CHEMICAL MODIFICATION OF POLYPHENOL AND ITS
APPLICATION FOR WOOD TREATMENT: NATURAL
CATECHIN MODIFICATION
FEBRINA DELLAROSE BOER
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT
I declare that this thesis, entitled Chemical Modification of Polyphenol and Its Applications for Wood Treatment: Natural Catechin Modification, is my own work with the direction of the supervising committee and has not been submitted in any form for any college except in AgroParisTech centre de Nancy and Université de Lorraine, France (required by the Double Degree Master Program—the joint Master program held between the Program Study of Forest Products Science and Technology of Bogor Agricultural University and Bois Forêt et Développement Durable of AgroParisTech centre de Nancy and Université de Lorraine). Information and quotes from journals and books have been acknowledged and mentioned in the thesis where they appear. All complete references are given at the end of the paper.
I understand that my thesis will become part of the collection of Bogor Agricultural University. My signature below gives the copyright of my thesis to Bogor Agricultural University.
Bogor, Februari 2017
Febrina Dellarose Boer
SUMMARY
FEBRINA DELLAROSE BOER. Chemical Modification of Polyphenol and Its Application for Wood Treatment: Natural Catechin Modification. Supervised by DODI NANDIKA and CHRISTINE GÉRARDIN.
Catechin is one of the naturally-occurred polyphenols which possesses powerful scavanger related to its antioxidant trait. Catechin itself can be modified to increase its potential utilization with several compounds such as epoxy resin through urethane synthesis and fatty acids through esterification. With the current trend of increasing efforts to develop non-isocyanate-based polyurethanes (NIPUs), the first objective of this study is to chemically modify catechin in order to synthesize the urethane. Meanwhile, in order to develop an environmental friendly wood preservative, the second objective of this study is to chemically modify the catechin’s hydroxy groups with the fatty acid chains in the context of its potential utilization as a wood preservative.
The methods used in the urethane synthesis consisted of four steps: glycidilation of catechin, hydrolysis of epoxide, carbonate synthesis, and a test for carbamate synthesis through condensation of butylamine. The resulting products then analyzed and characterized using FTIR (Fourier Transform Infrared) and NMR (Nuclear Magnetic Resonance) spectroscopy. It was determined that we have successfully obtained carbamate (urethane) with a model amine (butylamine) through this four-steps method, thus opening the door to further polyurethane synthesis using the modification of catechin.
Meanwhile, the esterification study has evaluated the methods of grafting commercial catechin with three types of fatty acids: capric acid (C10), myristic acid (C14), and lauric acid (C12). Specimens of beech wood (Fagus sylvatica L.) were impregnated by both the catechin and modified catechin at 5% concentration level using vacuum pressure treatment and subjected to leaching. Specimens were tested against white rot fungi Corioulus versicolor for twelve weeks. Catechin modified with C10, C12, and C14 fatty acids were evaluated through the analysis of their weight percent gain before (WPG) and after leaching (WPAL), percentage of leaching (PL), and the decay resistance of treated and untreated sample before and after leaching exposed to C. versicolor. Results show that the specimens treated with catechin modified with fatty acids possess significant results on PL while the specimens treated with catechin—C14 present best PL after the leaching compared to the others. It is believed that the addition of fatty acid, and the increasing length of its chain, will lead to the increasing of the leaching resistance. However, it is recommended to increase the concentration level of modified catechin for obtaining the significant effect on the decay resistance.
RINGKASAN
FEBRINA DELLAROSE BOER. Modifikasi Kimia Senyawa Polifenol dan Aplikasinya pada Perlakuan Kayu: Modifikasi Katekin Alami. Dibimbing oleh DODI NANDIKA dan CHRISTINE GÉRARDIN.
Katekin merupakan salah satu polifenol alami yang dikenal karena sifat antioksidannya. Katekin sendiri dapat dimodifikasi dengan tujuan untuk mening-katkan potensi kegunaannya dengan berbagai senyawa lain, seperti resin epoksi, melalui sintesis uretan dan asam lemak melalui reaksi esterifikasi. Dengan me-ningkatnya usaha dalam mengembangkan poliuretan bebas isosianat (NIPUs), tujuan pertama dalam penelitian ini adalah untuk memodifikasi katekin secara kimiawi dengan tujuan untuk sintesis uretan. Sementara itu, untuk pengemba-ngan bahan pengawet kayu ramah lingkupengemba-ngan, tujuan kedua dari penelitian ini adalah untuk memodifikasi secara kimia gugus hidroksi katekin dengan rantai asam lemak dalam konteks kegunaannya sebagai bahan pengawet kayu.
Metode yang digunakan dalam sintesis uretan, terdiri dari empat langkah: glisidasi katekin, hidrolisis epoksida, sintesis karbonat, dan sintesis karbamat. Produk yang diperoleh kemudian dianalisis menggunakan alat spektroskopi FTIR (Fourier Transform Infrared) dan NMR (Nuclear Magnetic Resonance). Hasil penelitian menunjukkan bahwa melalui keempat metode tersebut, penelitian ini telah berhasil memperoleh karbamat (uretan) dengan menggunakan model aminasi sederhana (butilamin), yang membuka peluang ke depan dalam penelitian lebih lanjut.
Sementara itu, dalam proses esterifikasi telah dievaluasi metode grafting menggunakan katekin komersial dengan tiga macam asam lemak: asam kaprat (C10), asam miristat (C14), dan asam laurat (C12). Selanjutnya sampel kayu beech (Fagus sylvatica L.) diimpregnasi dengan menggunakan katekin dan katekin termodifikasi pada konsentrasi 5% dengan menggunakan proses vakum kemudian diberikan perlakuan pencucian sesuai standar ENV 1250-2. Contoh uji kayu kemudian diuji ketahanannya terhadap jamur pelapuk putih Corioulus versicolor selama dua belas minggu. Efektivitas katekin yang dimodifikasi dengan asam lemak C10, C12, dan C14 dievaluasi melalui pengukuran Weight Percent Gain (WPG) sebelum dan sesudah pencucian, persentase ketercucian (Percentage of Leaching, PL), dan ketahanan terhadap jamur Corioulus versicolor sebelum dan sesudah pencucian. Hasil penelitian menunjukkan bahwa sampel kayu yang dimodifikasi dengan asam lemak memiliki penurunan nilai PL yang signifikan. Dalam hal ini, sampel yang diberi perlakuan katekin—C14 memiliki nilai WPG yang lebih tinggi, ketercucian paling rendah, dan ketahanan jamur yang lebih tinggi dibandingkan dengan perlakuan lainnya. Hal ini menunjukkan bahwa penambahan rantai asam lemak pada katekin dapat meningkatkan efektivitasnya sebagai bahan pengawet kayu khususnya dalam meningkatkan ketahanan kayu beech terhadap pencucian. Untuk penelitian selanjutnya, disarankan untuk meningkatkan konsentrasi katekin termodifikasi untuk mendapatkan tingkat ketahanan kayu terhadap jamur yang signifikan.
©Copyright IPB, 2017
Copyright Reserved by Law
Prohibited quoting part or all of this paper without mentioning or citing the sources. Quotation is only for educational purposes, research, writing papers, preparing reports, writing criticism, or review of an issue, and the citations will not harm the interests of IPB
Thesis
In partial fulfillment of the requirements for the degree of Master of Science
at
Forest Products Science and Technology Study Program
CHEMICAL MODIFICATION OF POLYPHENOL AND ITS
APPLICATION FOR WOOD TREATMENT: NATURAL
CATECHIN MODIFICATION
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2017
FOREWORD
All praise and gratitude to Allah SWT so that this scientific work, entitled Chemical Modification of Polyphenol and Its Application for Wood Treatment: Natural Catechin Modification, could be finished. The study is expected to provide scientific information on the methods of the modification of catechin in order to improve its utilization.
I would first like to thank my supervisor, Professor Dodi Nandika and Professor Christine Gérardin, for their expert advices and encouragement throughout this work, as well as Dr. Hubert Chapuis for his helpfulness and guidance in both technical and practical ways on my work.
