THE SYSTEMATIC OF WILD BANANA SPECIES (
MUSA
L.)
IN SULAWESI: MORPHOLOGY AND MOLECULAR
STUDIES
LULUT DWI SULISTYANINGSIH
THE GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
LETTER OF STATEMENT
I express that thesis entitled:
THE SYSTEMATIC OF WILD BANANA SPECIES (MUSA L.) IN
SULAWESI: MORPHOLOGY AND MOLECULAR STUDIES
Is true represent result of my own research and have never been published. All information and data that used have been expressed clearly and can be checked its truth.
Bogor, April 2013
Lulut Dwi Sulistyaningsih
SUMMARY
LULUT DWI SULISTYANINGSIH. The systematic of wild banana species (Musa L.) in Sulawesi: morphology and molecular studies. Supervised by RITA MEGIA and ELIZABETH A. WIDJAJA
Information of wild banana species in Sulawesi was lack since there were no systematic study have been done before. Therefore, the systematic study of wild banana species (Musa L.) in Sulawesi was carried out. The study consist of (a) species delimitation of wild banana species (Musa L.) in Sulawesi to provide information of diversity and distribution of wild banana species in Sulawesi, and to provide species description and an identification key; (b) phylogenetic studies based on morphological characters and on internal transcribed spacer (ITS) regions of nrDNA sequences to determine the phylogenetic relationship between the species.
A total of 110 specimen consisted of old and new collections deposited in BO and digital type specimen from MEL were examined morphologically. Phylogenetic tree used fifty three morphological characters was performed by Maximum Parsimony (MP) method using PAUP*4.0b10. While phylogenetic tree reconstruction based on ITS regions sequences used MP and Bayesian (MrBayes ver. 3.0) methods. Ensete and Musella were selected as the outgroup, whereas the ingroup represented by Musa species obtained from species delimitation study. Total DNA was extracted from silica-gel dried leaves by the modified CTAB method. ITS-5 and ITS-4 primers were used to amplify the ITS regions. Data of sequences were edited by using ChromasPro programme and were aligned by Muscle software.
Species delimitation based on morphological characters showed 6 Musa and 1 incompletely known specimen housed in Sulawesi. There were 2 species (Musa acuminata var. tomentosa and M. celebica) considered as endemic to Sulawesi. The existence of M. balbisiana and M. itinerans are noted as new records. In Sulawesi, M. balbisiana represented by a specimen collected from Manado (North Sulawesi), while M. itinerans represented by specimens collected from Mt. Nokilalaki (Central Sulawesi), and Lore Lindu National Park (Central Sulawesi). Observation on the morphology of each species in this study enables the selection of important characters for species delimitation and identification. Position of suckers, petiole canal leaf, bract imbrications, bract behavior before falling, bract colour, fruit shape, and seed surface are important characters for delimiting and identifying taxa of wild banana species (Musa) in Sulawesi.
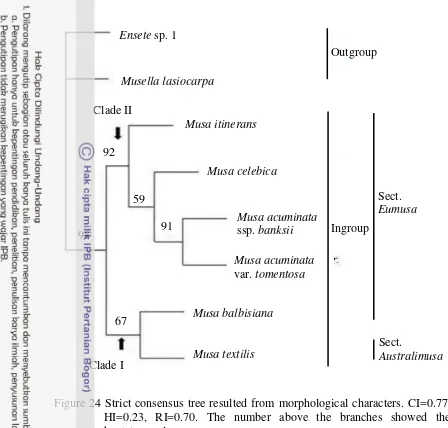
Phylogenetic tree used morphological characters showed M. balbisiana and
M. textilis placed in clade I in which they shared some similar characters for instance bract imbrication and behavior before falling. While Musa acuminata ssp.
banksii, M. acuminata var. tomentosa, M. celebica, and M. itinerans were placed in clade II. The phylogenetic study based on morphological characters is incongruent with the phylogenenetic study based on ITS regions of nrDNA sequences.
balbisiana, M. itinerans, M. celebica, M. acuminata ssp. banksii, and M. acuminata var. tomentosa. Nevertheless, M. textilis and M. balbisiana have closed relationship. By using MP analysis, M. itinerans together with M. acuminata ssp.
banksii, M. acuminata var. tomentosa and M. celebica were placed in the same clade, whereas Bayesian analysis showed M. itinerans was separated from M. acuminata ssp. banksii, M. acuminata var. tomentosa as well as M. celebica, and was placed in different clade.
This study revealed that phylogenetic study of wild banana species (Musa) in Sulawesi based on morphological characters and ITS regions of nrDNA sequences support the monophyly of genus Musa and also support the taxonomic status of M. acuminata ssp. banksii as intraspecific taxa of M. acuminata. Moreover, analysis of ITS regions also showed that sequences of ITS regions of nrDNA could be used for phylogenetic tree reconstruction at the species level.
RINGKASAN
LULUT DWI SULISTYANINGSIH. Sistematika pisang-pisang liar (Musa L.) di Sulawesi: kajian morfologi dan molekuler. Dibimbing oleh RITA MEGIA dan ELIZABETH A. WIDJAJA
Ketersediaan informasi mengenai pisang-pisang liar di Sulawesi masih sangat sedikit mengingat belum ada penelitian sistematika yang pernah dilakukan sebelumnya. Oleh karena itu, penelitian sistematika pisang-pisang liar (Musa L.) di Sulawesi ini dilakukan. Penelitian meliputi (a) kajian delimitasi jenis pisang-pisang liar (Musa L.) di Sulawesi untuk memberikan informasi keanekaragaman dan distribusi pisang-pisang liar di Sulawesi, serta menyajikan deskripsi jenis dan kunci identifikasi; (b) kajian filogenetik berdasarkan karakter morfologi dan urutan basa nrDNA daerah internal transcribed spacer (ITS) untuk melihat hubungan kekerabatan antar jenis.
Sebanyak 110 spesimen yang terdiri atas koleksi lama dan baru yang tersimpan di BO serta gambar digital spesimen tipe dari MEL diperiksa karakter morfologinya. Konstruksi pohon filogenetik menggunakan 53 karakter morfologi dianalisis dengan metode Maximum Parsimony (MP) menggunakan program PAUP*4.0b10. Sementara itu, konstruksi pohon filogenetik berdasarkan urutan basa daerah ITS menggunakan metode MP dan Bayesian (MrBayes ver. 3.0).
Ensete dan Musella dipilih sebagai outgroup sedangkan ingroup direpresentasikan oleh jenis-jenis Musa hasil kajian delimitasi jenis. DNA total diekstraksi dari daun yang tersimpan dalam gel silika menggunakan metode CTAB yang dimodifikasi. Primer ITS-5 dan ITS-4 digunakan untuk mengamplifikasi daerah ITS. Data urutan basa diedit dengan program ChromasPro dan disejajarkan dengan software Muscle.
Kajian delimitasi jenis berdasarkan karakter morfologi menunjukkan 6
Musa dan 1 jenis yang belum diketahui ditemukan di Sulawesi. Terdapat 2 jenis endemik Sulawesi, yaitu Musa acuminata var. tomentosa dan M. celebica. Ditemukannya M. balbisiana dan M. itinerans merupakan catatan baru. Di Sulawesi, keberadaan M. balbisiana direpresentasikan oleh spesimen yang dikoleksi dari Manado (Sulawesi Utara), sedangkan keberadaan M. itinerans
direpresentasikan oleh spesimen yang dikoleksi dari gunung Nokilalaki (Sulawesi Tengah) dan Taman Nasional Lore Lindu (Sulawesi Tengah). Dari hasil observasi karakter morfologi pada masing-masing jenis diperoleh karakter-karakter penting untuk memberikan batasan jenis dan identifikasi. Posisi tumbuh anakan, lekukan pada tangkai daun, imbrikata pada braktea, tipe braktea sebelum gugur, warna braktea, bentuk buah, dan permukaan biji merupakan karakter penting untuk memberi batasan dan identifikasi jenis pisang-pisang liar (Musa) di Sulawesi.
Konstruksi pohon filogenetik berdasarkan urutan basa nrDNA daerah ITS menggunakan metode MP dan Bayesian menunjukkan M. textilis berada pada klade I dan terpisah dari M. balbisiana, M. itinerans, M. celebica, M. acuminata
ssp. banksii, dan M. acuminata var. tomentosa. Meskipun demikian, M. textilis
berkerabat dekat secara filogenetik dengan M. balbisiana. Dengan menggunakan metode MP, M. itinerans bersama dengan M. acuminata ssp. banksii, M. acuminata var. tomentosa dan M. celebica berada pada klade yang sama, sedangkan metode Bayesian menunjukkan M. itinerans terpisah dari M. acuminata ssp. banksii, M. acuminata var. tomentosa dan M. celebica serta berada pada klade yang berbeda.
