i
EXTRACTION AND DETERMINATION OF ANTIOXIDANT
ACTIVITIES OF PHENOLIC COMPOUNDS
IN DEFATTED PERILLA SEED MEAL
KURNIA NUR FARIDAH
DEPARTMENT OF FOOD SCIENCE AND TECHNOLOGY
FACULTY OF AGRICULTURAL ENGINEERING AND TECHNOLOGY BOGOR AGRICULTURAL UNIVERSITY
iii
STATEMENT LETTER OF UNDERGRADUATE THESIS AND
SOURCES OF INFORMATION
Hereby I declare that this undergraduate thesis entitled Extraction and Determination of Antioxidant Activities of Phenolic Compounds in Defatted Perilla Seed Meal is authentic work of mine with supervise from academic advisor and has not been submitted in any forms to any universities. Source of information which has been cited from published or unpublished work from another writer has been mentioned in text and has been included in references in the last page.
Hereby, I bestow the copyright of my undergraduate thesis to Bogor Agricultural University and Mae Fah Luang University.
iii
ABSTRACT
KURNIA NUR FARIDAH. Extraction and Determination of Antioxidant Activities of Phenolic Compounds in Defatted Perilla Seed Meal. Supervised by HARSI DEWANTARI KUSUMANINGRUM and SIRIRUNG WONGSAKUL.
Perilla seed (Perilla frutescens) is an herb that usually used in cooking and traditional medicine. Defatted perilla seed meal (DPSM) is a by-product from extraction of perilla seed oil using screw press. This by-product might contain some bioactive compounds such as polyphenol that can be used as antioxidant. This research was conducted to study the extraction condition of polyphenol from DPSM which extracted using ethanol 70 % and 50 %, acetone 70 % and 50 %, n- hexane, and mixture of hexane:ethanol (50:50 v/v). This study also determines the antioxidant activities of DPSM extracts. The proximate analysis showed that raw perilla seed contained 18.84 % crude protein and 28.63 % crude fat, while DPSM contained 27.11 % crude protein and 4.49 % crude fat. DPSM was extracted using solvents with different polarity at various extraction times. The total phenolic compounds of DPSM extracts were measured by Folin-Ciocalteu assays as well as antioxidant activities using 2,2-diphenyl-1-picrylhydrazyl (DPPH) and ferric reducing antioxidant power (FRAP). Longer extraction time yielded higher TPC and better antioxidant activity. Total phenolic content of DPSM extract which be extracted using acetone 70 % for 90 minutes (1031.99 ± 92.45 µmol GAE/100 ml) resulted in the highest yield. It was not significantly different (p<0.05) from extracting using ethanol 70 % for 90 minutes. While extraction using n- hexane for 30 minutes (58.92 ± 7.60) indicated the lowest yield but it was not significantly different from extracting using n- hexane for 60 and 90 minutes as well as hexane:ethanol for 30 minutes at p<0.05. Extraction using acetone 70 % for 90 minutes exhibited the high FRAP (1269.44 ± 134.45 µmol AAE/100 ml) and DPPH values (1029.52 ± 7.4 µM Trolox). While n- hexane extraction for 30 minutes showed the lowest FRAP (216.20 ± 33.13 µmol AAE/100 ml) and DPPH values (18.48 ± 1.65 µM Trolox). Another polar components and phenolic compounds that have high activity in DPSM extracts can influence the antioxidant capacities. Based on the results, it is likely that hexane or mixture of hexane:ethanol have low yield of phenolic compounds but the antioxidant activities were not always low. When the extraction processes using polar or non-polar solvent, it is suggested to use another method other than FRAP and DPPH which more suitable to know the antioxidant capacity in DPSM extracts. To optimize the solubility of polar or non-polar compounds of DPSM, the continuous solvents can be used. This research is expected to explore more to know the application of this result into food products.
i
Undergraduate Thesis
submitted as a compliment of the requirement for degree of Sarjana Teknologi Pertanian
at the Department of Food Science and Technology
EXTRACTION AND DETERMINATION OF ANTIOXIDANT
ACTIVITIES OF PHENOLIC COMPOUNDS
IN DEFATTED PERILLA SEED MEAL
KURNIA NUR FARIDAH
DEPARTMENT OF FOOD SCIENCE AND TECHNOLOGY
FACULTY OF AGRICULTURAL ENGINEERING AND TECHNOLOGY BOGOR AGRICULTURAL UNIVERSITY
v
PREFACE
All of praise, mercy, and graceful to our God, Allah subhanahuwata’ala which give all of guidance so I can complete this research and undergraduate thesis. The research entitled “Extraction and Determination of Antioxidant Activities of Phenolic in Defatted Perilla Seed Meal” was done in Mae Fah Luang University from August to November 2014. By completion of this research and undergraduate thesis, the author would like to express high appreciation and sincere thanks to:
1. Beloved parents, sisters, and family for their supports, cares, and awesome loves,
2. Dr Ir Harsi Dewantari Kusumaningrum as academic advisor in Bogor Agricultural University for her help and care so that I can complete this undergraduate thesis,
3. Sirirung Wongsakul, PhD as academic advisor in Mae Fah Luang University for her advice and support while I was doing my research,
4. Dr Endang Prangdimurti and Dr Sukarno as examiners for the advice and suggestion,
5. Ditjen DIKTI for full financial support during research and all of committee of AIMS Program in IPB (Bapak Eko, Ibu Dias, Bapak Pungky, Mbak Tika) and MFU (Ajjarn of Food Technology Aj. Natt, P’Noi, P’Yoh, P’Ciap) for all of this great chance in my life,
6. All of staff in S4 Laboratory (P’Tic, P’Nan, P’Pin, P’Khwan, P’Sud, P’To) also all of staff in Department of Food Science and Technology,
7. Rufnia Ayu Afifah as my dearest senior and friend for all of great support and help,
8. AIMS students Thailand batch 2014 (Cynthia, Mujahid, Aldila, Ayendha, Adimas, Gilang, Anggun, Samsul, Rasadi) and MFU friends for help and unforgettable memories during stay in Thailand,
9. DPPI family for supports and loves,
10. All of Food Science and Technology friends Batch 48 for supports, helps, and cares in a good or bad time.
Last but not least, hopefully this undergraduate thesis is useful and gives benefit for the readers.
