemberti (Nouadri et al. 2010) and Pseudomonas sp. (Liu et al. 2011) resulting enzyme with yield value 34,6% and 44%, while purified Brevibacterium sp. amylase only 4,9%.
Characterization of enzyme conducted to find out the enzyme’s characteristics. The test of temperature effects on amylase activity showed that amylase activity increasing until the optimum temperature on 50 oC. Low activity on lower temperature was caused by the lack of activation energy available. Amylase activity was then decreased significantly on 60 oC, and keep decreasing on higher temperature. Higher temperature affected the conformation of substrate. It makes the active site of substrate inhibited to
enter the enzyme’s active site which makes
the enzyme activity low. Beside that, the higher temperature was also caused enzyme denaturation. Denaturation makes the folding structure of the enzyme opened on the surface and change the active site and causing low enzyme activity (Hames & Hooper 2000).
The effects of pH showed the same pattern as temperature effects on amylase activity. Amylase activity increasing until its optimum pH on 6,4. An extreme pH change could make the enzyme denaturated. pH change can also disturb non kovalen interaction that keep the
stability of enzyme’s three dimension
structure (Hames & Hooper 2000). The purified amylase has optimum activity on pH 6,4 and temperature 50oC.
Each bacteria has unique characteristic based on their living environment. Therefore, each of them produce enzyme with different characteristic as well. In his research, Ashwini et al. (2011) found that the amylase extracted from Bacillus sp. marini has optimum activity on 40 oC and pH 7. This result indicated a similarity with the characterization result of Brevibacterium sp. amylase. Both bacteria have a similar type of living environment. Both bacteria live on marine environment, near the land.
6
Various result of amylase optimum pH can be seen in many researches as well. The differences of the amylase optimum pH of each bacteria was caused by the differences of its living environment. pH of living environment known to affect the synthesis and secretion of amylase just like its stability (Sivaramakrishnan et al. 2006). The research about amylase production from Aspergillus sp. JGI 12 (Alva et al. 2007) showed that the optimum pH of medium growth for the bacteria was same to the optimum pH of its crude enzyme. The optimum pH of medium growth and crude enzyme were 5,8 ; 7,5 and 9. In another research, Penicillium camemberti has optimum pH on pH 5, which is an acidic environment (Nouadri et al. 2010). This variety of amylase characteristic is the reason why amylase has been widely used in various industrial fields.
Molecular weight of the enzyme was determined using SDS PAGE method. It is an electrophoresis technique that use polyacrilamide as its separation material. The SDS PAGE result showed that there were four bands obtained. The appearance of four bands occured possibly because the purified enzyme was an isozyme. Isozyme was enzymes that have different structure but catalyze the same reaction. Another possibility is because another proteins with different molecular weight that entered the same fraction with the enzyme fraction. Among the others, the second band was the thickest. According the analysis and calculation, this band has molecular weight 79 kDa, while the other three have 238 kDa, 55,7 kDa and 33,6 kDa of molecular weight.
CONCLUSION
The purification and characterization process of Brevibacterium sp. amylase were performed using amonium sulphate precipitation and gel filtration chromatography method. The purification process resulting an enzyme with optimum activity on temperature 50oC and pH 6,4.
SUGGESTION
Further purification and characterization need to be conducted to get more about Brevibacterium sp. amylase character.
Zymogram is also necessary to know which protein band that possess the catalytic feature.
BIBLIOGRAPHY
Alva S et al. 2007. Production and characterization of fungal amylase enzyme isolated from Aspergillus sp. JGI 12 in solid state culture. African Journal of Biotechnology 6:576-581.
Ashwini K, Gaurav K, Karthik L, Bhaskara RKV. 2011. Optimization, production and partial purification of extracellular
α-amylase from Bacillus sp. Marini. Archives of Applied Science Research 3:33-42.
Bugg TDH. 2004. Introduction to Enzyme and Coenzyme Chemistry. Oxford: Blackwell Publishing.
Gruner E, Pfyffer GE, Graevenitz A. 1993. Characterization of Brevibacterium spp. from clinical specimens. Journal of Clinical Microbiology 31:1408-1412.
Hames BD, Hooper NM. 2000, Biochemistry: The Instant Notes. Hongkong:Springer-Verlag.