Penelitian ini menunjukkan bahwa kajian filogenetik pisang-pisang liar (Musa) di Sulawesi berdasarkan karakter morfologi dan urutan basa nrDNA daerah ITS mendukung sifat monofiletik dari marga Musa dan juga mendukung status taksonomi M. acuminata ssp. banksii sebagai infraspesifik taksa dari M. acuminata. Lebih jauh lagi, analisis daerah ITS juga menunjukkan bahwa urutan basa nrDNA daerah ITS dapat digunakan untuk rekonstruksi pohon filogenetik pada tingkat jenis.
Copyright©2013, Bogor Agricultural University Copyright are protected by law
1. It is prohibited to cite all parts of this thesis without referring to and mentioning the source
a. Citation only permitted for the sake of education, research, scientific writing, critical writing or reviewing scientific problems.
b. Citation does not inflict the name and honor of Bogor Agricultural University.
Thesis submitted
As partial fulfillment requirement for the Master Degree
In Plant Taxonomy
THE SYSTEMATIC OF WILD BANANA SPECIES (
MUSA
L.)
IN SULAWESI: MORPHOLOGY AND MOLECULAR
STUDIES
THE GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2013
Title : The Systematic of Wild Banana Species (Musa L.) in Sulawesi: Morphology and Molecular Studies
Name : Lulut Dwi Sulistyaningsih
NRP : G353100031
Certified by Supervisor Committee
Dr. Rita Megia, D.E.A. Chairman
Prof. (R.) Dr. Elizabeth A. Widjaja, M.Sc Member
Approved by Head of Plant Biology
Study Program
Dr. Ir. Miftahudin, M.Si
Dean of Graduate School
Dr. Ir. Dahrul Syah, MSc. Agr.
ACKNOWLEDGEMENT
It would not have possible to write this thesis without the help and support of many people around me, to only some of whom it is possible to give particular mention here.
First and foremost, I would like to express my gratitude to my supervisors, Dr. Rita Megia, D.E.A. and Prof. (R.) Dr. Elizabeth A. Widjaja, M.Sc. for their valuable advices, guidance, and encouragement throughout my study. Many thanks also goes to Dr. Nunik Sri Ariyanti, M.Si., Dr. Rugayah, M.Sc., Dr. Himmah Rustiami, M.Sc. for their suggestions, advices, and criticisms.
I gratefully acknowledge the Karyasiswa Ristek scholarship from Ministry of Research and Technology (RISTEK); DIPA from Division of Botany, Research Centre for Biology – LIPI with entitled Revision of Selected Taxa in Sulawesi for supporting field research; Molecular Systematic Laboratory, Division of Botany, Research Centre for Biology – LIPI for supporting sequencing fee. I would like to thank to Dr. Witjaksono, M.Sc., Dr. Yuyu Purba, M.Sc., Fajarudin Ahmad, S.Si. for their kindly help in DNA materials.
I would like to thank to all taxonomists and technicians of Herbarium Bogoriense who gave their support and assist when conducted this study. I also express my gratitude to my colleagues and friends in Research Centre for Biology
– LIPI and IPB.
Last but not the least, my special thanks go to my family, my parents, my husband Dhani Yudhana and my daughter Nafeeza Armaghan El-Firdausy for their spirit, patience, and support in my study.
Bogor, April 2013
TABLE OF CONTENTS
LIST OF TABLES vi
LIST OF FIGURES vi
LIST OF APPENDICES vii
1 INTRODUCTION 1
2 LITERATURE REVIEW 2
Taxonomy of Musa L. 2
General Morphology of Musa L. 3
Distribution of Musa L. 5
Internal Transcribed Spacer (ITS) 7
3 MATERIALS AND METHODS 9
Species Delimitation of Wild Banana Species (Musa L.) in Sulawesi
Materials 9
Methods 9
Phylogenetic Study of Wild Banana Species (Musa L.) in Sulawesi Based on Morphology Characters
Materials 9
Methods 11 Phylogenetic Study of Wild Banana Species (Musa L.) in Sulawesi
Based on Internal Transcribed Spacer Region of nrDNA Sequences
Materials 12 Methods
DNA Extraction and Amplification 13
DNA Purification and Sequencing 13
Phylogenetic Analysis 14
4 RESULT AND DISCUSSION 14 Species Delimitation of Wild Banana Species (Musa L.) in Sulawesi
General Morphology of Musa in Sulawesi
Pseudostems 14
Leaves 15
Inflorescences 15
Male Buds 15
Flowers 16
Fruits 16
Seeds 16
Distribution 16
Key to the Species and Infraspecific Taxa of Musa from Sulawesi
Key to the Species 17
Key for Infraspecific Taxa of Musa acuminata Colla 17
Taxonomic Treatment 18
Based on Morphology Characters 35 Phylogenetic Study of Wild Banana Species (Musa L.) in Sulawesi
Based on Internal Transcribed Spacer Region of nrDNA Sequences
DNA Extraction and Amplification 37
Nucleotide Sequences of ITS Region of nrDNA and Variation
Within Species 38
Phylogenetic Analysis 42
General Discussion 46
5 CONCLUSION 47
REFERENCES 48
APPENDICES 52
LIST OF TABLES
1 Morphological characters state used in phylogenetic analysis 10
2 Source of ITS sequence utilized in the study 12
3 Sequence length variation of ITS region within outgroup and ingroup 39
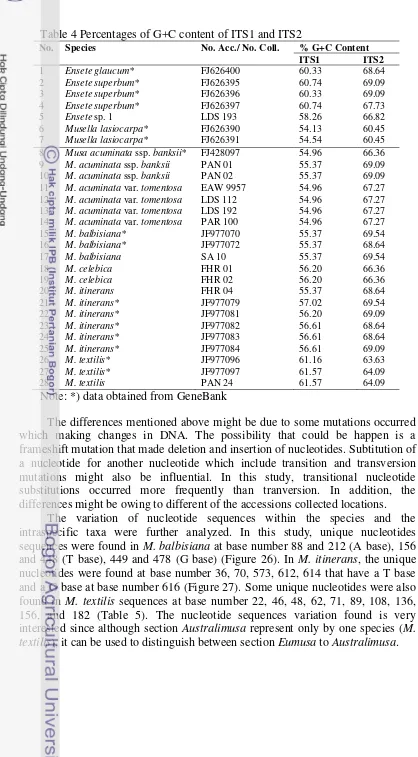
4 Percentages of G+C content of ITS1 and ITS2 40
5 Nucleotide sequences variation of ITS region within species showed unique nucleotides of species from section Eumusa and Australimusa 42
LIST OF FIGURES
1 Variation on leaf base 4
2 Variation on leaf canal margin 4
3 Variation on bract 5
4 Distribution map of Musa sections 6
5 Distribution map of Musa sections in Indonesia 6
6 Organization of ITS region of nrDNA 7
7 Taxonomic level of utility of nuclear DNA regions used in phylogenetic
reconstruction based on angiosperms 8
8 Leaf bases shapes 15
9 Bract imbrications on male bud 15
10 Shape of seeds 16
11 Distribution map of wild banana species in Sulawesi 17 12 Musa acuminata ssp. banksii (F. Muell.) N.W. Simmonds 19 13 Distribution map of M. acuminata ssp. banksii in Sulawesi 20 14 Distribution map of M. acuminata var. tomentosa in Sulawesi 22
15 Musa balbisiana Colla 24
16 Distribution map of M. balbisiana in Sulawesi 25 17 Musa celebica Warb. ex K. Schum. 27
18 Distribution map of M. celebica in Sulawesi 28
19 Musa itinerans Cheesman 30
20 Distribution map of M. itinerans 31
21 Musa textilis Née 32
22 Distribution map of M. textilis in Sulawesi 33
23 Distribution map of Musa sp. 1 in Sulawesi 34
24 Strict consensus tree resulted from morphological characters 36
25 Visualization of PCR product 38
26 Nucleotide sequences variation of ITS regions within species that showed some unique sequences of Musa balbisiana was compared with
other taxa 41
27 Nucleotide sequences variation of ITS regions within species that showed some unique sequences of Musa itinerans was compared with
other taxa 41
28 Strict consensus tree of the ITS sequence region resulting from MP
analysis 44
29 Strict consensus tree of the ITS sequence region resulting from
LIST OF APPENDICES
1 Matrix of morphological characters for phylogenetic analysis 52 2 The ITS region of nrDNA sequence alignment from position 272 to 373
showed part of the conserved region 53
3 The ITS region of nrDNA sequence alignment from position 485 to 575
1
INTRODUCTION
Bananas belong to Musaceae, a small family which consists of three genera,
Ensete Bruce ex Horan, Musa L., and Musella (Franchet) H.W. Li. In general, bananas (Musa L.) are grouped into wild seeded bananas that consist of approximately 70 species (Häkkinen 2008) and edible seedless bananas consisting of approximately 500 cultivars (Valmayor et al. 2002). Cultivated bananas have considerable economic value since they have a high level of consumption, whereas wild banana species have potential value as a genetic resource. Cultivated bananas have been largely evolved from two wild banana species, Musa acuminata Colla and M. balbisiana Colla. M. balbisiana is known to be resistant to drought, while the intraspecific of M. acuminata, namely M. acuminata var.
malaccensis is known to be resistant to fusarium wilt (Nasution 1991). Elucidating the phylogeny and taxonomy of Musa and its family is important as phylogenetic relationships can provide valuable information for the collection and utilization of genetic resources for further banana improvement.