Bogor, February 2016
vii
TABLE OF CONTENTS
LIST OF TABLES viii
LIST OF PICTURES viii
LIST OF APPENDICES viii
INTRODUCTION 1
Background 1
Objectives 1
LITERATURE REVIEWS 1
Perilla frutescens 1
Perilla frutescens seed 2
Perilla seed meal 2
Antioxidant 3
Antioxidant mechanisms of polyphenolics 3
METHODOLOGIES 4
Materials 4
Instruments 4
Methods 4
Extraction of defatted perilla seed meal 4
Method of analysis 5
Determination of moisture content 5
Determination of ash content 5
Determination of crude protein 5
Determination of crude fat 5
Determination of crude fiber 6
Determination of carbohydrate (by difference) 6
Determination of total phenolic content (TPC) 6
Determination of ferric reducing antioxidant power activity (FRAP assay) 6 Determination of DPPH radical scavenging activity (DPPH assay) 7
Statistical analysis 7
RESULTS AND DISCUSSION 7
Proximate composition of RPS and DPSM 7
Total phenolic content of DPSM extracts 8
Antioxidant activity of DPSM extracts 10
CONCLUSION AND RECOMMENDATION 14
Conclusion 14
Recommendation 15
REFERENCES 15
APPENDICES 19
LIST OF TABLES
1 Proximate composition of raw perilla seed and defatted perilla seed meal 8 2 Total phenolic and antioxidant activities of DPSM extracts 11
LIST OF PICTURES
1 Content of total polyphenols in DPSM extracts depending on solvent and
extraction time 9
2 Effect of total phenolic to FRAP assay of DPSM extracts 13
3 Effect of total phenolic to DPPH assay of DPSM 14
LIST OF APPENDICES
1 Moisture content of RPS and DPSM 19
2 Ash content of RPS and DPSM 19
3 Protein content of RPS and DPSM 19
4 Crude fat of RPS and DPSM 19
5 Crude fiber of RPS and DPSM 20
6 Standard curve for TPC of defatted perilla seed meal extract 20 7 Standard curve for FRAP of defatted perilla seed meal extract 20 8 Standard curve for DPPH of defatted perilla seed meal extract 21
1
INTRODUCTION
Background
Natural antioxidant can be found in various parts of plants such as fruits, leaves, seeds and oils. Polyphenol compounds are the most common groups in plants that have a wide range of biological activities which are related to the conventional antioxidant action. One of the biological properties of polyphenols in plants include antioxidant (Ferrazzano et.al. 2011). Perilla frutescens (Shiso) is an herb of the Mint family (Lamiaceae) native to East Asia that usually used for cooking and traditional medicine (Kurowska et.al. 2003). Polyphenol compound can be found in stems, leaves, and seeds of Perilla frustescens. Perilla seed extract is rich in polyphenols such as luteolin, chrysoeriol and apigenin as aglycons (Oryza 2007).
Defatted Perilla seed meal (DPSM) is a by-product from extraction of perilla seed oil using screw press. This by-product might contain some bioactive components such as polyphenol that can be used as natural antioxidant (Zhu and Fu 2012). However, defatting process can influence chemical composition and total phenolic content of the seed (Manukumar 2014). There are some studies on extraction of polyphenol from different materials by solvent extraction (Khodami 2013). Several factors affecting polyphenol yield and activity were examined, including solvent type, solvent composition, time, temperature, ratio, particle size, etc. (Durling 2007).
In this study, defatted perilla seed meal was extracted using different solvents and time to find out the suitable condition to extract polyphenol compounds of defatted perilla seed meal with the highest antioxidant activities. Solvents allowed to use in food were chosen so that the extracted bioactive compounds might be further applied in food products.
Objectives
The objectives of this research were to extract phenolic of DPSM using different solvent in various times and to determine the total phenolic content and antioxidant activities of DPSM.
LITERATURE REVIEWS
Perilla frutescens
2
has been known as a rich source of α-linolenic acid (Siriamornpun et al. 2006). In Uttarahand, the plants grow naturally and the local villagers collect the seeds for preparing the food chatney, making’roti’ and the seed oil for frying purposes. In some areas perilla also cultivated on a small scale as a garden herb (Singh 2013). Perilla frutescens is an Asian plant that usually used in cooking and traditional medicine (Kurowska et al. 2003). Plants are erect to 3 feet (1 m) in height, with an equal spread. Leaves are broadly ovate to 5 inches (12.5 cm) long and deeply toothed. They are extremely fast growers. The leaves are used as a vegetable or flavoring in Asia (Pink 2004). Perilla is also known has a capability as anti-bacterial, anti-carcinogenic, antiseptic, antipyretic and anti-asthmatic properties, also as immunomodulatory action (Zekonis et al. 2008).