My study has been impossible to complete without the support from the Ministry of Education and Culture of Indonesia (Kementerian Pendidikan dan Kebudayaan Indonesia) in the form of a scholarship, ―Beasiswa Unggulan‖, which supported my double degree Master education both in Indonesia and France, and CampusFrance with French Ministry of Foreign Affairs for their social coverage scholarship. I would also like to thank Prof. Wayan Darmawan, Prof. Philippe Gérardin, Prof. Mériem Fournier, and Dr. Holger Wernsdörfer for their support and assistance during my study both in Indonesia and in France.
I would also like to thank to my colleagues, especially in LERMaB, Nancy, France: to Aurélia Imbert, Thomas, and Wissem Sahmim for their help in the laboratory and inputs during my internship. To my lab-mates: Azza Zeriaa, Marwa Ben Hasinne, Amal Ben Hadj, and Huo Wenjie, for the sweet friendship during my stay in Nancy, and encouragement in every crisis moment.
Finally I would like to sincerely thank my parents and all of my family, for their love and support during my study.
I recognize that this paper is still far from perfect. Therefore, suggestions and constructive criticism are expected to improve this work.
Bogor, Februari 2017
TABLE OF CONTENTS
TABLE OF CONTENTS vi
LIST OF TABLES vi
LIST OF FIGURES
1 INTRODUCTION 1
Background 1
Formulation 3
Objective 3
Benefits 4
2 MATERIALS AND METHODS 4
Location and Period of Research 4
Tools and Materials 4
Data Analyzing 9
3 RESULTS AND DISCUSSIONS 10
Reaction of Catechin and Epoxide 10
Grafting of Catechin and Fatty Acid 16
Application as a Wood Preservative 20
4 CONCLUSIONS 25
Conclusion 25
Suggestions 25
REFERENCES 26
LIST OF TABLES
1 Classes of natural durability of wood to fungal attack using laboratory
tests based on EN 113 9
2 Yield of catechin modified with fatty acids 20
3 Percentage of weight gain after impregnation, weight gain after
leaching, and percentage of leaching 20
4 P-value for T-test 22
5 Weight loss of beech wood specimens exposed to C. versicolor 23
LIST OF FIGURES
1 Chemical modification of catechin through grafting with link ester. 2 2 Illustration of vacuum impregnation of the wood specimens using
desiccator 7
3 Leaching process under continuous shaking method based on ENV
1250-2 8
4 Reaction of catechin and epichlorohydrin 10
5 1H NMR spectrum of catechin 10
6 1H NMR spectrum after glycidylation of catechin 10 7 FTIR transmissionspectrum after glycidilation of catechin 11
8 Hydrolysis of epoxide 11
9 FTIR transmission spectrum: comparison between epoxide and diol 12 10 1H NMR spectrum after epoxide opening reaction (C27H38O14) 12
11 Synthesis of cyclic carbonate derivatives (C31H30O18) 13
12 FTIR transmissionspectrum after carbonation 13
13 1H NMR spectrum after carbonation reaction (C31H30O18) 14
14 Synthesis of carbamate with butylamine 14
15 Comparison between carbamation followed and unfollowed by a
liquid/liquid extraction step 15
16 1H NMR spectrum after carbamation reaction with butyl amine
(C47H74N4O18) 15
17 Catechin 16
18 Catechin modified with capric acid (monoester) 16
19 Catechin modified with capric acid (diester) 17
20 Catechin modified with myristic acid (monoester) 17
21 Catechin modified with myristic acid (diester) 18
22 Catechin modified with lauric acid (monoester) 18
23 Catechin modified with lauric acid (diester) 19
24 Boxplot results of WPG, WPAL, and PL 21
1
INTRODUCTION
Background
Lately, many studies aiming to create biobased, environmentally friendly, and non-toxic green wood preservatives have been conducted. This comes as a result of environmental issues and regulations pushing the development of new technologies to create new sustainable wood preservative which also reduces the utilization of biocide (Schultz et al. 2007; González-Laredo et al. 2015; Pizzi 2016). Not only for that, said studies also focused on creating biobased and green chemistry polymers, one of them was the effort to develop non-isocyanate based polyurethanes (NIPUs) by minimizing the utilization of isocyanates in polyurethane synthesis to reduce the severe toxicity issues (Zhenhua and Dong 2007; Maisonneuve et al. 2015; Datta and Włoch 2016). This is related to the production of conventional polyurethanes which are prepared through a polyaddi-tion reacpolyaddi-tion of isocyanates and polyols that remained a major concern as it in-volved generally the use of toxic petrochemical diisocyanates (Bayer 1947).
The chemical modification of biosourced polyphenols is interesting for its environmentally friendly traits (Peng et al. 2013; Pizzi 2016; Delgado-Sanchez 2016, Rangel et al. 2016). Catechin, a flavonoid-type compound, is an interesting molecule to be studied for its natural hydrophilic antioxidant characteristics (Ad-itya et al. 2015). It has been determined that this molecule has a powerful scaven-ger concerning to its antioxidant properties which seems to be related to its chem-ical structure, particularly with the presence of the catechol moiety on ring B and the presence of a hydroxyl group activating the double bond on ring C (Gadkari and Balaraman 2015). In the context of said issues, polyphenols such as catechin have the potential to be developed as a main compound in the synthesis of isocyanate-free polyurethane (Nouailhas et al. 2011; Aouf et al. 2013; Arbenz and Averous 2015) and its potency as a wood preservative (Malterud et al. 1985; Laks
et al. 1988).
The idea of utilizing catechin as a main compound in the synthesis of isocyanate-free polyurethane came from the work by Benyahya et al. (2013) and Thébault et al. (2014). In their study, natural polyphenols were used to replace bisphenol A, the most common phenol derivative used in epoxy resin formula-tions (Benyahya et al. 2013); and to replace isocyanate, the main compound in the making of polyurethane (Thébault et al. 2014). The methods used by Benyahya et al. (2013) led to the modification of catechin towards the synthesis of epoxide through glycidilation process. Additionally, the methods used by Thébault et al.
2
involved four steps: the glycidylation of catechin, the opening of epoxide, the carbonate synthesis which was conducted in order to obtain carbonate cyclic from the diol; and then the condensation of amine on this cyclic carbonate. This last step has been tested in this study by condensation of simple amine. In the future, if the synthesis of polyurethane has been successfully done, this technic can be develop in order to find the non biocidal alternative in the field of wood preservation.
Meanwhile as a wood preservative, studies conducted by Harun and Labosky (2007); Kawamura et al. (2010); and Vek et al. (2013) reported that cat-echin can be applied in the field of wood preservation due to its antimicrobial and antifungal properties. Moreover, this type of flavonoid is also proven as a valuable biomarker of wood decay (Mounguengui et al. 2007). The chemistry of flavo-noids is dominated by the presence of multiple phenol groups which furthermore give them strong complexing abilities and antioxidant properties. Their antifungal trait can be explained by the process of depolymerization of wood macromolecules that can be initiated by free radicals which is involving many oxidation process. In contrary, fungicidal action, free radical scavenging/ antioxi-dant, and metal chelating, as the properties of phenols can effectively protect the wood (Malterud et al. 1985; Schultz et al. 2008).
In the field of wood preservation, it is unlikely to find a single compound or natural antifungal to inhibit the various biological agents capable of colonizing wood and wood products. Previous study by Malterud et al. (1985) also reported that the effect of flavonoids on rot-producing fungi, particularly the catechin compound, is selective (active against only one of the fungi tested). In this context, it would be seen necessary to selectively changing its properties, for example through chemical modification, in order to increase the range of potential utility. Modification with compounds such as fatty acids, for example, through esterifica-tion or formaesterifica-tion of an ester linkage (Figure 1) could be interesting to study.
3 that low molecular weight, aliphatic fatty acids such as pentanoic (C5) to decanoic acids (C10) are highly effective against a wide range of fungal pathogens. These compounds have the potential to be used as environmentally friendly antifungal agents. Moreover, other study had been conducted in order to develop fungicide formulation through the combination of low molecular weight fatty acids with se-lected adjuvants such as organic acids or other natural compounds with more re-cent work applied to wood preservation (Clausen et al. 2010).