Indonesia has a large number of bananas. This country is the center of bananas origin (Simmonds 1966) as well as of its diversity (Daniells et al. 2001). At least 325 cultivars have been recorded in Indonesia (Valmayor et al. 2002), whereas only 12 wild banana species has been documented (Nasution and Yamada 2001). Presumably, there are wild banana species that have not been recorded and well documented.
In Indonesia, wild banana species grow widespread in Sumatra, Java, Lesser Sunda Islands, Kalimantan, Sulawesi, Moluccas, and Papua. Biogeographycally, Sulawesi has a unique characteristic because it is located in the Wallace line which is the transition region between Asia and Australia. Sulawesi is also known to have a large number of endemic flora and fauna (Mittermier et al. 1999).
Musaceae that have been reported as an endemic flora in Sulawesi are M. celebica
Warb. ex K. Schum. and M. acuminata Colla var. tomentosa (K.Sch.) Nasution (Nasution 1991; Nasution and Yamada 2001).
As a taxa that can undergo self crossing, hybridization, and mutation, bananas has a complex genome structure. To analyze bananas genetic diversity, molecular characterization was required to support morphological characters. During the past decades, molecular markers such as RFLPs (Gawel and Jaret 1991), AFLP (Wong et al. 2002), HAT-RAPD (Ruangsuttapha et al. 2007), trnL-F (Liu et al. 2010; Li et al. 2010), and ITS (internal transcribed spacer) (Liu et al. 2010; Li et al. 2010; Hřibova et al. 2011) have been used for phylogenetic analysis of Musaceae. Nowadays, ITS is used more often by researchers to conduct a molecular phylogenetic analysis of plant in order to understand the diversity and to answer phylogenetic problems. This is because ITS region is easy to be isolated, amplified, and analyzed, due to its small size (300-800 bp) and high copy number in the genome (Baldwin et al. 1995).
specimens from Sulawesi that stored in BO have been identified. Therefore, systematic studies to reveal the Musaceae diversity in Sulawesi need to be done.
The aims of the study were 1) to provide information of the diversity and the distribution of wild banana species in Sulawesi, 2) to provide species description and an identification key and 3) to determine the phylogenetic relationship between the species using morphological characters and ITS regions of nrDNA sequences.
2
LITERATURE REVIEW
Taxonomy of Musa L.
APG III (Angiospermae Phylogeny Group) places genus Musa L., together with Ensete Bruce ex Horan and Musella (Franchet) H.W. Li into family
Musaceae, the member of Zingeberales (APG 2009). Genus Musa is the largest genus in the family that is firstly established by Linnaeus (1753). Some botanists believed that the name of genus Musa is thought to be derived from the Arabic name for the plant (mouz) which in turn may have been applied in honour of Antonius Musa who is physician to Octavius Agustus, the first emperor of Rome (Hyam and Pankhurst 1995). Whereas the name “banana” is derived from the Arabic banan that means finger (Boning 2006) and was thought to be used in Guinea (West Africa) concomitant with the introduction of the fruit by the Portuguese and then the name spread to the New World (Cheesman 1948a).
The taxonomic complexity at the family level continues down to the genus level and there are inconsistencies in the number of sections and number of species proposed for inclusion in the genus Musa. The first classification made by Sagot (1887) that divided the genus into three groups: (1) giant bananas (type: M. ensete J.F. Gmel.), (2) bananas with fleshy fruit and often edible (type: M. sapientum L.), (3) ornamental bananas with erect inflorescences and brightly coloured bracts (type: M. coccinea Andrews).
In 1893, Baker divided genus Musa into three subgenera: (1) Physocaulis, characterized by stem bottle-shaped; flowers many to a bract; petal usually tricuspidate; fruit not edible. (2) Eumusa, characterized by stem cylindrical; flower many to a bract; petal ovate-acuminate; bracts green, brown or dull violet; fruit edible. (3) Rhodoclamys, characterized by stem cylindrical; flower few to a bract, petal linier; bracts bright-coloured; fruit usually not edible.
Cheesman (1947) elevated the first subgenus to the generic level as the genus Ensete and then made new classification. The classification is based on the haploid number of the chromosome that followed by the similarity and the differences on morphological characters. He treated the genus into four sections as follows:F
A. Chromosome number x=11
purple. Pseudostems commonly exceeding three meters high
…...………Section Eumusa
2. Inflorescence erect, or at least at the base, so that the fruits do not reflex in development but point toward the apex of the rachis. Flowers few to a bract, usually in a single serie. Bract brightly coloured, often red.
Pseudostems less than three meters high…...………...
………...Section Rhodochlamys B. Chromosome number x=10
3. Seeds subglobose, or more or less dorsiventrally compressed, smooth, striate, tuberculate, or irregularly angulate with a marked or obsolete umbo opposite to the hilum corresponding to small periperm chamber
within………...Section Australimusa
4. Seeds cylindrical, barrel-shaped, or top shaped, marked externally by transverse line or grove, above which are warted, tuberculate, or variously patterned, below usually smooth; internally a well-developed perisperm, chamber above the same line, this chamber empty in the ripe
seed……….Section Callimusa
Although this classification was widely accepted by most botanists, its validity has been questioned, in any case for some sections. Some newly described species which is already count the chromosome number also appeared problematic. Argent (1976) proposed to establish one more section in Musa,
Ingentimusa (x=7) to include the single species M. ingens N.W. Simmonds. Based on morphological and cytological characters, Simmond and Weatherup (1990) found a very low level of consistency among the characters and suggested that section Eumusa is heterogeneous and then divided it into two informal subgroups
“Eumusa-1” and “Eumusa-2. Recently, molecular data have been used to solve such problems in taxonomy of Musa. By using RFLPs data, Gawel and Jaret (1991) and Gawel et al. (1992) found that a molecular phylogeny was inconsistent with traditional classification and they proposed that section Rhodochlamys
should be merged with section Eumusa. Wong et al. (2002), by using AFLP data suggested that section Rhodochlamys should be placed in section Eumusa, and section Australimusa should be merged in section Callimusa.
General Morphology of Musa L.
Musa is a large perennial herb and they grow in clump with rhizome and false areal stem, cylindrical pseudostem that consisted of sheath leaves wrapped together with each other, has a short underground stem (corm). The root system is adventitious spreading out laterally.
Figure 1 Variation on leaf base. Both sides rounded (a); one side rounded, one pointed (b); both sides pointed (IPGRI 1996)
Figure 2 Variation on leaf canal margin. Open with margins spreading (a); wide with erect margins (b); straight with erect margin (c); margins curved inward (d) (Nasution and Yamada 2001)
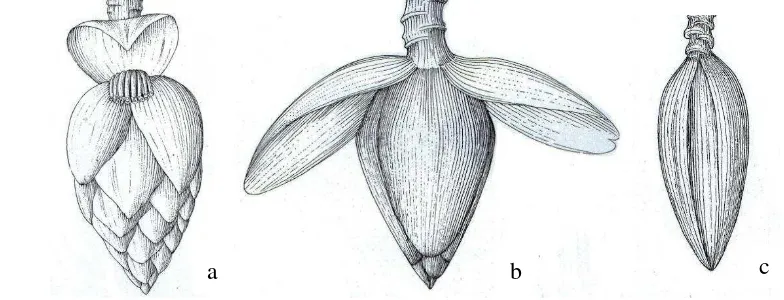
Inflorescence springs from the rhizome and emerges at the top of the stem, either erect or pendulous; the immature inflorescence is encased inside bracts that give the appearance of large bud. Bracts are plane or sulcate, revolute or not revolute before falling, imbricate or not imbricate (Figure 3). Flowers produce nectar, on each bract there is one or two rows of flower. Basal flower is female or hemaprodhite, consist of ovary that protected by compound tepal (calyx) and free tepal (corolla), style and staminode; compound tepal essentially tubular but split to the base on the adaxial side, 5 toothed at the apex (3-lobed at the apex with 2 accessory teeth between the main lobes; free tepal inserted within the compound tepal and opposite to it (i.e. in the adaxial position). Male flower have 5 fertile stamen that falling down with the bract. They are reduced to staminodes in female flower. Female and male flowers are morphologically indistinguishable until the inflorescence is about 12 cm long. At this point, the ovary in the male flower fails to develop any further (Simmonds 1959).