Perilla is an edible plant and has medicinal properties. The leaves have a very pleasant sweet taste and are used as a spice, combined with fish, rice, vegetables, and soups as well as giving color and flavor to many pickled dishes. It is also chopped and combined with ginger root and salads in many Asian countries. The seeds from the plant also supplies nutritious cooking oil. The essential oil of the plants is used as a food flavoring. The entire plant is very nutritious with vitamins and minerals (Asif and Kumar 2010).
Perilla frutescens seed
Perilla seed or also known as Ebara seed has the highest content of ɑ-linolenic acid (Longvah and Deosthale 1991). The perilla seeds are rich in minerals, vitamins, and especially on poly-unsaturated fatty acids. The oil comprises up to 51 % of the seed’s weight, it’s a very rich source of the omega-3 fatty acid; alpha-linolenic acid (ALA), about 50 to 60 % of the oil
consists of ALA. On the other hand, it’s also contained other poly-unsaturated fatty acid derivatives; omega-6 (linolenic acid) and omega-9 (oleic acid) (Gunstone 1994).
Perilla seed meal
3
Antioxidant
Antioxidant compounds in food play an important role as a health protecting factor. Scientific evidence suggests that antioxidants reduce the risk for chronic diseases including cancer and heart disease. Primary sources of naturally occurring antioxidants are whole grains, fruits and vegetables. Plant sourced food antioxidants like vitamin C, vitamin E, carotenes, phenolic acids, phytate and phytoestrogens have been recognized as having the potential to reduce disease risk. Most of the antioxidant compounds in a typical diet are derived from plant sources and belong to various classes of compounds with a wide variety of physical and chemical properties. Some compounds, such as gallates, have strong antioxidant activity, while others, such as the mono-phenols are weak antioxidants.
The main characteristic of an antioxidant is its ability to trap free radicals. Highly reactive free radicals and oxygen species are present in biological systems from a wide variety of sources. These free radicals may oxidize nucleic acids, proteins, lipids or DNA and can initiate degenerative disease. Antioxidant compounds like phenolic acids, polyphenols and flavonoids scavenge free radicals such as peroxide, hydroperoxide or lipid peroxyl and thus inhibit the oxidative mechanisms that lead to degenerative diseases.
There are a number of clinical studies suggesting that the antioxidants in fruits, vegetables, tea and red wine are the main factors for the observed efficacy of these foods in reducing the incidence of chronic diseases including heart disease and some cancers. The free radical scavenging activity of antioxidants in foods has been substantially investigated and reported (Miller and Rigelhof et al. 2000). chemical stability than the initial radical. The interaction of the hydroxyl group of phenolics with the π-electrons of the benzene ring gives the molecules special properties, most notably the ability to generate free radicals where the radical is stabilized by delocalization. The formation of these relatively long-lived radicals is able to modify radical-mediated oxidation processes (Parr and Bolwell 2002).
4
enzymes involved in radical generation, such as various cytochrome P450 isoforms, lipoxygenases, cyclooxygenase, and xanthine oxidase (Parr and Bolwell 2002). Additionally, synergistic effects of phenolics with other antioxidants, namely ascorbic acid, β-carotene, and α-tocopherol (Croft 1998), and regulation of intracellular glutathione levels have also been described (Seabra et al. 2006).
METHODOLOGIES
Materials
Raw perilla seed which were obtained from Mae Hong Son Province Thailand, defatted perilla seed meal (using German screw press method). Folin- Ciocalteu reagents, trichloroacetic acid (TCA) were from LobaChemieTM India. Sodium carbonate Na2CO3, potassium dihydrogen phosphate NaH2PO4, disodium hydrogenphosphate Na2HPO4, sodium hydroxide NaOH pellets grade AR were from QRëC® New Zealand.Trolox (6-Hydroxyl-2,5,7,8-tetramethyl-chromane-2-carboxylic acid), gallic acid, DPPH (1,1-diphenyl-2-picrylhydrazyl ) were from Sigma-Aldrich® China. Methanol and ascorbic acid were from Ajax Finechem® Seven Hills Australia. Potassium ferricyanide K3[Fe(CN)6], ferric chloride FeCl3were from Fisher Scientific Qualigens® United Kingdom. Ethanol, acetone, and n- hexane AR Grade were from J.T. Baker (USA).
Instruments
Soxhlet apparatus, distillation apparatus, spectrophotometer uv vis, centrifugator, shaker, rotary evaporator, incubator, digital balance, grinder, oven, muffle furnace, flask, heater, volumetric flask, filter paper, glass dish, water bath, desiccator, porous crucible, Buchner funnel, Whatmann No. 40 filter paper.
Methods
Extraction of defatted perilla seed meal
5
and then diluted in methanol to a volume of 15 ml for further analysis. The extract samples were kept in dark. for at least 6 hours to constant weight. The cans were placed in desiccator for 30 minutes to cool down. The moisture content in the samples was then calculated on wet basis.
Determination of ash content
Ash content was determined by oven drying method according to AOAC (2003) with slightly modification. RPS and DPSM samples weighed to the nearest 2 g. The samples were placed in crucible and evaporate water to dryness in an oven 105 oC for 15 minutes and heat in muffle furnace at 550 oC for 4 hours to grayish white residue were obtained then the crucible was cooled in desiccator to cool. Finally, total ash content was calculated in wet basis.