Beech wood (Fagus sylvatica L.) is one of the commercially most im-portant tree species in Europe that has been widely used in the furniture, plywood, particleboard, and bentwood industry (Gryc et al. 2008). Its natural durability is low (class 5) with high permeability and a low dimensional stability properties according to EN 350-2 standard (1994). According to its properties, this wood is also suitable for impregnation treatment due to its high treatability. The aim of this study was to chemically modify the catechin’s hydroxy groups with the fatty acid chains and to investigate the possibility of its utilization in wood preservation. Different parameters such as weight percent gain after impregnation, resistance to leaching, percentage of leaching, and decay resistance to the white rot fungus
Coriolus versicolor were investigated to evaluate the efficacy of the modified cat-echin as a wood preservative.
Formulation
Catechin is one of the naturally-occurred polyphenols possessing powerful scavanger related to its antioxidant trait (Aditya et al. 2015; Gadkari and Balara-man 2015). By selectively changing its properties through chemical modification, for example through uretahane synthesis with epoxy resin and through esterification with several fatty acids, the range of its potential utility will increase. Several studies have been conducted in modifying polyphenols compound both as a main compound in the synthesis of isocyanate-free polyurethane and as a wood preservative (Malterud et al. 1985; Laks et al. 1988; Thébault et al. 2014; Thébault et al. 2015). However, only a few of them have focused on the catechin modification. Therefore, the questions that need to be asked: what is the best method in syntesyzing catechin with epoxy resin to form urethane or polyurethane without using isocyanate? How about the fatty acids in the context of wood preservation? How would the resulted products’characteristics impact the wood properties, particularly to their resistance of leachability and against fungi? How much of those impacts are significant?
Objective
4
Benefits
The study is expected to provide scientific informations, original data, and statistical analysis on the methods of modifying the catechin’s hydroxy groups and its derived products. Further, the results of this study could become a reference on the methods and providing supporting data for the future study in the field of polyphenols modification and its application, particularly in the making of isocyanate-free polyurethane and in the development of natural wood preservative.
2
MATERIALS AND METHODS
Location and Period of Research
This study was conducted at the Laboratory of Wood Material Research and Study (Laboratoire d’Etudes et de Recherche sur le Matériau Bois) in Nancy, France from February to July 2016.
Tools and Materials
(+)-Catechin hydrate, epichlorohydrin (99.0%), benzyltriethylammonium chloride (≥98.0%), sodium hydroxide (≥98.0%), potassium carbonate (K2CO3),
butylamine, capric acid, myristic acid, lauric acid, and all reactants were obtained from Sigma–Aldrich Chimie, France.
Synthesis Procedures
Reaction of Catechin and Epoxide
Procedure of glycidilation of catechin
A 100-mL two-necked flask equipped with a condenser, a septum cap, and a magnetic stirring bar was charged with 1 g of catechin (3.445 mmol). Catechin was mixed in epichlorohydrin (5 Mequiv./OH), heated under a reflux condenser at 100°C, then benzyltriethylammonium chloride (0.05Mequiv./substrate) was added. After one hour, the resulting solution was cooled down to 30°C and an aqueous solution of NaOH 20 wt% (2 Mequiv./OH) with 0.05 Mequiv. of phase transfer catalyst (BnEt3NCl) was added drop by drop to prevent a massive precipitation of
the reaction mixture. The mixture was stirred vigourously for 90 minutes. The organic layer was separated, dried over MgSO4, and vacuum-concentrated.
Procedure of hydrolysis of epoxide (C27H30O10) in hot water
In a 100-mL round-bottom flask equipped with a condenser, C27H30O10
(1.03 g, 2 mmol) was added to 12 mL of distilled water; then, 6 mL of tetrahydrofuran (THF) was added. The solution was stirred at the temperature of 80°C and monitored using thin-layer chromatography (TLC). Generally, by increasing the heating temperature to T=80°C, the reaction of water, C27H30O10,
evapo-5 rator to remove the organic solvent (THF). The remaining water was then removed using a freeze dryer.
Procedure of carbonate synthesis from the diol derivative (C27H38O14)
In a 100-mL three-neck flask fitted with a condenser and a CaCl2 guard
tube, C27H38O14 was stirred with 24 eq. of dimethyl carbonate (3.64 mL, 43.18
mmol) and 0.03 eq. of potassium carbonate (K2CO3) (0.07 g, 0.054 mmol). The
mixture was then refluxed at T=80°C for 24 hours. The excess of dimethyl carbonate was then concentrated under reduced pressure.
Procedure of one-pot carbamate synthesis from C31H30O18
In a 100-mL three-neck flask fitted with a condenser and a CaCl2 guard
tube, butyl amine and C31H30O18 were stirred, and then 5 to10 mL methanol was
added to lower the viscosity of the reaction mixture. The reaction medium was subsequently, vigorously stirred using magnetic stirring and heated at 80°C for 8 hours. After completion, methanol was removed using a rotary evaporator. The progress of the reaction was monitored by Fourier transform infrared (FTIR) analysis. The reaction mixture was then extracted using 150 mL of ethyl acetate; this organic phase was then washed with 2×35 mL of HCl 1N and 35 mL of a saturated NaCl solution before being dried over using MgSO4, filtered and then
concentrated under reduced pressure.
Nuclear Magnetic Resonance (NMR) analysis
The products were identified using NMR performed on a Bruker AC-200 MHz and 400 MHz. All samples were dissolved either in methanol-d4 or in chloroform-d or in DMSO-d6 for 1H NMR analysis. Chemical shifts were expressed in parts per million (ppm) with reference to the solvent peak.
Fourier Transform Infrared (FTIR) analysis
FTIR spectra were recorded on a Perkin Elmer Spectrum One FT-IR Spectrometer with transmittance mode. The spectra were obtained by 4 scans/analysis, 4 cm-1 resolution, data intervals of 1 cm-1, and 0.2 cm/s sweeping speed. Start scan from 4000 cm-1, end scan 650 cm-1.Double beam method was used.
Grafting of Catechin and Fatty Acid
Procedure for reaction of catechin and capric acid (C10)
A 100-mL two-necked flask equipped with a condenser, a septum cap and a magnetic stirring bar was charged with 1 g of catechin. Catechin, capric acid 1.5 eq., and N,N'-Dicyclohexylcarbodiimide (DCCI) 1.5 eq. were dissolved in ace-tonitrile. The reaction was carried out with agitation at ambient temperature for 5 hours. Product was then extracted with ethyl acetate, then washed with a saturated NaHCO3 solution, a saturated NaCl solution, and then dried over MgSO4. After
6
Procedure for reaction of catechin and myristic acid (C14)
A 100-mL two-necked flask equipped with a condenser, a septum cap and a magnetic stirring bar was charged with 1 g of catechin. Catechin, myristic acid 1.5 eq., and DCCI 1.5 eq. were dissolved in acetonitrile. The reaction was carried out with agitation at ambient temperature for 5 hours. The product was then washed with ethyl acetate, a saturated NaHCO3 solution, a saturated NaCl solution,
and then dried over MgSO4. After completion, the products were separated using
column chromatography (acetate ethyl:hexane/50:50).
Procedure for reaction of catechin and lauric acid (C12)
Catechin, lauric acid 1 eq., and DCCI 1 eq. were dissolved in acetonitrile. The reaction was carried out in an ice bath with magnetic stirring during 20 minutes. Soon after, the ice was removed, and the reaction was continued under ambient temperature during 48 hours under N2 condition. After completion, quick
filtration under vacuum was conducted and produced yellow solid after evapora-tion. The product was then washed with ethyl acetate, a saturated NH4Cl solution,
a saturated NaHCO3 solution, a saturated NaCl solution, and then dried over
MgSO4. The solution then concentrated under reduced pressure and purified using
column chromatography (cyclohexane:ethyl acetat/8:2).
Spectroscopic characteristics
The resulting products were identified using Nuclear Magnetic Resonance (NMR) both 1H NMR and 13C NMR, performed on a Bruker AC-200 MHz and 400 MHz; and Fourier Transform Infrared FTIR spectra which were recorded on a Perkin Elmer Spectrum One FT-IR Spectrometer.