Figure 3 Variation on bract. Not imbricate (a); imbricate (b) (Nasution and Yamada 2001)
Fruits are berry, some dehiscent and some are not, with numerous seeds (except in the parthenocarpic form). Each fruit is known as a “finger”. Each
cluster of fruits at node is known as a “hand” and the entire collection of hands is known as a “bunch”. The number of hands varies each others. The outer
protective layer of each fruit known as the “skin” or “peel” is fusion of the hypanthium (floral receptacle) and outer layer (exocarp) of the pericarp (fruit wall derived from the ovary wall). This peel is easily removed from the fleshly pulp that originates mainly from the endocarp (innermost layer of the pericarp) (Simmonds 1953). During the development of the fruit from the ovary, the tepals, style, and staminodes abscise leaving a characteristic calloused scar at the tip of the fruit. Fruits develop only after pollination. Fruit size depends on the number of seeds and parenchymatous pulp develops around each seed. The growth volume curve is sigmoidial (Simmonds 1953). Wild banana species have little flash and is filled with black or brown seed. The seeds have linier embryos, large amounts of endosperm and a thick hard testa (Ellis et al. 1985).
Distribution of Musa L.
The section Australimusa is well-known in Brunei Darussalam, Indonesia, Malaysia, Papua New Guinea, and Philippines, while section Callimusa can be found in Brunei Darussalam, China, Cambodia, Indonesia, Malaysia, Papua New Guinea, and Vietnam. The biggest section, Eumusa is widespread in Australia, Bhutan, Cambodia, China, Eastern and SouthEast India, Indonesia, Japan, Laos, Malaysia, Papua New Guinea, Philippine, Samoa, Sri Lanka, Thailand, and Vietnam, wheread section Rhodochlamys grows well in Bangladesh, China, Malaysia, and Thailand (Figure 4).
Figure 4 Distribution map of Musa sections. Eumusa; Australimusa;
Callimusa; Rhodoclamys (Pollefeys et al. 2004)
Indonesia is situated in the centre of origin and diversity of Musaceae and has a large number of both wild banana species and cultivated bananas. The bananas widespread in Sumatra, Java, Lesser Sunda Islands, Borneo, Sulawesi, Moluccas, and Papua. Pollefeys et al. (2004) made distribution map of Musa
sections in Indonesia using MGIS (Musa Germplasm Information System) and DIVA-GIS. Almost all of the accessions that they used come from Nasution’s (1991) study and the rest come from Musalogue. Because of insufficient
geographical data for some of Nasution’s accessions, no entry is available in MGIS for Kalimantan (Borneo) although the expedition recorded wild species in all major islands (Figure 5).
Figure 5 Distribution map of Musa sections in Indonesia (Pollefeys et al. 2004) Legend
Australimusa Callimusa Eumusa
Rhodo- chlamys
Eumusa
Australimusa Callimusa
Pasific Ocean
Internal Transcribed Spacer (ITS)
The major ribosomal RNA (rRNA) genes of plants are localized in clusters on highly repeated sequences. Each repeat consist of sequences from 18S, 5.8S and 25S ribosomal subunits and each copy contains a transcribed region that is separated by the long non-transcribed intergenic spacer (IGS). These genes show little sequence divergence between closely related species. Within each repeat, these conserved regions are separated by internal transcribed spacer (ITS) which occur in the following order: 5’–18S–ITS-1–5.8S–ITS-2-26S(or 25S)–3’ that show higher rates of divergence (Figure 6).
Figure 6 Organization of ITS region of nrDNA (Soltis and Soltis 1998) The internal transcribed spacers including ITS-1 and ITS-2 regions are part of the nuclear rDNA (nrDNA) transcript but are not incorporated into ribosomes. In particular, the 5.8S rRNA is separated from 18S, the SSU (small ribosomal subunit) rRNA, by the first of two ITSs (ITS-1), and from 25-28S, the LSU (large ribosomal subunit) rRNA, by the second ITS (ITS-2). Sequencing of the ITS region, however has an exciting potential as a source of nuclear DNA characters for phylogenetic reconstruction in plants. This promise was heightened recently by encouraging result from ITS sequence-based phylogenies of protoctistans (Lee and Taylor 1991), apes and humans (Gonzales et al. 1990). White et al. (1990) have taken advantage of polymerase chain reaction (PCR) technology to promote sequencing of nrDNA in fungi. Baldwin (1992) have described the usefulness of these primers for PCR amplification and sequencing of the ITS region in angiosperms and also described the utility of ITS DNA sequences as a source of phylogenetic data in the subtribe Madiinae of Asteraceae.
ITS regions take a role in the maturation of nuclear rRNAs, bring ITS of 18S–26S nrDNA have a particularly valuable marker for phylogenetic analysis at
intraspecific level and intergeneric level among angiosperms and other eukaryotes (Baldwin et al. 1995). In general, the ITS region present some advantages of being a multicopy locus (100-200 copies), having a small size (300-800 bp), varying from one taxon to another but highly conserved in size in a given taxon, and making it a preferred diagnostic target for a universal test (Baldwin et al. 1995).
ITS sequences are proven to be valuable for phylogenetic reconstruction in angiosperms, algae, and ferns. Recent work indicates that ITS-1 and ITS-2 sequences are inherently G+C rich in which portions of these regions are quite conserved among angiosperms. Thus, the ITS regions not only possess high information content at lower taxonomic level, but also exhibit conserved sequence patterns and high alignability across angiosperms (Figure7).
3
MATERIALS AND METHODS
Species Delimitation of Wild Banana Species (Musa L.) in Sulawesi
Materials
A total of 110 sheets (46 number of collections) of herbarium specimens consist of the dry and spirit collections were used in this study. It composed of all specimens of Musaceae in Sulawesi which are stored in Herbarium Bogoriense (BO) and the new specimens collected from Central, North, South, and SouthEast Sulawesi. The digital data that consist of type specimens from Royal Botanic Garden (MEL) and JSTOR Plant Science were also evaluated.
Methods
Field research to collect new specimens were carried out by using exploration method. Sampling method used was purposive random sampling, in which sampling localities were randomly selected by considering factors that influence the existence of Musa. Data or information recorded from the field include location; altitude, longitude and latitude; vernacular name; plant general habit; pseudostem (height, diameter, colour, appearance, predominant underlying colour, pigmentation of the underlying pseudostem, sap colour, wax on leaf sheaths); suckers (number of suckers, position); petiole (blotches at the petiole base, colour of blotches, length); leaves (length, width, colour, and appearance of leaf upper surface, colour, and appearance of leaf lower surface, wax, colour of midrib dorsal and ventral surface); inflorescence/male bud (peduncle length, bunch position, rachis position, colour of the bract, wax on the bract); flower (compound tepal basic colour, lobe colour of compound tepal, free tepal colour, filament colour, anther colour, stigma colour); fruit (number of fruits, length, immature or mature colour). Morphological comparative study was done in Herbarium Bogoriense (BO).
All the herbarium specimens were studied based on their morphological similarity following de Vogel (1987) and Rifai (2008) using comparative morphology data as main source.
Phylogenetic Study of Wild Banana Species (Musa L.) in Sulawesi Based on Morphological Characters
Materials
The result of species delimitation study will be used as the ingroup, while
Table 1 Morphological characters state used in the phylogenetic analysis
No Characters Character s State
1 Habit 0. Single 1. Clumping
2 Sucker 0. Absent 1. Present
3 Pseudostem height 0.≤ 100 cm 1. > 100 cm 4 Pseudostem aspect 0. Slender 1. Robust 5 Pseudostem colour 0. Medium
green
1. Green 2. Red-purple 3.Blue
6 Pseudostem
appearance
0. Dull (waxy)
1. Shiny (not waxy)
7 Predominant underlying colour of the pseudostem
0. Green 1. Green-yellow
2. Pink purple
8 Pigmentation of the underlying pseudostem
0. Absent 1. Present
9 Sap colour 0. Watery 1. Milky 10 Blotches at the
petiole base
0. Absent 1. Present
11 Petiole canal leaf III
0. Wide with erect
margins
1. Straight with erect margins
2. Margins curved inward
12 Leaf sheaths 0. Lax 1. Clasping 13 Leaf habit 0. Erect 1. Intermediate 14 Colour of leaf
upper surface
0. Medium green
1. Green 2. Dark green
15 Appearance of leaf upper surface
0. Dull 1. Shiny
16 Colour of leaf lower surface
0. Light green 1. Medium green 17 Appearance of leaf
lower surface
0. Dull 1. Shiny
18 Shape of leaf blade base
0. Both sides rounded
1. Both sides pointed 19 Colour of midrib
dorsal surface
0. Green-yellow
1. Light green 2. Green
20 Colour of midrib ventral surface
0. Medium green
1. Light green
21 Inflorescence habit 0. Erect 1. Pendulous 22 Inflorescence
length [cm]
0. ≤ 100 1. 101-200 2. ≥201
23 Peduncle length [cm]
0. ≤ 30 1. 31-60 2. ≥ 61
24 Peduncle hairiness 0. Absent 1. Present
25 Bunch appearance 0. Loose 1. Compact 26 Bract appearance 0. Integral
with each other
1. Inserted independently on the axis
27 Bract habit 0. Persistent 1. Deciduous
28 Bract behavior 0. Not
revolute
Table 1 Morphological characters state used in the phylogenetic analysis (continue)
No Characters Character s State
29 Bract imbrication 0. Not
imbricate
1. Imbricate
30 Bract apex shape 0. Slightly Pointed
1. Intermediate
31 Colour on the bract apex
0. Not tinted with yellow
1. Tinted with yellow
32 Wax on the bract 0. Absent 1. Present
33 Male bud shape 0. Like a top 1. Lanceolate 2. Ovoid 34 Male flower
behavior
0. Persistent 1. Deciduous
35 Compound tepal of male flower shape
0. Linier 1. Tubular
36 Lobe of compound tepal of male flower
0. 3 –toothed 1. 5-toothed
37 Lobe colour of compound tepal of male flower
0. Yellow 1. Orange
38 Free tepal colour of male flower
0. Translucent white
1. White
39 Free tepal shape of male flower
0. Oval 1. Fan-shaped
40 Free tepal apex development of male flower
0. Little or no visible sign
1. Developed
41 Free tepal apex shape of male flower
0. Obtuse 1. Triangular
42 Style shape of male flower
0. Straight 1. Curved
43 Stigma colour of male flower
0. White 1. Cream
44 Ovary shape of male flower
0. Straight 1. Arched
45 Fruit shape 0. Straight 1. Curved 46 Fruit apex 0. Pointed 1. Blunt-tipped 47 Floral relicts 0. Absent 1. Present 48 Immature fruit
peel colour
0. Light green 1. Green
49 Mature fruit peel colour
0. Yellow 1. Bright yellow
50 Fruit fall from hands
0. Persistent 1. Deciduous
51 Seed diameter 0. >1 cm 1. ≤ 1 cm
52 Seed shape 0. Globular 1. Angular
53 Seed surface 0. Smooth 1. Wrinkled
Methods
and floral characters were selected for the analysis of which 45 were scored as binary and 8 as multistate. Some characters were scored as missing data when the data were unavailable. All characters are treated as unorder data and have an equal
weight. The missing data are symbolized with “?”. Starting tree (S) was obtained
via stepwise addition. Number of trees held at each step during stepwise addition = 1. Branch swapping algorithm was run by using tree-bisection-reconnection (TBR) and was evaluated using 100 bootstrap replicates and a complete Hsearch. Clade support values were obtained by using bootstrap. Bootstrap support (BS) was categorized as strong (>85%), moderate (70%-85%), weak (50%-69%), or poor (<50%) (Kress et al. 2002). The complete matrix is shown in Appendix 1.
