170 Susalit and Sidabutar MedJ Univ Indon
Experience
with
the
Once
- Daily
Calcium Antagonist
Amlodipine
on
24
-
Hour Ambulatory
Blood Pressure in Hypertensive Patients
E. Susalit, R.P. Sidabutar
Abstrak
Telah diteliti efekrivitos dan tolerabilitas penberian dosis tunggal antagonis kalsium antlodipin (5-10 nry) pada 20 penderita hipertensi ringandansedang. Untukmenilai efekanlodipin, selaindipakaisfig,nomanometer air raksajugadipergunakanalat pengukur teka nan darah 24
ian
ambulatoar. Penguktran tekanan darah secara antbulatoar selatna 24 jam setelah pengobatan anlodipin selatna 8 ninggu, menperlihatlcan bahwa obat ini efektif menurunkan tekanan darah selana 24 jam, tanpa nerubah pola nonnal sirkadian.Abstract
The antihypertensive efficacy and tolerability of once-daily calciunt antagonist antlodipine (5-10 ng) were studied in 20 patients with ttrild to moderate hypertensiotr. 24-hour anbulatory blood pressure nonitoring in conjunction with sphygnotnanonrctric
ur"^ur"-,nent were used to study the effects of anilodipine. 24-hour anbulatory blood pressure nonitoring nade after 8 weeks of treatrnent with amLodipine revealed that anilodipine effectively reduced blood pressure throughout the whole 24-h period without altering the nornnl circadian pattern.Keyw ords : Anlodipine, hypertensi on, 24-hour anbulatory blood pressure
INTRODUCTION
Amlodipine,
a structural analogof nifedipine,
thefirst
of
the
l,4-dihydropyridine calcium
antagonists,
hasbeen
sho
of
hyper-tension.
I
shown in
Fig.t.2A
nelimina-tion half-life
of 35
h following
a singleoral
dose andto
bereadily
absorbedfollowing oral
administration,
with
peak plasma levels
being
achieved 6-12
h
posf
dose.''* The pharmacokinetic
profile
of
amlodipine
allows once
daily administration,
which has
beenshown
to
reduce
blood
pressure(BP)
measured24 h
postdose.s The effective àntihypertensive dose appears to be 5or
10 mg oncedaily, with
simple
one-step doseadjustment
required
to meet
individual patient's
re-qurrements."
The
purpose
of
this study was
to evaluate
theantihypertensive efficacy
andtolerability
of once-dailyamlodipine during normal
daily
activity using
24-h
ambulatory BP
monitoring
and
periodic
clinic
Bp
[image:1.595.311.556.346.652.2]measurements.
Figure 1. The sffucture ofanùodipine
MATERIALS
AND
METIIODS
Patient
selection
Men
and women (notof child
bearing
potential)
agedbetween
2l
and 65 years,who
had
mild to
moderatehypertension
with
supine and standing diastolic Bp
H3COOC
Hsc
ct
cooc2Hs
cH2ocH2cH2NH2
N
IH
/\
l,
-,
VoI 3, No 3, JuIy - Septetnber 1994
(DBP)
of
95-115 mm
Hg
after
aminimum of
2 weekswith
no therapy were
enteredinto
the study.Patients
with
malignant
or
acceleratedhyperten-sion, and/or any other
severeconcomitant pathologic
condition
wereexcluded
from
thestudy.
The patients were informed
of
the nature
andpurpose
of
the study and were
asked
to
give
their
consent to
participate.
Study
design
A
2-week
placebo
run-in period was followed by
8weeks
of
active treatment
with
5-10
mgof amlodipine
in
asingle-blind
fashion.
Patients
visited the
clinic
at 2-week
intervals.
Standing and supine pulse rate and
BP
were measured intriplicate
at eachvisit.
Hematologic
andbiochemical
tests
were carried out
at the endof
the placeborun-in
phase, and
after
8 weeksof
treatmentwith
amlodipine.
A
12-leadelectrocardiogram
(ECG)
was obtained
onentry to
thestudy
and at the endof
thetreatment
with
amlodipine.
At
the
end
of
the placebo
run-in
phaseand
thetreatment
with
amlodipine, ambulatory BP
was measuredfor
24-h period using
an automated,nonin-vasive device
(9O2O7ABP
Monitor,
SpaceLabs Inc,,
Houston,
TX, U.S,A.).
MEAN SUPINEBLOOD
PRESSURE
Placebo Amlodipine therapy
-
-
-
-:
Blood
Pressure
(mmHg)
Entry
Week0
Week 2*
= P < 0.05 comPared witb week 0
**
[image:2.595.51.548.421.737.2]= P
(
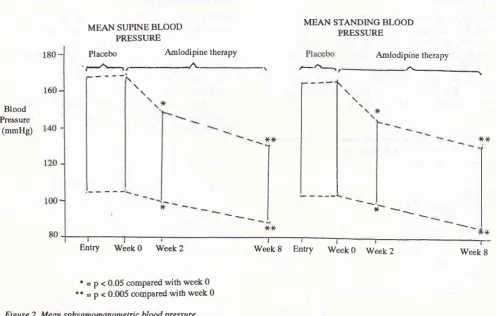
0.005 comPared with week 0Figure 2. Mean sphygmomanontetric blood pressure
Anlodipine on 24-Hour Atnbulatory Blood
Pressure
17l
A two-tailed
t
test was used to compare the meanbaseline
final
changes
in
clinic BP,
daytime
andnightime 24-h ambulatory BP, heart rate,
and ECG
parameters.