Wood Treatment and Measurement
Sample preparation
Ninety-six of wood specimens from beech wood (Fagus sylvatica L.) were cut into dimensions of 2.5 cm × 1.5 cm × 0.5 cm (L × R × T). 64 specimens were used for wood treatment, and 31 specimens were used as a control in the decay fungi test. All of the wood specimens were oven dried at 103±2°C for 48 hours (m0).
Wood impregnation
Wood specimens of 2.5 cm × 1.5 cm × 0.5 cm were oven-dried at 103±2°C for 48 hours and weighed (m0). Dried wood specimens (16 specimens
7
Figure 2 Illustration of vacuum impregnation of the wood specimens using desic-cator
Weight percent gain
After their mass were stable, all wood specimens were then weighed (m1)
and their weight percentage gain (WPG) were calculated following this equation: WPG (%) = (m1-m0)/m0×100 (equation 1)
where:
WPG = weight percent gain of the wood specimen after the impregnation m0 = initial oven-dried weight wood specimen before the impregnation
m1 = oven-dried weight of wood specimen after the impregnation Leachability
Leaching process was evaluated according to the European standard ENV 1250-2 (1994). Eight wood specimens from each treatment methods were immersed in 90 mL distilled water and subjected to six leaching periods of increasing duration under continous shaking at 20°C (Figure 3). In the first period, the specimens were shaken for 1, 2, and 4 hours, and in each periods, the water were replaced. Afterwards, the specimens were removed and kept without water for 16 hours. Leaching process was then continued to the next periods for 8, 16, and 48 hours. After all the leaching periods completed, all the wood specimens were oven dried at 103±2°C for about 24 hours and their mass were measured (m2). Weight percentage gain after leaching (WPAL) and percentage of mass loss
after leaching (PL) were calculated following this equation:
WPAL (%) = (m2 – m0)/m0× 100 (equation 2)
PL (%) = (m1 – m2)/m1× 100 (equation 3)
where:
WPAL = weight percentage gain after leaching
PL = mass loss percentage of the wood specimen after leaching
m0 = initial oven-dried weight of the wood specimen before the impregnation
Vacuum hose
Input hose for the impregnation solution
Desiccator
8
m1 = oven-dried weight of the wood specimen after the impregnation
m2 = oven-dried weight of wood specimen after leaching
Figure 3 Leaching process under continuous shaking method based on ENV 1250-2
Decay measurement
Untreated and treated wood specimens were exposed to the white rot fun-gus C. versicolor according to the EN 113 standard (1986). Sterile culture medi-um (20 mL), prepared from malt (25 g) and agar (40 g) in distilled water (1.0 L), were placed in a 9 cm petri dish, inoculated with fungus and cultivated in an incu-bator at 22±2°C of temperature and 70±5% of relative humidity for approximately 15 days to allow the colonization of the petri dish surfaces by the mycelium. After being sterilized at the temperature of 110°C for 20 min, all wood specimens in-cluding untreated wood as a control and wood specimens before and after leach-ing were put in the petri dish and incubated for 12 weeks. After this period, myce-lia were cleaned from the wood specimen and then oven dried at 103±2°C for 24 hours and weighed. Decay resistance was measured by the weight loss due to the fungal attack according to the following equation:
WL (%) = (ma– mb)/ma× 100 (equation 4)
where:
WL = percentage of weight loss
ma = dried weight of the wood specimen before being exposed to fungi
mb = dried weight of the wood specimen after being exposed to fungi
After obtaining the percentage of weight loss, durability class (DC) of each treatment then calculated and classified based on their durability index ―x‖ (Table 1) according to EN 350-1 (1994).
Bottle filled with water and wood specimens
9 Table 1 Classes of natural durability of wood to fungal attack using laboratory test
based on EN 113
Durability class (DC) Description Results expressed in X* value
1 Very durable x ≤ 0.15
2 Durable x > 0.15 but ≤ 0.30
3 Moderately durable x > 0.30 but ≤ 0.60 4 Slightly durable x > 0.60 but ≤ 0.90
5 Not durable x > 0.90
*x is the durability index expressed as weight loss of the test specimens / weight loss of the reference specimens.
Data Analyzing
10
3
RESULTS AND DISCUSSIONS
Reaction of Catechin and Epoxide
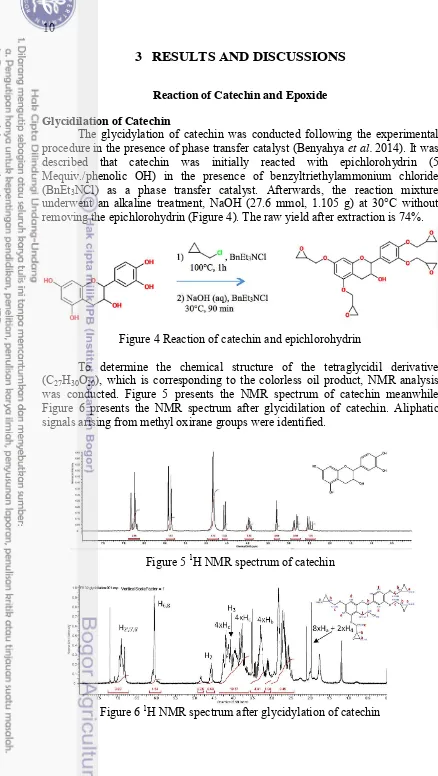
Glycidilation of Catechin
The glycidylation of catechin was conducted following the experimental procedure in the presence of phase transfer catalyst (Benyahya et al. 2014). It was described that catechin was initially reacted with epichlorohydrin (5 Mequiv./phenolic OH) in the presence of benzyltriethylammonium chloride (BnEt3NCl) as a phase transfer catalyst. Afterwards, the reaction mixture
underwent an alkaline treatment, NaOH (27.6 mmol, 1.105 g) at 30°C without removing the epichlorohydrin (Figure 4). The raw yield after extraction is 74%.
Figure 4 Reaction of catechin and epichlorohydrin
To determine the chemical structure of the tetraglycidil derivative (C27H30O10), which is corresponding to the colorless oil product, NMR analysis
was conducted. Figure 5 presents the NMR spectrum of catechin meanwhile Figure 6 presents the NMR spectrum after glycidilation of catechin. Aliphatic signals arising from methyl oxirane groups were identified.
Figure 5 1H NMR spectrum of catechin
11 Indeed Ha protons appeared to overlap with H4 protons belonging to the
flavonoid skeleton as a multiplet between 2.75 and 2.95 ppm. The multiplet be-tween 3.20 and 3.40 ppm corresponded to Hb protons. The two signals at 3.68–
3.85 ppm and 4.05–4.35 ppm both corresponded to Hc protons. The methylene
and methyne ring proton signals appeared in the 2.73–4.65 ppm range and the CH2-O protons gave resonances signals in the 3.92–4.65 ppm spectral range.The
chemical modification of the catechin structure also influenced the H6 and H8
sig-nals that almost merge with each other around 6.03 ppm.
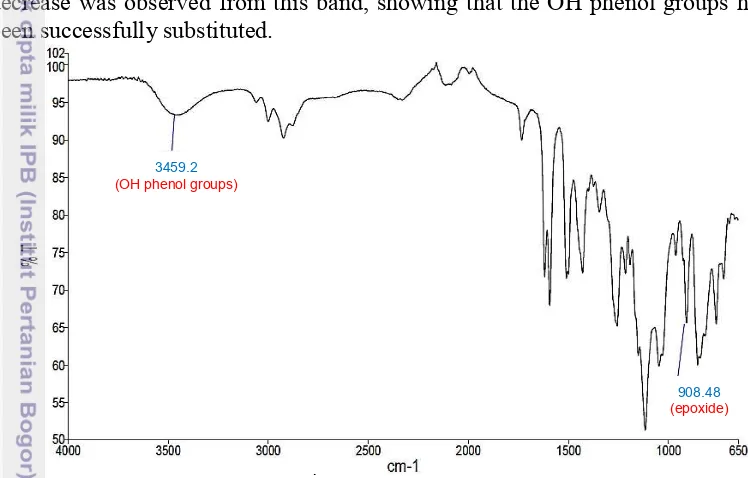
Figure 7 shows the evidence of epoxide formation at 908.5 cm-1 presented by FTIR measurements. This peak is assignable to the vibration of epoxy groups. The comparison between before and after glycidilation can also be seen here which the intensity of the stretching vibration bands of alcohol (phenol) groups in the 3000—3500 cm-1 area differ. In the (tetra)glycidylated product, a drastic decrease was observed from this band, showing that the OH phenol groups have been successfully substituted.