Determination of crude protein
Protein in sample was determined by Kjeldahl method with slightly modification. The 0.25 gram of dried sample was taken in protein tube. Add 12 ml of concentrated H2SO4 and 5 g of digestion mixture K2SO4 and Cu2SO4 (10:1). The tube is swirled order to mix the contents then placed on heater 420 oC to start digestion until the mixture become clear (blue green in color). It needs 1 hour to complete. The digest was cooled and volume was made up to mark by the addition 50 ml of distilled water. Distillation of the digestion performed in distillation apparatus. The digest was introduced in the distillation tube then 50 ml of NaOH is gradually added through the same way. Distillation was continued for at least 4 min and NH3 produced was collected NH4OH in a conical flask containing 25 ml of 4 % boric acid solution with few drops of modified methyl red indicator. It was done until it produces a green color. The distillate was then titrated against standard 0.980 N HCl solutions until the appearance of pink color. A blank was also run through all steps as above. The result was calculated using correction factor of seed 5.30 based on FAO standard.
Determination of crude fat
6
washing and evaporated ether. The dish in placed in oven at 105oC for 2 hours and cooled in desiccator.
Determination of crude fiber
Crude fiber was determined by AOAC (2003) method with slightly modification. RPS and DPSM samples were weighed 1 g and placed into porous crucible. Then placed the crucible into Dosi-fiber unit and kept the valve in “CLOSE” position. After that added 150 ml of preheated 1.25 % H2SO4 solution and some drops of foam-suppresser to each column. Then opened the cooling circuit and turned on the heating elements (power at 90 %). When it started boiling, reduced the power at 30 % and left it for 30 min. Valves are opened for drainage of acid and rinsed with distilled water thrice to completely ensure the removal of acid from sample. The same procedure was used for alkali digestion by using 1.25 % NaOH. The samples were dried at 105 oC overnight. Then allowed the sample to cool in a desiccator and weighed. The crucibles were kept in muffle furnace at 525 oC for 4 hours. Cooled the samples in desiccator and
The total phenolic content (TPC) was determined by spectrophotometry, using Gallic acid as a standard, according to the method described by Sultana et al. (2012). Sample extracts were diluted (100 fold) with distilled water and 0.5 ml portions of the diluted solution were placed in triplicate to separate tubes μmol/100 ml. The TPC was expressed as Gallic acid equivalents (GAE), i.e. μmol GAE/100 ml.
Determination of ferric reducing antioxidant power activity (FRAP assay)
7
100 ml volumetric flask to make standard solution of FRAP assay (1000 μmol/100 ml). The antioxidant capacity was calculated and expressed as ascorbic acid equivalents (μmol AAE/100ml sample). The control and standard were subjected to the same procedures as the sample except that, for the control, only distilled water was added, and, for the standard, the extract was replaced with 0-1,000 µM ascorbic acid standard. The calibration curve was plotted between ascorbic acid concentration (µM) and absorbance at 700 nm.
Determination of DPPH radical scavenging activity (DPPH assay)
The DPPH assay was determined according to the modification method from Anesini et al. (2008) and Molyneux (2004). 50 μl of diluted sample extract was mixed with 1950 μl of a 60 μM DPPH (1,1-diphenyl-2-picrylhydrazyl C18H12N5O6 M=394.33) solutions in methanol. Then, the mixtures were left in the dark place for 30 minutes and measured the absorbance at 517 nm using methanol as blank and Trolox as standard. The radical scavenging activity was expressed as % inhibition. DPPH concentration was derived from the % inhibition vs. concentration plot. Preparation of standard solution was 0.0250 g Trolox diluted with methanol in 100 ml volumetric flask as standard stock solution (10000 μM). The DPPH 60μM reagent was made by dilute 0.0024 g of DPPH into 100 ml of methanol.
Statistical analysis
Data were expressed as means ± standard deviation. The data were also subjected to analysis of variance (ANOVA) and Duncan’s multiple range tests using SPSS 20.0 for Windows. The significance level of p<0.05 was considered significantly different.
RESULTS AND DISCUSSION
Proximate compositionofRPS and DPSM
Proximate analysis was done to know the differences between RPS and DPSM characteristics. The proximate compositions of samples are shown in Table 1. The moisture content (in wet basis) of RPS and DPSM were not significantly different. However, moisture content of RPS (6.86 %) is lower in comparison to those reported by Dhyani (2014) which has 7.6 %. The DPSM has higher moisture content of 6.98 % with no significantly different (p<0.05). This low moisture content may support longer shelf life for the seed meal (Ogunbusola 2012).
Total ash content refers to the total mineral composition of sample. The DPSM showed higher ash content in wet basis (5.22 %) than RPS (3.29 %) with significantly different at p<0.05. The high ash content is an indication of high inorganic mineral content (Oloyede 2005).
8
crude protein of seed. Crude fat in DPSM decrease to 4.49 % from that of 28.63 % in RPS as a result of defatted process. The defatting process may prolong the quality of the seed meal because the rancidity as results of lipid degradation may be less occurred. The defatted seed meal may also be used in food preparation where protein supplementation is needed (Ogunbusola 2012).
Table 1 Proximate composition of raw perilla seed and defatted perilla seed meal
Parameter RPS DPSM
Different letters in the same row indicate significant difference at p < 0.05.
Total phenolic content of DPSM extracts
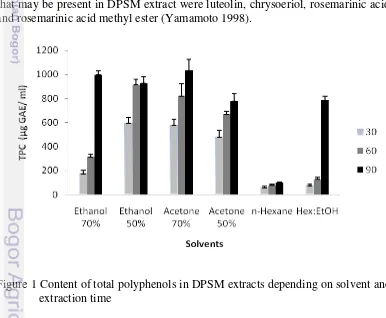
9
(Kosar 2005). Figure 1 showed significant difference (p<0.05) in total phenolic compound of DPSM in various solvents and times. In general, phenolic compounds in plants are polar compounds which usually are extracted by polar solvents such as aqueous acetone and ethanol (Wissam 2012).