Phylogenetic Study of Wild Banana Species (Musa L.) in Sulawesi Based on Internal Transcribed Spacer Region of nrDNA Sequences
Materials
A total of 28 ITS sequences were used in this study (Table 2). Twelve nrDNA were successfully sequenced while the rest sixteen ITS sequences were obtained from GeneBank. The outgroup represented by Ensete and Musella while the ingroup consists of Musa species obtained from species delimitation study. Table 2 Source of ITS sequence utilized in the study
No Taxon
Code for Phylogenetic Tree GeneBank Acc number/ Coll No Location Outgroup
1 Ensete glaucum* EGL* FJ626400a Yunnan, China
2 E. superbum* ESB 1* FJ626395a Yunnan. China
3 E. superbum * ESB 2* FJ626396a India
4 E. superbum* ESB 3* FJ626397a Thailand
5 Ensete sp. 1 ESP LDS 193 Bogor Botanic Garden,
Indonesia
6 Musella
lasiocarpa*
MSL 1* FJ626390a Yunnan
7 Musella
lasiocarpa*
MSL 2* FJ626391a Myanmar
Ingroup
8 Musa acuminata
ssp. banksii*
ABS 1* FJ428097b
Papua New Guinea
9 M. acuminata ssp.
banksii
ABS 2 PAN01 Tomohon, N. Sulawesi,
Indonesia
10 M. acuminata ssp.
banksii
ABS 3 PAN 02
Tomohon, N. Sulawesi, Indonesia
11 M. acuminata var.
tomentosa
ATO 1 EAW 9957 Mt. Mekongga, Kolaka,
S.E. Sulawesi, Indonesia
12 M. acuminata var.
tomentosa
ATO 2 LDS 112 Poraboa, Kolaka, S.E.
Sulawesi, Indonesia
13 M. acuminata var.
tomentosa
ATO 3 LDS 192 Mangolo, Kolaka, S.E.
Table 2 Source of ITS sequence utilized in the study
No Taxon
Code for Phylogenetic Tree GeneBank Acc number/ Coll No Location Ingroup
14 M. acuminata var.
tomentosa
ATO 4 PAR 100 Gowa, S. Sulawesi,
Indonesia
15 M. balbisiana* BAL 1* JF977070c China
16 M. balbisiana* BAL 2* JF977072c China
17 M. balbisiana BAL 3 SA 10 Manado, N. Sulawesi,
Indonesia
18 M. celebica CEL 1 FHR 01 Lore Lindu National Park,
C. Sulawesi, Indonesia
19 M. celebica CEL 2 FHR 02 Lore Lindu National Park,
C. Sulawesi, Indonesia
20 M. itinerans ITE 1 FHR 04 Lore Lindu National Park,
C. Sulawesi, Indonesia
21 M. itinerans* ITE 2* JF977079c China
22 M. itinerans* ITE 3* JF977081c China
23 M. itinerans* ITE 4* JF977082c China
24 M. itinerans* ITE 5* JF977083c China
25 M. itinerans* ITE 6* JF977084c China
26 M. textilis* TEX 1* JF977096c China
27 M. textilis* TEX 2* JF977097c China
28 M. textilis TEX 3 PAN 24 Tomohon, N. Sulawesi,
Indonesia *)
Sequence data from GeneBank a
Liu et al. (2010) b
Li et al. (2010) c
Li et al. (2011)
.
Methods
DNA Extraction and Amplification
Total DNA was extracted from silica-gel dried leaves by modification of CTAB method (Doyle and Doyle 1987). Amplification of ITS regions refers to Liu et al. (2010) by using one pair of ITS-5 (5’–TAGAGGAAGGAGAAGTCGT AACAA–3’) as forward primer and ITS-4 (5’–CCCGCCTGACCTGGGGTCGC–
3’) as reverse primer. The PCR process was started with heat shock at 95oC for 3’
. Three steps amplification process were done for 35 cycles. DNA were denaturized at 95oC for 30 seconds. Annealing at 55oC for 30 seconds. DNA extension were done at 72oC for 60-90 seconds. The final extension was performed at 72oC for 7 minutes.
DNA Purification and Sequencing
programme (Technelysium Pty, Ltd). The Muscle software (Edgar 1994) was used to align all the sequences.
Phylogenetic Analysis
All data matrices were analyzed with parsimony approach using PAUP*4.0b10 (Swofford 1998). Maximum Parsimony Heuristic Search were conducted with the following setting: all characters were treated as unorder data and have equal weight; random stepwise addition; branch swapping algorithm was run by using tree-bisection-reconnection (TBR); gaps were treated as missing; a strict consensus tree was produced from the resulting trees. Clade support values were obtained by using bootstrap. Bootstrap support (BS) was categorized as strong (>85%), moderate (70%-85%), weak (50%-69%), or poor (<50%) (Kress et al. 2002).
Mr. Bayes version 3.0 (Ronquist and Huelsenbeek 2003) were used to Bayesian analysis. A general time reversible model (rates= gamma, nst=6) was used. Markov Chain Monte Carlo (MCMC) runs of one millions generations each, starting from different random point in parameter space to verify consistency in our results. Trees were sampled every 100th cycle from chain. All samples points that occurred before stationary score was achieved were discarded as part of the
burn period. Nodes with posterior probability values ≥ 95% were retained in the
50% majority role consensus tree.
4
RESULT AND DISCUSSION
Species Delimitation of Wild Banana Species (Musa L.) in Sulawesi
By examining 110 sheets specimens, 6 taxa consist of 5 species, 1 subspesies and 1 variety of Musa in Sulawesi have been identified and 1 specimen that incompletely known was found. They were M. acuminata Colla ssp. banksii
(F.Muell.) N.W. Simmonds, M. acuminata Colla var. tomentosa (K.Sch.) Nasution, M. balbisiana Colla, M. celebica Warb. ex K. Schum., M. itinerans
Cheesman that belong to section Eumusa, and M. textilis Née from section
Australimusa. Those taxa can be recognized by several important morphological characters. The general morphology, distribution, an identification key, and taxonomic treatment of wild banana species in Sulawesi are discussed below: General Morphology Musa of Sulawesi
Pseudostems
Leaves
Petiole with small to large blotches at base; petiole canal leaf wide with erect margin or straight with erect margin or margin curved inward. Leaf sheaths clasping, erect, oblong or lanceolate, truncate at apex, upper surface medium to dark green, lower surface light to medium green, both surfaces shiny or dull or upper surface slightly dull and lower surface shiny. Leaf bases asymmetric or slightly asymmetric, both sides rounded or pointed, midrib dorsally green-yellow to green and ventrally medium to light green.
Figure 8 Leaf bases shapes. Asymmetric and both sides pointed (a); slightly asymmetric and both sides rounded (b)
Inflorescences
Inflorescence pendulous; bunch hanging vertically, loose to very compact; peduncle glabrous or pubescent; rachis falling vertically or curved.