Figure 7 FTIR transmissionspectrum after glycidilation of catechin
Hydrolysis of Epoxide in Hot Water
Hydrolysis of epoxide was conducted by heating the crude product in hot water at a temperature between 60 and 80°C for 16 hours, in order to open the epoxide ring (Figure 8). Wang et al. (2008) reported that the ring opening reactions of epoxides can be promoted using hot water with a good yield. It was proposed that the hot water acts as a modest acid catalyst. In this study, THF was added to the mixture to increase the solubility in the water. The final product from this reaction corresponded to a colorless oil product.
Figure 8 Hydrolysis of epoxide
908.48 (epoxide) 3459.2
12
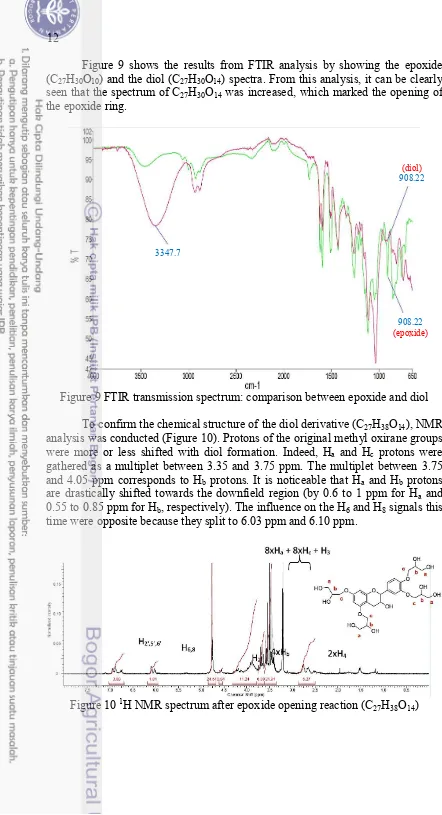
Figure 9 shows the results from FTIR analysis by showing the epoxide (C27H30O10) and the diol (C27H30O14) spectra. From this analysis, it can be clearly
seen that the spectrum of C27H30O14 was increased, which marked the opening of
the epoxide ring.
Figure 9 FTIR transmission spectrum: comparison between epoxide and diol To confirm the chemical structure of the diol derivative (C27H38O14), NMR
analysis was conducted (Figure 10). Protons of the original methyl oxirane groups were more or less shifted with diol formation. Indeed, Ha and Hc protons were
gathered as a multiplet between 3.35 and 3.75 ppm. The multiplet between 3.75 and 4.05 ppm corresponds to Hb protons. It is noticeable that Ha and Hb protons
are drastically shifted towards the downfield region (by 0.6 to 1 ppm for Ha and
0.55 to 0.85 ppm for Hb, respectively). The influence on the H6 and H8 signals this
time were opposite because they split to 6.03 ppm and 6.10 ppm.
Figure 10 1H NMR spectrum after epoxide opening reaction (C27H38O14) 3347.7
(diol) 908.22
13
Carbonate Synthesis
Latest studies reported that carbonation can be implemented from reaction with dimethyl carbonate (Panchgalle et al. 2011, Thébault et al. 2015). Carbonate synthesis was conducted in order to obtain carbonate cyclic (C31H30O18), which
afterwards, could be reacted with amines to provide carbamate function. The reaction with dimethyl carbonate can be seen in Figure 11 which was carried out by heating at 80°C for 24 hours. Dimethyl carbonate was used here both as a solvent and as a reagent, but because its solvating properties proved to be poor in this case, the amount of dimethyl carbonate was increased up to 24 eq.
Figure 11 Synthesis of cyclic carbonate derivatives (C31H30O18)
The expected product after carbonation shows up typically at 1797 to 1800 cm-1 on infrared spectra. According to the infrared analysis presented in Figure 12, it can be seen that the carbonate cyclic formation was marked by the signal that appeared at 1793 cm-1. The decrease of the alcohol wide band at 3000 to 3500 cm
-1
shows that the diol moieties reacted well. The signal at 1748.5 cm-1 may be due to some residual (or excess) of the dimethyl carbonate. The total raw yield after carbonation was 69%.
Figure 12 FTIR transmissionspectrum after carbonation
3445.6
Phenol
O-H stretch
Carbonate cyclic
C=O stretch 1792.7
1748.5
Carbonate non cyclic
14
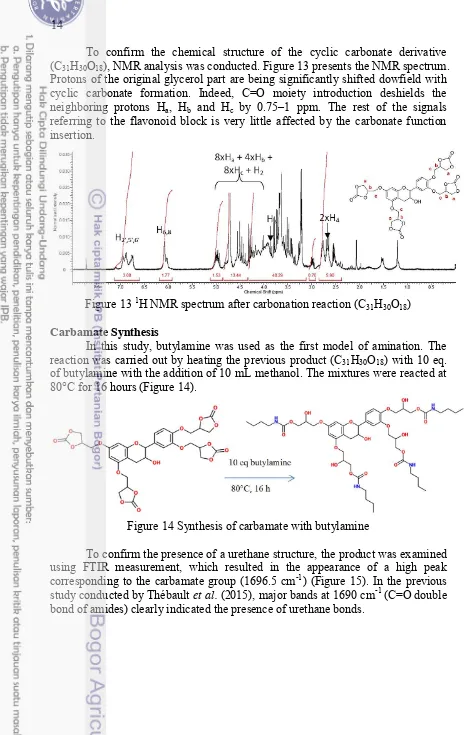
To confirm the chemical structure of the cyclic carbonate derivative (C31H30O18), NMR analysis was conducted. Figure 13 presents the NMR spectrum.
Protons of the original glycerol part are being significantly shifted dowfield with cyclic carbonate formation. Indeed, C=O moiety introduction deshields the neighboring protons Ha, Hb and Hc by 0.75–1 ppm. The rest of the signals
referring to the flavonoid block is very little affected by the carbonate function insertion.
Figure 13 1H NMR spectrum after carbonation reaction (C31H30O18) Carbamate Synthesis
In this study, butylamine was used as the first model of amination. The reaction was carried out by heating the previous product (C31H30O18) with 10 eq.
of butylamine with the addition of 10 mL methanol. The mixtures were reacted at 80°C for 16 hours (Figure 14).
Figure 14 Synthesis of carbamate with butylamine
15
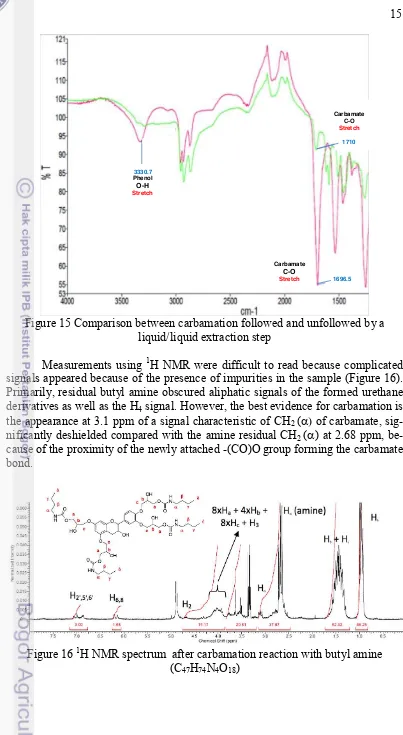
Figure 15 Comparison between carbamation followed and unfollowed by a liquid/liquid extraction step
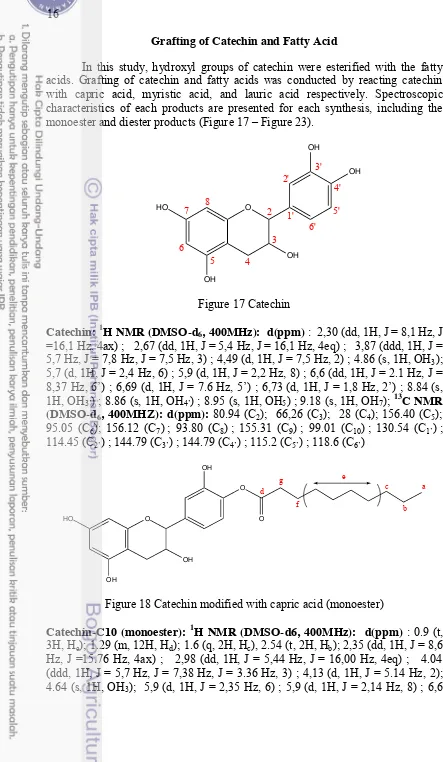
Measurements using 1H NMR were difficult to read because complicated signals appeared because of the presence of impurities in the sample (Figure 16). Primarily, residual butyl amine obscured aliphatic signals of the formed urethane derivatives as well as the H4 signal. However, the best evidence for carbamation is
the appearance at 3.1 ppm of a signal characteristic of CH2 (D of carbamate,
sig-nificantly deshielded compared with the amine residual CH2 (D) at 2.68 ppm,
be-cause of the proximity of the newly attached -(CO)O group forming the carbamate bond.