The results exhibited that total phenolic content of DPSM extracts varied and ranged from 58.92 to 1031.99 µmol GAE/100 ml. Total phenolic content of DPSM extract which be extracted using acetone 70 % for 90 minutes (1031.99 ± 92.45 µmol GAE/100 ml) was not significantly different from extracting using ethanol 70 % for 90 minutes (996.63 ± 35.38 µmol GAE/100 ml) at p<0.05. While extracting using n- hexane for 30 minutes (58.92 ± 7.60) was not significantly different from extracting using n- hexane for 60 (75.76 ± 10.59), n- hexane for 90 minutes (97.64 ± 8.25) and hexane:ethanol for 30 minutes (74.07 ± 10.43) at p<0.05 using a standard curve of gallic acid (R² = 0.9926).
The polarity of solvent affected the total phenolic content which showed that the polar solvent has higher capability to extract polyphenol from DPSM. Lu and Foo (2000) mentioned that aqueous mixtures of acetone are good solvents for polar polyphenols as well as other antioxidants. Increasing concentration of polar solvents resulted in increase of total phenolic content in DPSM extracts. In a mixture with non-polar solvent showed low yield of total phenolic content. As shown in Figure 1, as extraction time increased (30 to 90 minutes), the yields of total phenolic content in DPSM also increased. The interaction between type of solvent and extraction time has been proved that the prolongation of the extraction time increased extraction ability of each examined solvent (Druzynska 2007). The longer time that is used for extraction, the more phenolic compounds that can be extracted from the DPSM. Extraction of total phenolic content in DPSM extracts is depending on solvent composition and extraction time. Phenolic compounds that may be present in DPSM extract were luteolin, chrysoeriol, rosemarinic acid, and rosemarinic acid methyl ester (Yamamoto 1998).
10
Antioxidant activity of DPSM extracts
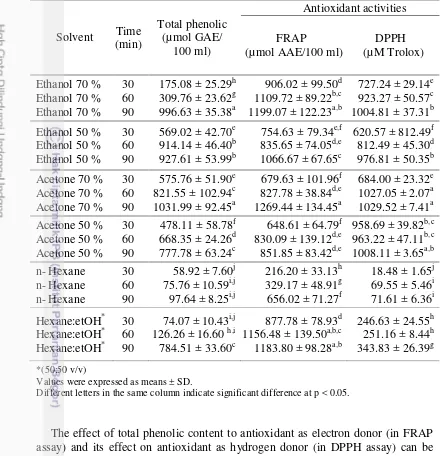
Antioxidant capacity should be measured using more than one method in order to obtain more reliable results (Fukumoto and Mazza 2000). In this study two methods, FRAP assay and DPPH assay were used. The ferric reducing antioxidant power (FRAP) assay was used to evaluate antioxidant capacities of the DPSM extracts. The FRAP assay was based on the capacity of antioxidants to reduce ferric (III) ions to ferrous (II) ions (Benzie and Szeto 1999). Higher FRAP values showed higher antioxidant capacity because FRAP value was based on reducing ferric ion, where antioxidants were the reducing agent. The results in reducing power demonstrate the electron donor properties (Beyhan et al. 2010).
As indicated in Table 2, extraction using acetone 70 % for 90 minutes (1269.44 ± 134.45 µmol AAE/100 ml), ethanol 70 % for 90 minutes, mixture of hexane:ethanol 50:50 (v/v) for 90 minutes, and hexane:ethanol 50:50 (v/v) for 60 minutes showed high FRAP values with no significantly different (p<0.05) among these extracts. While n- hexane extraction for 30 minutes showed the lowest FRAP value (216.20 ± 33.13 µmol AAE/100 ml).
Measurement of the antioxidant capacity using DPPH radical scavenging assay has been widely spread due to its simplicity and reproducibility. The DPPH radical scavenging activity (DPPH) measure the radical scavenging activity of antioxidants against the 2,2-diphenyl-1-picryhydrazyl (DPPH) radical. In the presence of antioxidants the purple color of the DPPH radical solution changes to a bright yellow and the intensity of this change can be monitored by spectrophotometer. DPPH radical scavenging activities in DPSM extracts may be attributed to hydrogen and electron donating abilities of phenolic compounds (Yang and Zhai 2010).
Results (Table 2) showed that extraction using acetone 70 % for 90 minutes (1029.52 ± 7.4 µM Trolox), acetone 70 % for 60 minutes, and acetone 50 % in 90 minutes showed high DPPH value with no significantly different at p<0.05. While n- hexane for 30 minutes showed the lowest DPPH value (18.48 ± 1.65 µM Trolox).
11
Table 2 Total phenolic and antioxidant activities of DPSM extracts
*(50:50 v/v)
Values were expressed as means ± SD.
Different letters in the same column indicate significant difference at p < 0.05.
The effect of total phenolic content to antioxidant as electron donor (in FRAP assay) and its effect on antioxidant as hydrogen donor (in DPPH assay) can be shown in Table 2. Overall, it showed that the antioxidant capacities from DPSM extracts present a better reducing power capability as electron donor than the free radical scavenging activity as hydrogen donor, except in acetone 70 % and acetone 50 % extracts.