Statistical significance
was calculated
at5%
level.
RESULTS AND DISCUSSION
Patients
Twenty
patients (14
men,6
women)
with
a mean ageof47
years (rangeof
29to
64 years) were enteredinto
the study.Three patients
failed
to
fulfill
the
BP criteria
at the endof
the placebo phase and werewithdrawn.
Onepatient failed to finish the study,
becausehe
had
tomove to
another
city
and
so
was excluded
from
the analysis.Of
theremaining
16patients,
sevenpatients
hadnot
received any previous therapy, three patients
hadreceived diuretics
only, three patients had
received
beta-blocker, and three patients had received
ace-in-hibitor.
Those drugs werewithdrawn
before
the study. Eleven patientsrequired
an increasein
doseto
l0
mg
of
amlodipine once
daily
andfive
remained
on
5mg daily.
MEAN STANDING BLOOD PRESSURE
Amlodipine therapy
\
.ttl+
-\
t72
Susalit and Sidabutarr20
100 Heatt rate
80 (beats/min)
Blood pressure (mmHg)
68107214161820222424
[image:3.595.57.552.57.404.2]Time of day (h)
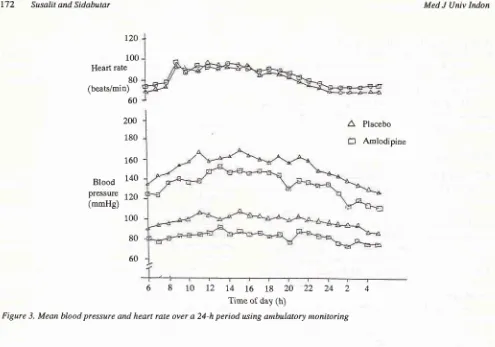
Figure 3. Mean blood pressure and heart rate over a 24-h period using anbulatory nonitoring
Sphygmomanometric
BP
Mean sphygmomanometric BP measurements
for
the16 patients at each
visit are
shownin Fig.
2. Significant decreasesin
BP were achieved
after 2 weeks (148196 mm Hgsupine,
146197 mmm Hg standing) and 8 weeks(133/88
mm Hg supine, 133189
mm Hg
standing)
of
treatmentwith
amlodipine
comparedwith
values at theend
of
the placebo
run-in
phase
(166/105 mm
Hg
supine,
1641106mm
Hg
standing).
Table
1. Mean daytime
and nighttime 24-h ambulatory
blood pressure and heart rate.
A
60200
180
Med
J
Univ IndonPlacebo
Amlodipine
24-h ambulatory BP
The
mean
hourly BP and
HR
over
24-h period
areshown
in
Fig. 3. A
reduction
in
BP
was
observedthroughout the 24-h period after
treatment
with
am-lodipine
compared
with
placebo
without altering
thecircadian pattem.
No significant
changesin
heart ratewas observed.
Comparison of mean daytime and mean
nighttime
BP values on amlodipine and on placebo are shownin Table
1.Both daytime
andnighttime
BP wassignificantly
reducedafter treatment
with
amlodipine,
with
no changein
HR.
Side effects
One
patient on
l0
mg
doseof amlodipine
developed ankle edemawhich
disappeared after reducing the doseup to 5 mg. There were no
significant
hematologic
or
biochemical
changes and no changes in theelectrocar-diograms.
CONCLUSIONS
The results
of
this study
clearly
show that once
daily
amlodipine provides
24-hBP control without
altering
160
140
r20
100
80
60
Placebo
AmlodipineDaytime
Systolic BP (mm Hg)
*
Diastolic BP (mm Hg)*
Heart rate (beats/min)**
NighttimeSystolic BP (mm Hg)
*
Diastolic BP (mm Hg)*
Heart rate (beats/min)**
l@
101 88
154
97 68
139
89 89
135
84 70
*pvalue<0.05
[image:3.595.58.291.584.761.2]Vol 3, No 3, July - September 1994
the
physiologic
circadian
patternof
BPvariation.
Am-lodipine
waswell
tolerated
in
this study.
REFERENCES
1.
rùy'ebsterI,
Robb OI, Ieffers A, Scott AK,Petrie IC, Towler
HM.
Oncedaily
amlodipinein
the treatmentof
mild
tomoderate hypertension. Br
I
Clin Pharmacol 1987 ;27 :7l3-9.
2.
Abemethy DR. Amlodipine: Pharmacokinetic profileof
a low c)earance calcjutn antagoùst.I
Cardjovasc Pharmaco)l99l;L7
(suppl 1):54-7.Amlodipine on 24-Hour Atnbulatory Blood
Pressure
173Faullcrer ID, McGibney D, Chasseaud LF, Perry IL, Taylor
IW. The pharmacokinetics of amlodipine in healthy volun-teers after single intravenous and oral doses and after 14
repeated oral doses given once daily, Br
I
Clin Pharmacol 1986;22:21-5.\ililliams DM, Cubeddu LX. Amlodipine pharmacokinetics
in healthy volunteers. J Clin Pharmacol 1988;28:990-4. Frick MH, McGibney D, Tyler HM. Amlodipine : A double-blind evaluation
ofthe
dose-response relationship in mild to moderate hypertension.I
Cardiovasc Pharmacol 1988;l2(suppl 7) : 576-9.
Iulius S.
Amlodilne in
hypertension; an overviewof
the clinical dossier. J Cardiovasc Pharmacol 1988;12(suppl 7) :s27-34.
4.