Figure 16 1H NMR spectrum after carbamation reaction with butyl amine (C47H74N4O18)
3330.7
Phenol
O-H Stretch
Carbamate C-O
Stretch
1710
Carbamate
C-O
16
Grafting of Catechin and Fatty Acid
In this study, hydroxyl groups of catechin were esterified with the fatty acids. Grafting of catechin and fatty acids was conducted by reacting catechin with capric acid, myristic acid, and lauric acid respectively. Spectroscopic characteristics of each products are presented for each synthesis, including the monoester and diester products (Figure 17 – Figure 23).
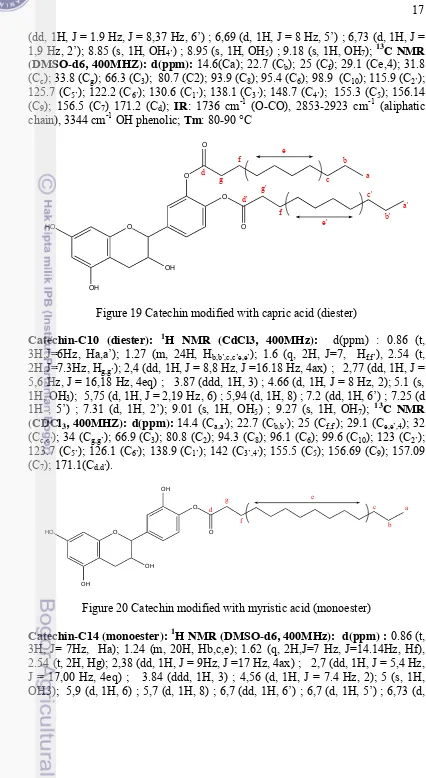
Figure 17 Catechin
Figure 18 Catechin modified with capric acid (monoester)
17
Figure 19 Catechin modified with capric acid (diester)
Catechin-C10 (diester): 1H NMR (CdCl3, 400MHz): d(ppm) : 0.86 (t,
Figure 20 Catechin modified with myristic acid (monoester)
18
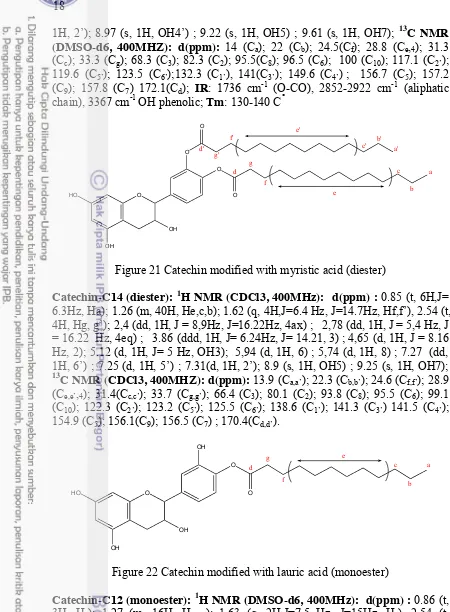
Figure 21 Catechin modified with myristic acid (diester)
Catechin-C14 (diester): 1H NMR (CDCl3, 400MHz): d(ppm) : 0.85 (t, 6H,J=
Figure 22 Catechin modified with lauric acid (monoester)
19
Figure 23 Catechin modified with lauric acid (diester)
Catechin-C12 (diester): 1H NMR (CDCl3, 400MHz): d(ppm) : 0.85 (t, 6H,J=
The reaction of catechin and capric acid was conducted by mixing catechin and capric acid in acetonitrile with the presence of DCCI. The reaction was conducted at ambient temperature with a magnetic stirrer during 5 hours. The resulting product was obtained by purification presenting 70% yield with solid form. The reaction between catechin and myristic acid was conducted as the same procedure with reaction catechin and capric acid, meanwhile reaction between catechin and lauric acid 1 eq., and DCCI 1 eq. were dissolved in acetonitrile and were carried out under a freezing condition with magnetic stirring during 20 minutes.
Modification of hydrophilic compounds such as catechin with hydrophobic compounds, including fatty acids, can result in products with an amphiphilic behavior. This trait will possess both hydrophilic and hydrophobic properties. This amphiphilic properties enable the hydrophobic part to control the release rate; in this case, it is believed that, inside the wood, these molecules would effectively control the release of the product while at the same time, the hydrophilic part form a stable suspension in water (Salma et al. 2010; Ding et al.
20
derivatives are able to self-assemble in the modification of chitosan chains (which are hydrophilic) with hydrophobic compounds.
The results corresponding to the yield of catechin modified with fatty acids are presented in Table 2. Generally, every treatment produces good yields, ranging from 65 to 70% of the monoester. The addition of C14 brought the highest yield, which produced 70% yield of the monoester and 8% of the diester. Monoester is formed when one molecule of acid has been added to the catechin while diester is formed when two of molecules of acid have been added instead of only one. The formation of diester is a secondary product, so it has a low yield which has no significance because the objective of adding them is to make the catechin as hydrophobic as it can be.
Table 2 Yield of catechin modified with fatty acids
Treatment Yield (%) and catechin modified with fatty acid in ethanol solution (w/w). The percentage of weight gain after impregnation (WPG), weight gain after leaching (WPAL), and percentage of leaching (PL) were presented in the Table 3. The statistical analysis were then conducted to determine the effects of each treatment.
Table 3 Percentage of weight gain after impregnation, weight gain after leaching, and percentage of leaching
21 that catechin compound modified with fatty acid will give the significant perfor-mance of weight gain compared to the treatment using catechin alone.
For WPAL, however, each treatment possesses results that are different from each other, which shows that each treatment has significant effects. The leaching test is required to ensure the fixation between the products inside the wood during its service use. Regarding the treatment A which has a negative val-ue of WPAL, it could be concluded that there are some wood components which are leached during the leaching process. It is possible that these components are water soluble extractive compounds. The results show that treating catechin with myristic acid produces the highest WPAL compared to others. It can be under-stood because this type of fatty acid has the longest chain which represents to hy-drophobic properties which means it is more resistant to water. This also con-firmed that the increase of hydrophobicity with longer chain length may reduce their solubility in aqueous systems (Coleman et al. 2010).
As has been said before, the modified product from catechin and fatty acid possess amphiphilic properties. It contrasts with the catechin-only treatment: its compound does not possess amphiphilic properties but instead only the hydro-philic ones. This is the reason why the catechin-only treatment did not produce good results through the leaching process. Catechin is a polar compound; it leach-es easily during the leaching procleach-ess. It could be possible that it fill the wood pores completely through their hydrogen bonding with wood but it was not a sta-ble fix. Amphiphilic properties enasta-ble them to be fixated more strongly in wood.
Small leaching values indicate that the bonding strength between wood and product were good than others, even though it was leached. The final results, PL, show that while specimens A produce high PL, the specimens that are treated with catechin and some fatty acids are much lower, with specimens C possess sig-nificantly lowest leaching percentage. This concludes that specimens C, which was treated with catechin and myristic acid, lost the lowest amount of preserva-tives after the leaching. Thus, specimens C’s preservative will last longer against open air and atmosphere.