Harsono Y. 2001. Pemurnian enzim α-amilase dengan menggunakan filtrasi gel. [skripsi]. Bogor: Fakultas Matematika dan Ilmu Pengetahuan Alam, Institut Pertanian Bogor.
Kandra L. 2003. α-Amylases of medical and industrial importance. Journal of Molecular Structure THEOCHEM 666-667:487-498.
Laderman et al 1993. The purification and characterization of an extremely thermostable α-amylase from the hyperthermophilic Archaebacterium Pyrococcus furiosus. The Journal of Biological Chemistry 268:24394-24401.
7
Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry 31(3):426– 428.
Mohapatra BR, Bapuji M, Sree A. 2003. Production of Industrial Enzymes (Amylase, Carboxymethylcellulase and Protease) by Bacteria Isolated from Marine Sedentary Organisms. Acta Biotechnologica 23:75-84.
Motta AS, Brandelli A. 2002. Characterization of an antibacterial peptide produced by Brevibacterium linens. Journal of Applied Microbiology 92:63-70.
Nielsen JE, Borchert TV. 2000. Protein engineering of bacterial alpha-amylases. Biochimica et Biophysica Acta 1543:253-274.
Nouadri T, Meraihi Z, Shahrazaed DD, Leila B. 2010 Purification and
characterization of the α-amylase isolated from Penicillium camemberti PL21. African Journal of Biochemistry Research 4(6):155-162.
Rodriguez VB, Alameda EJ, Gallegos JFM, Requena AR, Lopez AIG. 2006.
Enzymatic hydrolysis of soluble starch
witn an α-amylase from Bacillus licheniformis. Biotechnology Progress 22:718-722.
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A. 2006. α -Amylases from microbial sources – an overview on recent developments. Food Technology and Biotechnology 44 (2): 173–184.
Seftiono H. 2001. Pemurnian dan karakterisasi mananase dari Streptacidiphilus luteoalbus [skripsi]. Bogor: Fakultas Matematika dan Ilmu Pengetahuan Alam, Institut Pertanian Bogor.
Stanton P. 2004. HPLC of Peptides and Proteins, Methods in Molecular Biology. New York: Humana Press.
van der Maarel MJ, van der Veen B, Uitdehaag JC, Leemhuis H, Dijkhuizen L. 2002. Properties and applications of starch-converting enzymes of the alpha-amylase family. Journal of Biotechnology 94:137-155.
8
9
Appendix 1 Composition of bacteria growth medium : 1. Solid Artificial Sea Water (ASW)
- 3.8 g ASW - 0.1 g yeast extract - 0.5 g pepton - 1.5 g agar - 1.5 g starch - 100 ml akuades
2. Liquid ASW - 3.8 g ASW - 0.1 g yeast extract - 0.5 g pepton - 1.5 g starch - 100 ml akuades
Appendix 2 Composition of DNS solution : - 1 g DNS
- 30 g Na K tartat - 1.6 g NaOH - 100 ml akuades
Appendix 3 Amylase activity on ASW medium with pH 8 on room temperature
Hour Amylase
Activity (U/ml)
24 0,44
48 0,5
72 0,61
96 1,26
120 1,01
144 0,62
168 0,16
Appendix 4 Precipitation using Ammonium Sulphate
Ammonium suphate concentration
Amylase Activity (U/ml)
30% 0,2
40% 1,07
50% 3,36
60% 1,02
70% 0,63
Appendix 5 Temperature effects on amylase activity
Temperature Amylase Acivity (U/ml)
10
40 oC 0,87
50 oC 4,43
60 oC 1,67
70 oC 1,69
80 oC 1,54
Appendix 6 pH effects on amylase activity
pH Amylase Activity (U/ml)
6 0,46
6,2 1,03
6,4 1,27
6,6 0,63
6,8 0,71
7 0,69
Appendix 7 Protein marker standard data
No.
Marker
Marker Tracking Distance
B
Rf
Log BM
BM
1
1,5
68
0,02
2,41
260
2
5,5
68
0,08
2,15
140
3
9
68
0,13
2
100
4
18,5
68
0,27
1,85
70
5
21
68
0,31
1,7
50
6
27
68
0,4
1,60
40
7
34
68
0,5
1,54
35
8
41
68
0,60
1,4
25
9
54
68
0,79
1,18
15
10
63
68
0,93
1
10
Appendix 8 Sediment of protein after precipitation with 50% concentration of ammonium sulphate