12
activity as electron donor e.g. in DPSM that was extracted by ethanol 70 %. Medium TPC-medium FRAP group presented that medium value of total phenolic in the extract had medium antioxidant activity as electron donor e.g. in DPSM that was extracted by acetone 50 %. High TPC-medium FRAP group showed that high value of total phenolic in the extract possessed medium antioxidant activity as electron donor e.g. in DPSM that was extracted by acetone 70 %. While high TPC-high FRAP group exhibited the high value of total phenolic as well as high antioxidant activity as electron donor e.g. in DPSM that was extracted by ethanol 50 %. The results showed that there was another polar compound other than phenolic compound that may influence the antioxidant capacity by donor the electron. It may also show that there were phenolic compounds in the extracts that has high activity as antioxidant capacity by donor the electron. It can be showed in sample that be extracted by ethanol 70 % which showed high antioxidant capacity by electron donor activity. Lee et al. (2013) mentioned that rosmarinic acid-3-O-glucoside and rosmarinic acid were two major phenolic compounds in perilla seed extracts and they might be had high activity to increased antioxidant activity in DPSM extracts.
By plotting the total phenolic to DPPH assay, the result (Figure 3) also exhibited that there are 6 predominate groups i.e. low low DPPH, low high DPPH, medium medium DPPH, medium high DPPH, high TPC-low DPPH and high TPC-high DPPH. Low TPC-TPC-low DPPH group indicated that low value of total phenolic showed low antioxidant activity as hydrogen donor e.g. in DPSM that was extracted by n- hexane. Group with low TPC-high DPPH showed the low value of total phenolic which had high antioxidant activity as hydrogen donor e.g. in DPSM that was extracted by ethanol 70 %. Medium TPC-medium DPPH group represented that TPC-medium value of total phenolic affected medium antioxidant activity as hydrogen donor e.g. in DPSM that was extracted by acetone 70 %. Medium TPC-high DPPH group showed that medium value of total phenolic had high antioxidant activity as hydrogen donor e.g.in DPSM that was extracted by acetone 50 %. In high TPC-low DPPH group indicated that high value of total phenolic contributed in low antioxidant activity as hydrogen donor e.g. in DPSM that was extracted by hexane:ethanol. While high TPC-high DPPH group showed that high value of total phenolic and also high antioxidant activity as hydrogen donor e.g. in DPSM that was extracted by ethanol 50 %. The results showed that there was another polar compound other than phenolic compound that may influence the antioxidant capacity by donor the hydrogen. It may also show that there were phenolic compounds in the extracts that had high activity as antioxidant capacity by donor the hydrogen. It can be showed in sample that be extracted by ethanol 70 % which showed high antioxidant capacity by hydrogen donor activity.
13
Bolwell 2002). Folin-Ciocalteu assay gives a rough estimate of the total phenolic compounds present in extracts. It is not specific to polyphenols, but many interfering compounds may react with the reagent, giving elevated the apparent phenolic concentrations (Prior et al. 2005). It is likely that antioxidant activities is not limited to phenolic compounds, polar components other than phenolic can influence the results of antioxidant capacity. The activity may also come from the presence of other antioxidant secondary metabolites in the extracts such as carotenoids and vitamins (Javanmardi et al. 2003). The high activity of phenolic compounds also contributes in high results of antioxidant capacities. There were phenolic compounds that have high activity in DPSM extracts which might be come from rosmarinic acid-3-O-glucoside and rosmarinic acid.
Extraction using hexane or mixture of hexane:ethanol showed low yields of phenolic content, probably due to single extraction process which then be evaporated and be analyzed using FRAP and DPPH reagents so that the samples has low solubility and the antioxidant activities cannot measure optimally. Mixing with dimethyl sulfoxide (DMSO) could optimize the solubility of non-polar components that will be extracted by those solvents. This colorless liquid found immediate application as a polar, aprotic solvent miscible with water and able to dissolve an enormous catalog of polar and non-polar small molecules (Capriotti 2012). It will be better if used another method besides FRAP and DPPH which more suitable to know the antioxidant capacity in DPSM extracts. Extraction using another method e.g. CUPRAC (cupric ion reducing antioxidant capacity) method is more applicable to both hydrophilic and lipophilic antioxidants and completion of redox reactions (Apak et al. 2007). To optimize the solubility of polar or non-polar compounds of DPSM, the continuous solvents can be used.
14
Figure 3 Effect of total phenolic to DPPH assay of DPSM
CONCLUSION AND RECOMMENDATION
Conclusion
15
Recommendation
Some recommendation of this study are using another type of solvents, more various time, and temperature to know the best condition to extract DPSM. Another antioxidant method also can be used. Further research to support this can be done, include study about the application of DPSM extracts in food product.
REFERENCES
Alothman M, Bhat R. Karim AA. 2009. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. J Food Chem. 115:785-788.
Anesini C, Ferarro GE, Filip R. 2008. Total phenolic content and antioxidant capacity of commercially available tea (Camellia sinensis) in Argentina. J Agr Food Chem. 56:9225-9229.
Anil UT, Ganesh GT, Sneha K, Sanjay JS. 2011. Effect of solvents on total phenolics, antioxidant and antimicrobial properties of Bridelia retusa Spreng. stem bark. Indian J Nat Prod Resour. 2(4):442-447.
Apak R, Guclu K, Demirata B, Ozyurek M, Celik SE, Bektasoglu, Berker KI, Ozyurt D. 2007. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 12:1496-1547.
Asif M, Kumar A. 2010. Nutritional and Functional Characterisations of Perilla frutescens Seed Oil and Evaluation of Its Effect on Gastrointestinal Motility. Malay J Pharm Sci. 8(1):1–12.
AOAC. 2003. Official methods of analysis of the association of official’s analytical chemists, 17th edition. AOAC Arlington, Virginia.