Figure 24 Boxplot results of WPG, WPAL, and PL
Decay measurement
22
treated with catechin and fatty acid. On Figure 25, it can also be observed that there are seems to be no significant differences among the specimens treated with catechin with fatty acid, and between the leached and unleached specimens among them.
Figure 25 Boxplot results of weight loss for all treated samples
Explanation: A1: 5% catechin, leached; A2: 5% catechin, unleached; B1: 5% C10, leached; B2: 5% C10, unleached; C1: 5% C14, leached; C2: 5%
catechin-C14, unleached; D1: 5% catechin-C12 leached; D2: 5% catechin-C12 unleached
Table 4 P-value for T-test C10, leached; B2: 5% C10, unleached; C1: 5% C14, leached; C2: 5% catechin-C14, unleached; D1: 5% catechin-C12 leached; D2: 5% catechin-C12 unleached. Numbers written in bold means statistically significant difference between the paired sets of samples.
Moreover, in the Table 4—which shows the p-value for T-test—further analysis has been conducted and thus the statistical groupings of all the specimens have been calculated by determining whether or not two sets of data from each specimens are significantly different using T-test. The results can be seen, which presents the average weight loss of beech wood specimens treated with various kinds of treatments after being exposed to C. versicolor.
23 For untreated beech wood specimens (E), as were presented in the Table 5, the weight loss appears to be high, up to 51.492%. Statistically, it differs signifi-cantly compared to all the treated specimens, thus indicate the vigorous fungal activity of C. versicolor under test conditions. Meanwhile, the specimens treated with catechin alone especially after being leached (A1) was still given the poor weight loss up to 40.932%, and the treatment of catechin unleached (A2) pro-duced 37.062% of weight loss. This is related to the previous experiment conduct-ed by Lacks et al. (1988), whose catechin treatment was prepared using a full-cell method, gave a high percentage of weight loss up to 42% in 4% of concentration which, in their study, did not differ significantly from their controls. A different study conducted by Tascioglu et al. (2013) also showed the same trend, in which they used several commercial and environmentally friendly plant extracts such as Mimosa and Quebracho which gave a high percentage of weight loss in 3% and 6% of concentration (up to 37%-51% of weight loss). The high average value of weight loss on treatment A1 can be understood from its WPG which explains the possibility that catechin was washed off during the leaching process.
Table 5 Weight loss of beech wood specimens exposed to C. versicolor
Treatment Code Weight Loss (%) DC
Explanation: means within each column and factor followed by the same letter are not significantly different (p < 0.05).
For the treated wood, the modification of catechin with fatty acid increase their resistance against leaching process, it can be seen that the weight loss be-tween leached and unleached specimens do not differ significantly among them. Compared to each other, the specimens treated with catechin and C10 experience highest weight loss (30.249% and 30.193%), while specimens treated with cate-chin and C14 experience the lowest weight loss (29.621% and 28.795%) and specimens treated with catechin and C12 possess the weight loss of 30.778% and 28.766%. T-test results also show that the specimens treated with catechin and C14 stand out as a unique single letter group (―c‖), further show the significance of them as the specimens with the lowest weight loss.
24
relatively high, which was unacceptable according to EN 113 at values between 25 and 30% weight loss. The minimum weight loss required according to EN 113 is 20% for the fungal strain and its virulence on untreated wood control gave weight losses of more than 35%. However, this preliminary study can give some knowledge especially in the effect of leachability in order to study the environ-mental friendly wood preservative material that can be used for the outdoor appli-cation. This study shows that in 5% of concentration level, the treated wood with leaching still present a good weight loss result compare to the unleached ones. In the further study, it is highly recommended to increase the percentage of catechin modified concentration.
Figure 26. Decay resistance measurements of wood specimens Explanation: after 1 month of exposure (left) with leaching (right) without leaching; which
showed the vigorous fungal activity of C. versicolor under test conditions
25
4
CONCLUSIONS
Conclusion
The synthesis of carbamate using catechin has succeeded with a model amine (butylamine): the first through the fourth steps have been completed and, using FTIR, it can be seen that the carbamate (urethane) has been obtained from catechin.
Catechin modified with fatty acids can be used and developed due to their antifungal and hydrophobic properties. The specimens treated with catechin that has been modified with fatty acids produce a significant results on PL, with the specimen treated with catechin-C14 present best PL after the leaching compared to the others. It is believed that the addition of fatty acid, and the increasing length of its chain, will lead to the increase of resistance to leaching.
Suggestions
1. Further efforts should be conducted by extending the last step of this trans-formation to a diamine instead of butylamine to explore the trans-formation of pol-yurethanes.
26
REFERENCES
Aditya NP, Aditya S, Yang H, Kim HW, Park SO, Ko S. 2015. Co-delivery of hydrophobic curcumin and hydrophilic catechin by a water-in-oil-in-water double emulsion. Food Chem 173: 7–13. doi: 10.1016/j.foodchem.2014.09.131.
Aouf C, Le Guernevé C, Caillol S, Fulcrand H. 2013. Study of the O-glycidylation of natural phenolic compounds. The relationship between the phenolic structure and the reaction mechanism. Tetrahedron 69(4): 1345– 1353. doi: 10.1016/j.tet.2012.11.079.
Arbenz A, Avérous L. 2015. Chemical modification of tannins to elaborate aro-matic biobased macromolecular architectures. Green Chem 17(5): 2626– 2646. doi: 10.1039/C5GC00282F.
Bayer O. 1947. Das di-isocyanat-polyadditions verfahren (polyurethane). Angew. Chem 59: 257–272. doi : 10.1002/ange.19470590901.
Benyahya S, Aouf C, Caillol S, Boutevin B, Pascault JP, Fulcrand H. 2014. Func-tionalized green tea tannins as phenolic prepolymers for bio-based epoxy resins. Ind Crops Prod 53: 296–307. doi: 10.1016/j.indcrop.2013.12.045. Clausen CA, Coleman RD, Yang VW. 2010. Fatty acid–based formulations for
wood protection against mold and sapstain. For Prod J 60(3): 301–304. doi: 10.13073/0015-7473-60.3.301.
Coleman R, Yang V, Woodward B, Lebow P, Clausen C. 2010. Efficacy of fatty acid chemistry: candidate mold and decay fungicides, in: Proceedings One Hundred Sixth Annual Meeting of the American Wood Protection Associa-tion, Hyatt Regency Riverfront, Savannah, Georgia, May, Citeseer.
Datta J, Włoch M. 2016. Progress in non-isocyanate polyurethanes synthesized from cyclic carbonate intermediates and di- or polyamines in the context of structure–properties relationship and from an environmental point of view. Polym. Bull 73(5): 1459–1496. doi: 10.1007/s00289-015-1546-6. Delgado-Sanchez C, Letellier M, Fierro V, Chapuis H, Gérardin C, Pizzi A,
Cel-zard A. 2016. Hydrophobisation of tannin-based foams by covalent graft-ing of silanes. Ind Crops and Prod 92: 116–126. doi: 10.1016/j.indcrop.2016.08.002.
Ding X, Richter DL, Matuana LM, Heiden PA. 2011. Efficient one-pot synthesis and loading of self-assembled amphiphilic chitosan nanoparticles for low-leaching wood preservation. Carbohydr Polym 86(1): 58–64. doi: 10.1016/j.carbpol.2011.04.002.
[EN 113] European Committee for Standardization. 1986. Wood preservatives— determination of toxic values of wood preservatives against wood destroying basidiomycetes cultured on agar medium. NF EN 113. AFNOR. [ENV 1250-2] European Committee for Standardization. 1994. Wood
preservatives—methods for measuring losses of active ingredients and other preservative ingredients from treated timber—part 2: laboratory method for obtaining samples for analysis to measure losses by leaching into water or synthetic sea water. ENV 1250-2, Brussels, Belgium.
27 to the principles of testing and classification of the natural durability of wood. ENV 350-1, Brussels, Belgium.