Male Buds
[image:31.595.127.519.560.710.2]Male bud like a top or lanceolate or ovoid, purple or red purple or brown violet or yellow externally, imbricate or slightly imbricate or not imbricate, apex acuminate not tinted or tinted with yellow, revolute or not revolute before falling, shiny or dull.
Figure 9 Bract imbrications on male bud. Imbricate (a); slightly imbricate (b); not imbricate (c)
a b
Flowers
Basal flowers female or hermaphrodhite, one or two rows. Male flowers in one or two rows, falling with the bract; compound tepals translucent white or white with yellow or orange lobes; free tepals translucent white or white, oval or fan-shaped, apex obtuse or triangular, upper part serrate, no visible sign to developed; filaments white; anthers yellowish; styles straight or curved; stigmas white or cream; ovaries straight or arched.
Fruits
Individual fruits straight to slightly curved, apex pointed or blunt-tipped, with or without relictual floral remains, immature fruits light green to green, mature fruits peel yellow to bright yellow.
Seeds
Seeds numerous, irregularly angular or globular, smooth or wrinkled.
Figure 10 Shape of seeds. Globular and wrinkled (a); angular and smooth (b)
Distribution
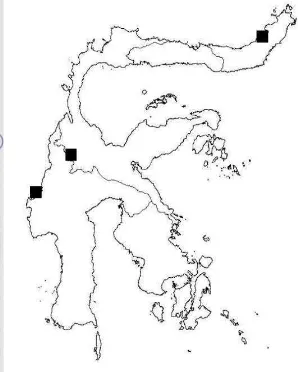
The distribution map (Figure 10) showed that M. acuminata ssp. banksii
was found in North and SouthEast Sulawesi, while M. textilis was found in North Sulawesi. M. acuminata var. tomentosa grow widespread in Sulawesi, whereas M. celebica was found in Central, North, and West Sulawesi. M. acuminata var.
tomentosa and M. celebica are endemic flora in Sulawesi since they are only found in this island (Nasution and Yamada 2001, Nasution 1991). There are two new records of Musa found in Sulawesi, M. balbisiana and M. itinerans. Cheesman (1948b) reported that M. balbisiana distributed from Burma, Ceylon, India, Java, Malaya, New Guinea, Philippines, and Siam. The occurrence of M. balbisiana in Sulawesi was proven by a specimen collected from North Sulawesi (SA 10, Manado). M. itinerans commonly grows continental Southeast Asia. It is distributed in Burma, India, Thailand (Cheesman 1949), China and Vietnam (Häkkinen et al. 2008). In Sulawesi, M. itinerans is represented by specimens stored at BO, Meijer 9979 collected from Mt. Nokilalaki and FHR03, FHR04, FHR05 collected from Lore Lindu National Park.
Figure 11 Distribution map of wild banana species in Sulawesi
Key to the Species and Intraspecific Taxa of Musa from Sulawesi
Key to the Species
1. Bract imbricate and not revolute before falling…..………...2 Bract not imbricate and revolute before falling…..………..3 2. Petiole canal leaf margins curved inward, bract red purple
………...Musa balbisiana Colla
Petiole canal leaf leaf straight with erect margins, bract brown violet or brown
greenis……….Musa textilis Née
3. Fruit slightly curved ………..……….Musa acuminata Colla Fruit straight in the distal part ……….……….4 4. Suckers close to parent………Musa celebica Warb. ex K. Schum
Suckers far to parent………..Musa itinerans Cheesman
Key for Intraspecific Taxa of Musa acuminata Colla
1. Petiole canal leaf wide with erect margins, seed smooth
………...ssp. banksii (F. Muell.) N.W. Simmonds
2. Petiole canal leaf straight with erect margins, seed wrinkled
.………..var. tomentosa (K.Sch.) Nasution
Musa acuminata ssp. banksii Musa acuminata var. tomentosa Musa balbisiana
Taxonomic Treatment
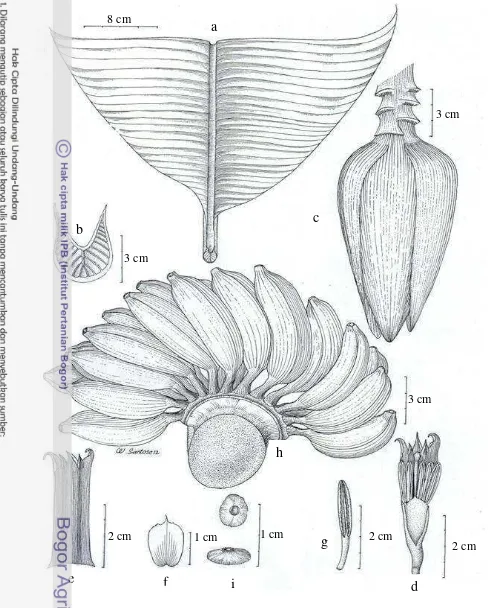
Musa acuminata ssp. banksii (F. Muell.) N.W. Simmonds (Figure 12)
Musa banksii F. v. Muell., Fragm. Phyto. Australie 4: 132. 1864. Baker, Ann. Bot. 7: 217-218. 1893; Chesmann, Kew Bull. 2: 154-157. 1948; Argent, Not. R. Gard. Edinb. 35, 1: 87. f. 5. 1956. – Musa acuminata Colla ssp. banksii (F. Muell.) N.W. Simmonds, Simmonds, Kew Bull. 11: 463. 1957. – Type: Mueller sn, Mount Eliot Fitzalan, Queensland, Mt. Elliot, Elliot Fitzalan, (holo: MEL!)
Plant normal, sucker close to parent, growing vertical; mature pseudostem 3-4 m high, slender, 15-25 cm diameter at base, red-purple, shiny, predominant underlying colour green-yellow with brown purple pigmentation, sap watery.
Petiole 60-70 cm, large red brownish blotches at base, petiole canal leaf wide with erect margin. Leaf sheaths clasping, leaf habit erect, lamina 150-300 cm long, oblong or lanceolate, truncate at apex, upper surface green, lower surface light green, both surfaces shiny, leaf bases asymmetric, both sides pointed, midrib dorsally light green and ventrally medium green, cigar leaf dorsal surface green, leaf of water sucker usually with purple blotches. Inflorescence pendulous, 210-250 cm long; bunch hanging vertically, very compact; peduncle 62-75 cm long, thinly pubescent; rachis with a curve, thinly pubescent. According to Cheesman (1948b), basal flower hermaprodhite, about 10 per bract in two rows; compound tepal white, about 3.5 cm long, lobes yellow; free tepal 2-2.5 cm long, strongly boat shaped; fertile stamen usually 2-4; ovary pale green, glabrous, 8-10 cm long.
Male bud lanceolate, 12-18 cm long, 4-7.5 cm diameter, bracts inserted on the axis, purple externally with yellow stripes internally, not imbricate, apex intermediate tinted with yellow, revolute before falling, shiny. Male flower 10-20 per bract in 2 rows, falling with the bract; compound tepal 2.7-4.5 cm long, 0.8-1.5 cm wide, white with yellow lobes, the two central lobes smaller than outer lobes; free tepal 1-2.1 cm long, 0.5-1.4 cm wide, translucent white, fan-shape, smooth, upper part serrate, apex triangular and developed; stamen 5, 2.2-4 cm long, 0.1-0.3 cm wide, yellow; filaments 0.5-2 cm long, white; anther 1.5-2.2 cm long, yellowish; style straight; stigma white; ovary arched, usually yellow. Fruit
bunch slightly lax, with 15-20 hands per bunch and 17-24 fruits per hands, in 2 rows; individual fruit 10-12 cm long, 2-2.5 cm diameter, slightly curved, pedicel 1.5-2.5 cm, fruit apex blunt-tipped, without relictual floral remains, immature fruit light green, mature fruit bright yellow. Seed ca. 4-6 mm diameter, flattened to angular, smooth.
Distribution
3 cm
a
b
c
d
e
f
g
h
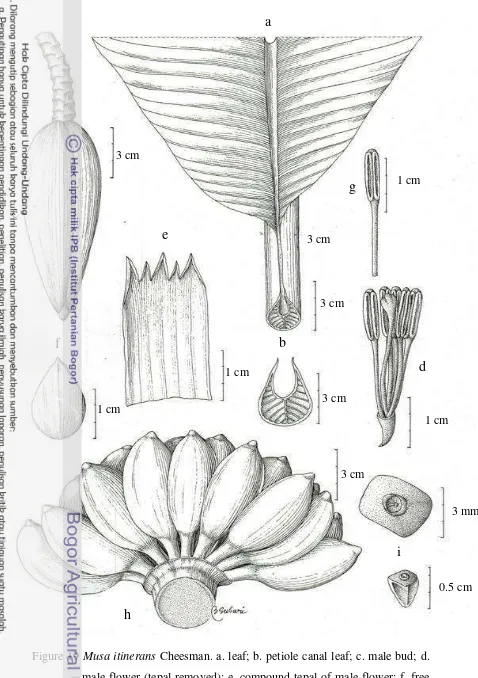
Figure 12 Musa acuminata ssp. banksii (F. Muell.) N.W. Simmonds. a. leaf; b. petiole canal leaf; c. male bud; d. male flower; e. compound tepal of male flower; f. free tepal of male flower; g. stamen; h. fruit; i. seed (LDS 182)
8 cm
3 cm
3 cm
2 cm 1 cm 1 cm
3 cm
2 cm
2 cm
[image:35.595.54.542.64.672.2]Figure 13 Distribution map of M. acuminata ssp. banksii in Sulawesi Habitat
The population of M. acuminata ssp. banksii are found on riverside at the plantation in Tomohon – North Sulawesi, and also on the open places in Singgere hill, Kolaka – SouthEast Sulawesi at 200-300 m above sea level.