Benzie IF, SzetoYT. 1999. Total antioxidant capacity of teas by the ferric reducing antioxidant power assay. J Agr Food Chem. 47:633-636.
Beyhan O, Elmastas M, Gedikli F. 2010. Total phenolic compounds and antioxidant capacity of leaf, dry fruit and fresh fruit of feijoa (Acca sellowiana, Myrtaceae). J Med Plants Res. 4(11):1065-1072.
Capriotti K, Capriotti JA. 2012. Dimethyl Sulfoxide history, chemistry, and clinical utility in dermatology. J Clinic Aesth Derm. 5(9):24-26.
Croft KD. 1998. The chemistry and biological effects of flavonoids and phenolic acids. J. Ann N Y Acad Sci. 854:435-442.
Dhyani D, Dhyani S. 2014. Nutritional, food and energy value of Perilla frutescens: an underutilized traditional oilseed crop of Western Himalaya, India J Agro food ind Hi-tech. 25(1):24-28.
16
Durling NE, OJ Catchpole, JB Grey, RF Webby, KA Mitchell, LY Foo, NB Perry. 2007. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol- water mixtures. J Food Chem. 101:1417–1424. FAO. 1970. Food Policy and Food Science Service, Nutrition Division:
Amino-Acid Content of Foods and biological data on proteins. FAO Nutritional Studies No. 24 - FAO Food and Nutrition Series No.21. FAO, Rome.
Ferrazzano GF, Ivana A, Aniello I, Armando Z, Gabriele P, Antonino P. 2011. Plant Polyphenols and their anti-cariogenic properties: a review. Molecules. 16:1486-1507.
Fukumoto LR, Mazza G. 2000. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J Agr Food Chem. 48:3597-3604.
Gunstone FD, Harwood J, Padley FB. 1994. The Lipid Handbook (2nd edition). London: Chapman & Hall.
Huang D, Ou B, Prior RL. 2005. The chemistry behind antioxidant capacity assays. J Agr Food Chem. 53:1841-1856.
Jackson D, Shelton K. 2002. Perilla (Perilla frutescens), alternative nature online herbal [internet]. [be downloaded in 2014 September 21]. Can be accessed in: http://www.altnature.comgallery/ perilla.htm.
Javanmardi J, Stushnoff C, Locke E, Vivanco JM. 2003. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. J Food Chem. 83:547-550.
Khodammi A, Wilkes MA, Roberts TH. 2013. Techniques for Analysis of Plant Phenolic Compounds. Molecules. 18:2328-2375.
Kosar M, HJD Dorman, R Hiltunen. 2005. Effect of an acid treatment on the phytochemical and antioxidant characteristics of extracts from selected Lamiaceae species. J Food Chem. 91:525-533.
Kurowska EM. 2003. Bioavailability of Omega-3 essential Fatty Acids from Perilla Seed Oil. Prostaglandins, Leukotrienes and Essential Fatty Acids. 68:207-212.
Lee JH, Park KH, Lee MH, Kim H, Seo WD, Kim JY, Baek I, Jang DS, Ha TJ. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 136:843-852.
Longvah T, Deosthale YG. 1991. Chemical and nutritional studies on Hanshi (Perilla frutescens) a traditional oil seed from Northeast India. J American Oil Chem Soc. 68:781-784.
Lu Y, Foo LY. 2000. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry. 55:263–267.
Maja Dent, Verica DU, Marija P, Mladen B, Tomislav B, Branka L. 2013. The effect of extraction solvents, temperature and time on the composition and mass fraction of polyphenols in Dalmatian wild sage (Salvia officinalis l.) extracts. Food Technol Biotech. 51(1):84–91.
17
Miller HE, Rigelhof F, Marquart L, Prakash A, Kanter M. 2000. Cereal Foods World. 45(2):59-63.
Molyneux P. 2004. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sco Technol. 26(2):211-219.
Moure A, Franco D, Sineiro J, Dom nguez H, NúñezM AJ, Lema JM. 2001. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res Int. 34:103-109.
Ogunbusola EM, Fagbemi TN, Osundahunsi OF. 2012. Chemical and Functional Properties of Full Fat and Defatted White Melon (Cucumero psismannii) Seed Flours. J Food Sci Eng. 2:691-696.
Oloyede OI. 2005. Chemical profile of Unripe Pulp of Carica Papaya. Pak. J Nutrition. 4(6):379-381.
Oryza. 2007. Perilla seed extract: antibacterial oral preparation. Oryza Oil & Fat Chemical Co. LTD.
Parr AJ, Bolwell JP. 2002. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J Sci Food Agr. 80:985-1012.
Pink A. 2004. Gardening for the Million. Project Gutenberg Literary Archive Foundation.
Prior RL, Wu X, Schaich K. 2005. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agr Food Chem. 53:4290-4302.
Singh U. 2013. Popularization of Perilla seed oil as functional food source. Curr sci. 104 (3):284-285.
Siriamornpun S, Li D, Yang L, Sutajit S, Suttajit M. 2006. Variation of lipid and fatty acid compositions in Thai Perilla seeds grown at different locations. Songklanakarin J Sci Technol. 28:17–21.
Teh SS, Bekhit AE, Birch J. 2014. Antioxidative Polyphenols from Defatted Oilseed Cakes: Effect of Solvents. J Antioxidants. 3:67-80.
Tura D, Robards K. 2002. Sample handling strategies for the determination of biophenols in food and plants. J Chromat A. 975:71-93.
