VARIATIONS IN THE DENSITY AND ULTRAMORPHOMETRIC
OF ANTENNAL SENSILLA BETWEEN
Apis andreniformis
AND
Apis cerana
IN THE THREE CASTES OF HONEY BEES
CUT FERAWATI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT
LETTER
I hereby declare that this thesis entitled “Variations in the Density and Ultramorphometric of Antennal Sensilla between Apis andreniformis and Apis cerana in the Three Castes of Honey Bees” is the original result of my own
research under supervision of an advisory commitee and has never been submitted in any form to any other institution before. All information from other authors cited here are mentioned in the text and listed in the reference list at the end of the thesis.
Bogor, October 2015
Cut Ferawati
SUMMARY
CUT FERAWATI. Variations in the Density and Ultramorphometric of Antennal Sensilla between Apis andreniformis and Apis cerana in the Three
Castes of Honey Bees. Supervised by RIKA RAFFIUDIN and BERRY JULIANDI.
Honey bees live in two different types of nest, the single comb open air nests of Apis andreniformis, A. florea and A. dorsata and multiple comb cavity
nests of A. cerana, A. koschevnikovi, A. nuluensis, A. nigrocincta, and Athra nests.
Honey bee that consist of three castes (queen, drone and worker caste) perform complex communications. The communication mostly mediate by antennal sensilla that receive odor or pheromone. However, the variations of the density, distribution, and ultramorphometric of antennal sensilla of the open and cavity nesting types is poorly understood and the available data is derived from the worker caste.
The objectives of this research were to analyse: (1) the variations of the type and density of antennal sensilla within the three castes of the open nesting A. andreniformis and cavity nesting A. cerana, (2) the sensilla distribution between
the anterior and posterior sides of antenna and between lower and upper end of each flagellar antenna within the three castes of A. andreniformis and A. cerana,
and (3) ultramorphometric of the length, width, and area of trichodea and placodea sensilla in anterior and posterior sides of flagella within the three castes in both species.
The honey bee samples of A. andreniformis and A. cerana were obtained
from Padang Pariaman (West Sumatera) and Bogor (West Java), Indonesia, respectively. Antennal sensilla of honey bees were analysed by using scanning electron microscope. The densities, distributions and ultramorphometric of antennal sensilla were examined using the ImageJ program. Furthermore, the ultramorphometric sensilla were analyzed with ANOVA and t-test.
This study observed six antennal sensilla types in A. andreniformis and A. cerana. Those are trichodea (variants A, B, C, and D), bassiconica, placodea,
campaniform, ampullacea, and coeloconica. The last two types of sensilla are the new report in the open nesting A. andreniformis.
Densities of sensilla in the cavity nesting honey bee A. cerana are higher
compare to the open air nesting A. andreniformis. This phenomena might be a
structure adaption due to the bees use antennal sensilla for communication inside of dark cavity hive.
The density of sensilla is varied among the three castes of open nesting A. andreniformis and cavity nesting A. cerana. The density of bassiconica in worker
caste is higher than in the other castes in both species. The density of placodea is the highest in the drone caste of both species. Sensilla density of trichodea C, trichodea D, and bassiconica are more abundant in worker flagella among other castes in both species, while placodea, ampulacea, coeloconica sensilla are more abundant in drone among other castes. Interestingly, this study did not find coeloconica in the queen of A. cerana. Density of trichodea A is the highest in A. cerana queen and trichodea B are more higher on in A. cerana worker.
This research also the first report of the variations of antennal sensilla distribution found between the anterior and posterior sides, as well as between the upper and the lower end of flagellar antenna. The number of sensilla on the anterior side is twice compared to the posterior side of flagellar antenna in A. andreniformis and A. cerana. This result suggests that anterior areas are more
prominent to receive odor comes from the frontal area of the bee head. This study also observed that the sensilla distributions are more prominent on the upper end of flagella compare to the lower end. This result might be due to the upper end of antenna is directly contact to the substrate, thus serve as the chemo- and mechanoreception.
Beside the variations of the density and distribution of antennal sensilla types, almost all of ultramorphometrics of trichodea and placodea sensilla are differ within the three castes of open nesting A. andreniformis and cavity nesting A. cerana. Ultramorphometric measurements of A. andreniformis drone placodea
are the highest compare to other caste. This suggest that they need highly efficient to detect the queen pheromones. The ultramorphometric sensilla on anterior and posterior sides are also different, that might imply the functional of sensilla as an effective ogan to capture the odor molecules exposure to the olfactory sensory from the outside environment. Therefore, the diversity in the density and distribution as well as the ultramorphometric of antennal sensilla might give an indication of the adaption of honey bees to different nesting environments.
RINGKASAN
CUT FERAWATI. Variasi Densitas dan Ultramorphometrik dari Sensilla Antena antara Apis andreniformis dan Apis cerana pada Ketiga Kasta Lebah
Madu. Dibimbing oleh RIKA RAFFIUDIN dan BERRY JULIANDI.