Notes
The taxonomy status of M. acuminata ssp. banksii is still questionable. Argent (1976) considered to place this taxa as species level. According to him, it was premature to assume a closed linked between M. banksii with forms of M. acuminata. Shepherd (1990), Simmonds and Weatherup (1990) supported Argent (1976) above opinion. Hotta (1989) considers that M. banksii is synonymous with
M. acuminata. Here, this taxa was treated as intraspecific level under M. acuminata. This is supported by phylogeny analysis using both of morphological and ITS data.
Specimens Examined
North Sulawesi: Manado, YS. Poerba, F. Ahmad, KU. Nugraheni PAN 01
Musa acuminata Colla var. tomentosa (K. Sch.) Nasution
Musa tomentosa K. Sch., Pflanz. 1. Musac. 22. 1900. – Musa acuminata
Colla var. tomentosa (K. Sch.) Nasution, Mem. of Tokyo Univ. of Agri. 32: 82. 1991.- Type: Warburg 15741, Bojong, Minahasa (holo: B, n.v.).
Plant normal; sucker close to parent, to 6 suckers, growing vertical; mature pseudostem 2-4.75 m high, slender, 10-15 cm diameter at base, blue, shiny, predominant underlying colour green-yellow with brown (sometime pink-purple) pigmentation, sap watery. Petiole 40-60 cm, large red brownish blotches at base; petiole canal leaf straight with erect margins. Leaf sheaths clasping, leaf habit erect; lamina 130-325 x 29-100 cm, oblong or lanceolate, truncate at apex, upper surface green, lower surface light green, shiny on both surfaces; leaf bases asymmetric, both sides rounded, midrib dorsally light green and ventrally medium green, cigar leaf dorsal surface green, leaf of water sucker usually with purple blotches around midrib. Inflorescence pendulous, 120-150 cm long, bunch hanging vertically, compact; peduncle 40-60 cm, pubescent; rachis falling vertically, thinly pubescent. Basal flower female, 10-11 cm long, 0.9-1.1 cm wide; ovary 6.3-6.5 cm long. 0.9-1.1 cm wide, yellowish green; style 2.6-2.7 cm long, sub-terete, yellowish; stigma capitate, slightly flat, cream, staminode 1.1 cm long, half the style, sub terete, yellowish; compound tepal 3.4-3.6 cm long, 1.5-1.6 cm wide, white at base but yellow at tip; free tepal ovate, 2-2.1 cm long, 1.5-1.6 cm wide, acuminate, translucent (Nasution 1991). Male bud lanceolate, 10-12 cm long, 4-7 cm diameter, bracts inserted independently on the axis, purple or yellow externally, not imbricate, apex intermediate tinted with yellow, revolute before falling, shiny. Male flower 16-20 per bract in 2 rows, falling with the bract; compound tepal 3.5-4.2 cm long, 1-1.5 cm wide, cream with yellow lobes, central lobes smaller than outer lobes; free tepal fan-shaped, 2-2.5 cm long, 1.5-1.8 cm wide, apex triangular and developed, translucent white; stamen 5, 4-4.2 cm long, 0.2-0.4 cm wide, yellow; anther 2-2.1 cm long, yellow; filament as long as anther; style straight; stigma whitish; ovary straight, yellowish. Fruit bunch lax or compact, 3-12 hands per bunch, 7-20 fruits per hands, in 2 rows; individual fruit 8.5-10 cm long, 1.8-2.5 cm diameter, pedicel 1.1-1.6 cm, apex blunt-tipped, slightly curved, without relictual floral remains, immature fruit green, mature fruit yellow. Seed many, 105-120 per fruit, 5-6.6 mm diameter, irregularly angular and flattened, wrinkled, black when ripe.
Distribution
Figure 14 Distribution map of M. acuminata var. tomentosa in Sulawesi
Habitat
M. acuminata var. tomentosa could be found on lowland up to 600 m above sea level, at open places but sometime could be found under shape as well. This taxa also found at slope of mountain and hills.
Notes
Variations found in M. acuminata var. tomentosa were high enough, this taxa have widely range for some characters such as number of hands per bunch and number of fruits per hands. Neotypification is needed as the type specimen of
M. acuminata var. tomentosa that is only stored at B were lost which probably have been burnt during the second World War.
Specimen Examined
Central Sulawesi: Palu, W. Meijer Meijer 9338 (BO!), leaf, fruit, seed; Lore Lindu, RE. Nasution Rusdy 1133 (BO!), N. Ariyanto, Sahlan and F. Ramadhan ASR01 (BO!), ASR02 (BO!), all specimen leaves, fruits, seeds (spiritus); Maros, Karaenta, RE. Nasution Rusdy 1134 (BO!), petiole, male bud, fruit, seed. North Sulawesi: Mt. Kawatak, Minahasa, Alston 16262 (BO!), sterile. South Sulawesi: Marioriwawo, Soppeng, YS. Poerba, F. Ahmad, Nurmansyah
Rusdy 1627 (BO!), Rusdy 1628 (BO!), all specimen male buds, fruits (spirit); Parangloa, Gowa, YS. Poerba, F. Ahmad, Nurmansyah PAR 100 (BO!), sterile. SouthEast Sulawesi: Suharjono and Maskuri sn (23-10-1973) (BO!), sterile; Hutan Silui, Poraboa, LD. Sulistyaningsih LDS 108 (BO!), LDS 112 (BO!), LDS 122 (BO!), LDS 123 (BO!), all specimen leaves, petioles, peduncles, male buds, male flowers, fruits, seeds (spirit); Mt. Watuwila, LD. Sulistyaningsih LDS 156
(BO!), LDS 167 (BO!), all specimen leaves, petioles, male buds, male flowers, fruits, seeds; Singgere, Tinondo, LD. Sulistyaningsih LDS 179 (BO!), LDS 180
(BO!), LDS 181 (BO!), all specimen leaves, petioles, peduncles, male buds, male flowers, fruits, seeds (spirit); Bolaang Mangondow, Pindol Lolok, Dransfield 3854 (BO!), leaf, petiole, male bud. fruit; Mt. Mekonga, EA. Widjaja and U. Hapid EAW 9956 (BO!), EAW 9957 (BO!), all specimen leaves, petioles, male buds, male flowers, fruits, seeds; Mangolo, EA. Widjaja and U. Hapid EAW 9850
(BO!), LD. Sulistyaningsih LDS 191 (BO!), LDS 192 (BO!), all specimen leaves, petioles, male buds, male flowers, fruits, seeds; Sulawesi: V. Balgooy 3490 (BO!), fruit.
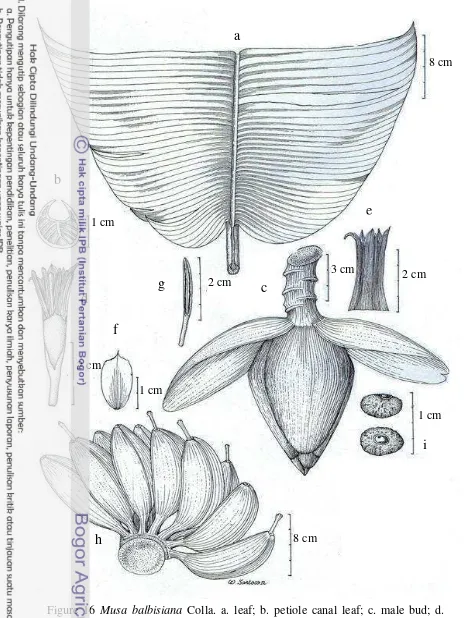
Musa balbisiana Colla (Figure 15)
M. balbisiana Colla, Mem. Gen. Musa. 56 (1820). M. XI Pissang batu seu pissang bidjii Rumph. Herb. Amb. 5, 132, t. 60 Figure f. (1750). M. troglodytarum Linn. Sp. Pl. ed. II. 1478 p.p. (1763). M. seminifera Lour. Fl. Cochinch. 644 p.p. (1790). M. sapientum L. (“the wild sort”) sensu Roxb. Hort.