[EN 350-2] European Committee for Standardization. 1994. Durability of wood and wood-based products—natural durability of solid wood— part 2: Guide to natural durability and treatability of selected wood species of importance in Europe. ENV 350-2, Brussels, Belgium.
Fray ME, Niemczyk A, Pabin-Szafko B. 2012. Chemical modification of chitosan and fatty acids. PCACD. 17:29-36.
Gadkari PV, Balaraman M. 2015. Catechins: sources, extraction and encapsulation: a review. Food Bioprod Process 93: 122–138. doi: 10.1016/j.fbp.2013.12.004.
González-Laredo RF, Rosales-Castro M, Rocha-Guzmán NE, Gallegos-Infante JA, Moreno-Jiménez MR, Karchesy JJ. 2015. Wood preservation using natural products. Madera y bosques 21: 63–76.
Gryc V, Vavrčík H, Gomola Š. 2008. Selected properties of European beech (Fagus sylvatica L.). J For Sci 54(9): 418–425.
Harun J, Labosky P. 2007. Antitermitic and antifungal properties of selected bark extractives. Wood Fiber Sci 17(3): 327–335.
Kawamura F, Ramle SFM, Sulaiman O, Hashim R, Ohara S. 2010. Antioxidant and antifungal activities of extracts from 15 selected hardwood species of Malaysian timber. Eur J Wood Wood Prod 69(2): 207–212. doi: 10.1007/s00107-010-0413-2.
Laks PE, McKaig PA, Hemingway RW. 1988. Flavonoid biocides: wood preserv-atives based on condensed tannins. Holzforschung 42(5): 299–306.
Maisonneuve L, Lamarzelle O, Rix E, Grau E, Cramail H. 2015. Isocyanate-free routes to polyurethanes and poly(hydroxy urethane)s. Chem. Rev 115(22): 12407–12439. doi: 10.1021/acs.chemrev.5b00355.
Malterud KE, Bremnes TE, Faegri A, Moe T, Dugstad EKS, Anthonsen T, Hen-riksen LM. 1985. Flavonoids from the wood of Salix caprea as inhibitors of wood-destroying fungi. J Nat Prod 48(4): 559–563. doi: 10.1021/np50040a007.
Mounguengui S, Dumarçay S, Gérardin P. 2007. Investigation on catechin as a beech wood decay biomarker. Int Biodeter Biodegrad 60(4): 238–244. doi: 10.1016/j.ibiod.2007.03.007.
Nouailhas H, Aouf C, Le Guerneve C, Caillol S, Boutevin B, Fulcrand H. 2011. Synthesis and properties of biobased epoxy resins. part 1. Glycidylation of flavonoids by epichlorohydrin. J Polym Sci Part Polym Chem 49(10): 2261–2270. doi: 10.1002/pola.24659.
Panchgalle SP, Kunte SS, Chavan SP, Kalkote UR. 2011. Exploration of l-proline-catalyzed α-aminoxylation of aldehyde to (s)-guaifenesin-related drug molecules. Synth Commun 41(13): 1938–1946. doi: 10.1080/00397911.2010.493631.
Peng Y, Zheng Z, Sun P, Wang X, Zhang T. 2013. Synthesis and characterization of polyphenol-based polyurethane. New J. Chem 37(3): 729. doi: 10.1039/c2nj41079f.
28
Pohl CH, Kock JL, Thibane VS. 2011. Antifungal free fatty acids: a review. Sci Microb Pathog Curr Res Technol Adv 1:61–71.
Rangel G, Chapuis H, Basso MC, Pizzi A, Delgado-Sanchez C, Fierro V, Celzard A, Gerardin-Charbonnier C. 2016. Improving water repellence and friabil-ity of tannin-furanic foams by oil-grafted flavonoid tannins. BioRes 11(3): 7754- 7768.
Sado-Kamdem SL, Vannini L, Guerzoni ME. 2009. Effect of α-linolenic, capric and lauric acid on the fatty acid biosynthesis in Staphylococcus aureus. Int J Food Microbiol 129(3): 288–294. doi: 10.1016/j.ijfoodmicro.2008.12.010.
Salma U, Chen N, Richter DL, Filson PB, Dawson-Andoh B, Matuana L, Heiden P. (2010). Amphiphilic core/shell nanoparticles to reduce biocide leaching from treated wood, 1 – leaching and biological efficacy. Macromol Mater Eng 295(5): 442–450. doi: 10.1002/mame.200900250.
Schultz TP, Nicholas DD, Preston AF. 2007. A brief review of the past, present and future of wood preservation. Pest Manag Sci 63(8): 784–788. doi: 10.1002/ps.1386.
Schultz TP, Nicholas DD, McIntyre CR. 2008. Recent patents and developments in biocidal wood protection systems for exterior applications. Recent Pat Mater Sci 1(2): 128–134.
Tascioglu C, Yalcin M, Sen S, Akcay C. 2013. Antifungal properties of some plant extracts used as wood preservatives. Int Biodeter Biodegrad 85: 23– 28. doi: 10.1016/j.ibiod.2013.06.004.
Thébault M, Pizzi A, Dumarçay S, Gerardin P, Fredon E, Delmotte L. 2014. Pol-yurethanes from hydrolysable tannins obtained without using isocyanates.
Ind Crops Prod 59: 329–336. doi: 10.1016/j.indcrop.2014.05.036.
Thébault M, Pizzi A, Essawy HA, Barhoum A, Van Assche G. 2015. Isocyanate free condensed tannin-based polyurethanes. Eur Polym J 67: 513–526. doi: 10.1016/j.eurpolymj.2014.10.022.
Vek V, Oven P, Poljanšek I. 2013. Quantitative HPLC analysis of catechin in wound- associated wood and knots of beech. Drv Ind 64(3): 231–238. doi: 10.5552/drind.2013.130.
Wang LL, Johnson EA. 1992. Inhibition of Listeria monocytogenes by fatty acids and monoglycerides. Appl Environ Microbiol 58(2): 624–629.
Wang Z, Cui YT, Xu ZB, Qu J. 2008. Hot water-promoted ring-opening of epox-ides and aziridines by water and other nucleopliles. J Org Chem 73(6): 2270–2274. doi: 10.1021/jo702401t.
29
CURRICULUM VITAE
Febrina Dellarose Boer was born on January 14, 1992 in Bogor, Indonesia, as the second daughter of four children from Mrs and Mr. Dirvamena Boer. She was attended high school in SMA Negeri 5 Bogor beginning in 2006, and in 2009, she took part in USMI (Undangan Seleksi Masuk IPB) in Bogor Agricultural University, then admitted to the Bachelor of Forestry program at Forest Products Department, Faculty of Forestry. During these bachelor years, she participated in many field and laboratory studies, became specialized in wood preservation against termite, and in consequence, wrote a Bachelor thesis in said subject. Graduated in 2013 as an honored student, she went on to work at Puter Indonesia Foundation, a non-profit organization aiming to improve the livelihood of rural people and the management of forestry in various Indonesian regions.
In 2014, she was awarded a scholarship from Indonesian Ministry of Education, Beasiswa Unggulan, to enroll at a double degree Master program held between Department of Forest Products, Bogor Agricultural University, and
Formation du Bois Forêt et Développement Durable at AgroParisTech in France. She took courses which deepen her understanding of wood preservatives, this time from the chemistry’s point of view. During her second year of Master (2016), she participated in a one-week Summer School of Wood Science at École Supérieure du Bois, Nantes, a French engineering school in wood science and technology. She also worked as an intern researcher at Laboratoire d’Étude de Matériaux Bois
(LERMaB), under the tutelage of Prof. Christine Gérardin (a professor and researcher at LERMaB and Université de Lorraine), for six months.
The results of her research during the aforementioned internship have been poured down into her Master thesis and two scientific articles, written with the partnership of Wissem Sahmim (a PhD student at LERMaB), Hubert Chapuis (a researcher and teacher at LERMaB), and two of her thesis advisors: Christine Gérardin and Dodi Nandika (a professor and researcher at Forest Products Department, Bogor Agricultural University). At the moment, one of two articles of this writing, entitled ―First Results for Feasibility Study of the Synthesis of Isocyanate-Free Polyurethanes from Flavan-3-ol‖ has been submitted to