Tinrat S, Akkarachaneeyakorn S, Singhapol C. 2014. Evaluation of antioxidant and antimicrobial activities of Momordicaco chinchinensis Spreng (gac fruit) ethanolic extract. J Int Pharm Sci Res Bangkok: King Mongkut’s University of Technology. 5(8):3163-3169.
Valentão P, Fernandes E, Carvalho F, Andrade PB, Seabra RM, Bastos ML. 2003. Hydroxylradical and hypochlorous acid scavenging activity of small centaury (Centauriumerythraea) infusion.
Wissam Z, BashourGhada B, Wassim A, Warid K. 2012. Effective extraction of polyphenols and proanthocyanidins from pomegranate’s peel. Int J Pharm Pharm Sci. 4(3):675 - 682.
Yamamoto H, Jinsaku S, Akito N, Keizo S. 1998. Inhibitors of Arachidonate Lipoxygenase from Defatted Perilla Seed. J Agr Food Chem. 46:862−865. Yang CS, Landau JM, Huang MT, Newmark HL. 2001. Inhibition of
carcinogenesis bydietarypolyphenolic compounds. AnnuRev Nutr. 21:381-406.
18
extracted from the seed and cob of purple corn (Zea mays L.). Innov Food Sci and Emerg Technol. 11:169–176.
Žekonis G. 2008. Effect of Perilla Frutescens Aqueous Extract on Free Radical Production By Human Neutrophil Leukocytes. Med Kaunas. 44:699-705. Zhu J, Fu Q. 2012. Optimization of ultrasound –assisted extraction process of
perilla seed meal proteins. Food sci biotech. 21(6):1701-1706.
19
APPENDICES
Appendix 1 Moisture content of RPS and DPSM
Sample Wo Ws W1
Appendix 2 Ash content of RPS and DPSM
Sample Wo Ws W1 %Ash Mean SD
Appendix 3 Protein content of RPS and DPSM Sample Ws V HCl %Protein Mean SD
Appendix 4 Crude fat of RPS and DPSM
Sample Ws F0 F1 %Fat Mean SD
RPS 1.0076 45.3923 45.6781 28.36 28.63 0.38 1.0064 45.3503 45.6410 28.89
20
Appendix 5 Crude fiber of RPS and DPSM
Sample W1 W2 W3 % Fiber Mean SD
RPS 1.0017 30.027 29.818 20.86
20.51 0.727255 RPS 1.002 30.5614 30.3653 19.57
RPS 1.0032 30.4913 30.2827 20.79 DPSM 1.0032 30.4429 30.1758 26.62
20.62 0.652976 DPSM 1.0036 30.3004 30.0463 25.32
DPSM 1.0033 30.9209 30.6603 25.97
Appendix 6 Standard curve for TPC of defatted perilla seed meal extract
21
Appendix 8 Standard curve for DPPH of defatted perilla seed meal extract
Appendix 9 Statistical analysis of DPSM extracts Water Content
Sum of Squares
df Mean Square F Sig.
Between Groups .022 1 .022 3.465 .136 Within Groups .025 4 .006
Total .047 5
Ash Sum of
Squares
df Mean Square F Sig.
Between Groups 5.568 1 5.568 9826.000 .000 Within Groups .002 4 .001
Total 5.570 5
Protein Sum of
Squares
df Mean Square F Sig.
Between Groups 102.589 1 102.589 210.038 .000 Within Groups 1.954 4 .488
Total 104.543 5
Fiber Sum of
Squares
df Mean Square F Sig.
Between Groups 46.426 1 46.426 97.869 .001 Within Groups 1.897 4 .474
22
Carbohydrate Sum of
Squares
df Mean Square F Sig.
Between Groups 287.042 1 287.042 552.996 .000 Within Groups 2.076 4 .519
Total 289.118 5
Fat Sum of
Squares
df Mean Square F Sig.
Between Groups 582.981 1 582.981 8289.812 .000 Within Groups .141 2 .070
24
AUTHOR BIOGRAPHY
Kurnia Nur Faridah was born in Malang, May 13th 1993. She is the middle daughter of 3 siblings from Mr. Johan Syaifudin, M.Sc and Mrs. Dra. Nurul Hasanah. She studied elementary school in SDI Al-Ma’arif 02 Singosari (1999-2005) , junior high school in SMPN 1 Singosari (2005-2007) and senior high school in SMAN 1 Malang (2008-2010) and continued her study in Department of Food Science and Technology Bogor Agricultural University.
During her study, she also joined non academic activities in some organization such as staff of HIMITEPA (Himpunan Mahasiswa Ilmu dan Teknologi Pangan) in Divisi Peduli Pangan Indonesia (DPPI) 2013 and Secretary 2 of Divisi Peduli Pangan Indonesia (DPPI) 2014, Himarema (Himpunan Mahasiswa Arek Malang), Rohis ITP 48, Lises Gentra Kaheman, Elodea dancing club Fateta, Komunitas Bicaradesa, Majalah Emulsi, and We are Siblings (2014). Author also active in organized it activities such as staff of consumption in Reds Cup (2011), chief of PDD in Suksesi (2012), Canteen Campus Auditor (2013), staff of PDD in Food Bowl Quiz competition 2013 (2013), staff of PDD in Lomba Cepat Tepat Ilmu Pangan XXI (2013), chief of PDD in Baur-Access ITP 49 (2013), staff of PDD in HACCP Plasma training (2013), staff of PDD in Drawing and Paper Competition Tropical Plant Curriculum Program SEAFAST Center IPB (2014), volunteer in Pekan Sarapan Sehat (2014), staff of consumption in International Students Party (2015). She also joining training programs such as Food Safety Expert training and inspection and CDA training.