Beng. 19 (1814); Corom. Pl. t. 275 (1819); Fl. Ind. 2, 484 (1824) et ed. 2. 663 (1832); non L. M. paradisiaca L. sec. Trimen, Flora of Ceylon 4, 265 (1898); non L. M. sapientum ssp. seminifera form. pruinosa King MSS ex Baker, Ann. Bot. 7, 214 (1893); Cheesman, Kew Bull.1948, 327. M. sapientum var. pruinosa King MSS ex Cowan and Cowan, Trees of North Bengal, 135 (1929). M. balbisiana
Colla, Cheesman, Kew Bull. 1948, 327. Type: India orientalis, ex H. Rip. 1820
Anonymous (lecto designed by Häkkinen and Väre: TO, n.v.).
Plant normal, sucker close to parent, growing vertical; mature pseudostem 4-6.5 m high, 20-30 cm diameter at base, green, dull, predominant underlying colour green with brown-purple pigmentation at base, sap milky. Petiole 45-60 cm, sparse blotches at base, petiole canal leaf margins curved inward. Leaf sheaths clasping, leaf habit erect, lamina 180-300 x 60-100 cm, oblong, truncate at apex, upper surface dark-green, lower surface medium green, dull on both surfaces, leaf bases asymmetric, both sides rounded, midrib dorsally green-yellow and ventrally light green, cigar leaf dorsal surface green. Inflorescence pendulous, 100-150 cm long; peduncle 30 cm, thinly pubescent; rachis thinly pubescent; bunch hanging vertically, compact. Basal flower female, about 10 (Cheesman 1948b). Male bud
a
b
c
d
e
f
h
i
Figure 16 Musa balbisiana Colla. a. leaf; b. petiole canal leaf; c. male bud; d. male flower; e. compound tepal of male flower; f. free tepal of male flower; g. stamen; h. fruit; i. seed (SA10)
8 cm
2 cm 3 cm
2 cm 1 cm
2 cm
1 cm
8 cm
[image:40.595.36.500.68.686.2]3.3 cm long, 0.5-0.8 cm wide; filament 1.4 cm long, white; anther 1.7-1.9 cm long, yellowish; style curved under stigma; stigma cream; ovary straight, yellow.
Fruit bunch lax, with 6 hands per bunch, 9-12 fruits per hands, in 2 rows; individual fruit 10.5-14 cm long, straight, apex blunt, persistent style, immature fruit green to dark green, mature fruit yellow. Seed 1-1.2 mm diameter, globular, wrinkled, minutely warty, brown.
Distribution
M. balbisiana is found on the roadside at Manado, North Sulawesi (Figure 16).
Figure 16 Distribution map of M. balbisiana in Sulawesi Habitat
Several population are found on open place at forest border in District Noongan, Minahasa Induk, North Sulawesi.
Notes
Specimen Examined
North Sulawesi: Manado, LA. Sukamto and F. Ahmad, SA10 (BO!), leaf, petiole, malebud (spirit), male flower (spirit), fruit (spirit), seed (spirit).
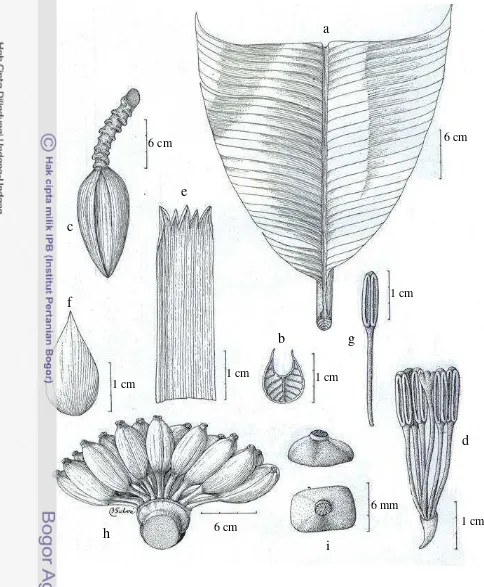
Musa celebica Warb. ex K. Schum. (Figure 17)
Musa celebica Warb., in Engler, Das Pflanzenreich IV. 45: 22. 1900. – Type: Warburg 15742, Bojong, Minahasa (holo: B, n.v.)
Plant normal, sucker close to parent, to 1-2 suckers, growing horisontal; mature pseudostem 2-3.5 m high, slender, 10-15 cm diameter at base, red-purple, shiny, predominant underlying colour green with brown-purple pigmentation, sap milky. Petiole 30-35 cm long, large red blackish blotches at base, petiole canal leaf straight with erect margins. Leaf sheaths clasping, leaf habit erect, lamina 180-200 x 45-50 cm, lanceolate, truncate at apex, upper surface green, lower surface light green, shiny on both surfaces, leaf bases asymmetric, both sides pointed, midrib dorsally light green and ventrally medium green, cigar leaf dorsal surface red-green. Inflorescence pendulous, 110-130 cm long; peduncle 40-60 cm, thinly pubescence; rachis thinly pubescence; bunch hanging vertically, loose.
Basal flower female. Male bud like a top, bracts inserted independently on the axis, yellow, not imbricate, apex intermediate tinted with yellow, revolute before falling, shiny. Male flower 16-20 per bract in 2 rows, falling with the bract; compound tepal 3-4 cm long, 1.5-2 cm wide, yellow lobes, central lobes smaller than outer lobes; free tepal 1.5-2 cm long, 1-1.2 cm wide, translucent white, fan-shaped, smooth, apex triangular, little or no visible sign, upper part serrate; stamen 5, filament white; anther yellowish; style straight; stigma cream; ovary straight. Fruit bunch lax, with 8 hands per bunch and 5-18 fruits per hands, in 2 rows; individual fruit 4-5 cm long, straight, 2.6-3 cm diameter, pedicel 0.5-0.9 cm, fruit apex blunt-tipped, without relictual floral remains, immature fruit green, mature fruit bright yellow. Seed ca. 3-4 mm diameter, angular, smooth. straight, cream.
Distribution
a
b
c
e
f
g
h
i
Figure 17 Musa celebica Warb. ex K. Schum. a. leaf; b. petiole canal leaf; c. male bud; d. male flower (tepal removed); e. compound tepal of male flower; f. free tepal of male flower; g. stamen; h. fruit; i. seed (FHR 02)
6 cm
1 cm
1 cm 1 cm
1 cm
6 cm
6 cm
6 mm
[image:43.595.69.553.70.657.2]Figure 18 Distribution map of M. celebica in Sulawesi Habitat
M. celebica is found on open places and also on slope of mountains at 700-800 m above sea level. It is never found at low land. This taxa can not grow well under shade and drought resistant.
Notes
Specimen Examined
Central Sulawesi: Palolo, Lore Lindu, RE. Nasution Rusdy 1135 (BO!), N. Ariyanto, Sahlan and F. Ramadhan ASR03 (BO!), ASR04 (BO!), all specimen leaves, fruits (spirit), seeds (spirit); Lore Lindu, Fahriadi FHR 01 (BO!), FHR 02
(BO!), all specimen leaves, petioles, malebuds, male flowers, fruits, seeds. West Sulawesi: Popangatalu, Kaluku, Mamuju, J.J. Afriastini 2087A (BO!), fruit, seed.
Musa itinerans Cheesman (Figure 19)
Musa itinerans Cheesman, Kew Bull. 4: 23. 1949. – Type: Parkinson 1761, Myitkyina, Myanmar (lecto designed by Liu et al. (2002): LT, K, n.v.)
Plant normal, sucker far to parent, to 2 suckers, growing vertical; mature pseudostem 3-4 m high, slender, 18-20 cm diameter at base, red-purple, shiny, predominant underlying colour green with brown pigmentation, sap milky. Petiole
35-50 cm, petiole canal leaf straight with erect margin, sparse brown blotches.
Leaf sheaths clasping, leaf habit erect, lamina 150-180 x 42-48 cm, lanceolate, truncate at apex, upper surface green, lower surface light green, shiny on both surfaces, leaf bases asymmetric, both sides pointed, midrib dorsally and ventrally light green; cigar leaf dorsal surface green, leaf of water sucker with purple blotches. Inflorescence pendulous, 110-150 cm long; peduncle 25-30 cm long, puberulent with short hairs; rachis glabrous; bunch hanging vertically, compact.
Basal flower female. Male bud like a top, 15 cm long, 7 cm diameter, bract inserted independently on the axis, red purple externally with yellow stripes, not imbricate, apex slightly pointed, revolute before falling, shiny. Male flower 12-22 per bract in 2 rows, falling with the bract; compound tepal 4-4.8 cm long, 1-1.5 cm wide, yellow lobes; free tepal 2-2.1 cm long, 1-1.4 cm wide, translucent white, fan-shaped, smooth, apex triangular, upper part serrate, little or no visible sign; stamen 5, filament white, anther yellowish, style straight, stigma cream, ovary straight, whitish. Fruit bunch lax, with 8 hands per bunch and 5-12 fruits per hands, in 2 rows; individual fruit 6-6.5 cm long, 2-2.5 cm diameter, straight, fruit apex pointed, without relictual floral remains, immature fruit light green, mature fruit bright yellow. Seed ca. 3-4 mm diameter, angular, smooth.