Lebah madu hidup pada dua tipe sarang yang berbeda, yaitu sarang terbuka dengan satu sisir seperti sarang Apis andreniformis, A. florea and A. dorsata dan sarang tertutup dengan beberapa sisir seperti A. cerana, A. koschevnikovi, A. nuluensis, A. nigrocincta, dan A. mellifera. Lebah madu terdiri
atas tiga kasta (ratu, jantan, dan pekerja) yang melakukan komunikasi kompleks. Komunikasi pada umumnya menggunakan sensila pada antenna untuk menerima bau atau feromon. Namun, masih sedikit pengetahuan tentang variasi densitas, distribusi, dan ultramorfometrik sensilla antena pada tipe sarang terbuka dan tertutup dan data yang tersedia hanya kasta pekerja saja.
Tujuan penelitian ini adalah untuk menganalisis: (1) variasi tipe dan densitas sensila antena pada ketiga kasta dari lebah sarang terbuka A. andreniformis dan lebah sarang tertutup A. cerana, (2) distribusi sensila antara sisi
anterior dan posterior serta antara pangkal dan ujung dari masing-masing flagela antena pada ketiga kasta A. andreniformis dan A. cerana, dan (3)
ultramorfometrik panjang, lebar dan area dari sensila trichodea dan placodea pada sisi anterior and posterior flagela pada ketiga kasta dari kedua spesies.
Sampel lebah madu A. andreniformis dan A. cerana diperoleh dari Padang
Pariaman (Sumatera Barat) dan dari Bogor (Jawa Barat), Indonesia, secara berurutan. Analisis Sensila antena lebah madu menggunakan scanning electron microscope. Pengujian densitas, distribusi dan ultramorfometrik sensila antena
diuji menggunakan program ImageJ. Selanjutnya, data ultramorfometrik sensila dianalisis menggunakan ANOVA dan uji t.
Pada penelitian ini ditemukan enam tipe sensila pada A. andreniformis dan A. cerana, yaitu sensila trichodea (variasi A, B, C, dan D), bassiconica, placodea,
campaniform, ampullacea, dan coeloconica. Dua tipe sensilla terakhir merupakan laporan baru pada lebah sarang terbuka A. andreniformis.
Total densitas sensila lebah sarang tertutup A. cerana memiliki yang lebih
banyak dibandingkan lebah sarang terbuka A. andreniformis. Fenomena ini
kemungkinan karena adanya adaptasi struktur karena lebah menggunakan sensilla untuk berkomunikasi di dalam sarang yang gelap.
Densitas sensila bervariasi diantara ketiga kasta pada lebah sarang terbuka
A. andreniformis dan lebah sarang tertutup A. cerana. Densitas sensila bassiconica
pada kasta pekerja lebih tinggi dibandingkan kasta lainnya pada kedua spesies. Densitas placodea paling tinggi ditemukan pada kasta jantan pada kedua spesies. Densitas sensila trichodea C, trichodea D, dan bassiconica lebih banyak pada flagela kasta pekerja dibandingkan kasta lainnya pada kedua spesies, sementara itu sensila placodea, ampulacea, coeloconica lebih banyak pada kasta jantan dibandingkan kasta lainnya. Menariknya, pada penelitian ini tidak ditemukan sensila coeloconica pada kasta ratu A. cerana. Densitas trichodea A paling tinggi
pekerja A. cerana. Selanjutnya, bassiconica tidak ditemukan pada kasta jantan A. cerana.
Penelitian ini juga pertama kali mencatat adanya variasi distribusi sensila antara sisi anterior dan posterior antena, sama halnya dengan sensila antara ujung dan pangkal flagela antena. Densitas sensila pda sisi anterior dua kali lipat lebih banyak dibandingkan sisi posterior dari flagela antena pada A. andreniformis dan A. cerana. Hasil ini diperkirakan karena area anterior lebih dominan untuk
menerima bau dari area depan kepala lebah. Penelitian ini juga menemukan bahwa distribusi sensila lebih dominan pada bagian ujung flagela dibandingkan pangkal. Hasil tersebut diperkirakan karena bagian ujung antena kontak langsung dengan substrat, dalam mengindera chemo dan mechanoreception.
Selain variasi densitas dan distribusi tipe sensila antena, hampir semua ultramorfometrik sensila trichodea dan placodea berbeda pada ketiga kasta dari lebah sarang terbuka A. andreniformis dan lebah sarang tertutup A. cerana. Hasil
pengukuran ultramorfometrik placodea pada kasta jantan A. andreniformis lebih
panjang dibandingkan kasta lainnya. Hal ini diperkirakan karena jantan membutuhkan sensila tersebut agar lebih efisien dalam mendeteksi feromon ratu. Ultramorfometrik sensila pada anterior dan posterior berbeda, diperkirakan fungsi sensila agar efektif menangkap molekul bau dari lingkungan luar oleh sensorik olfaktori. Oleh karena itu, keragaman densitas dan distribusi maupun ultramorfometrik sensila antena mungkin mengindikasikan bahwa adaptasi lebah madu terhadap perbedaan lingkungan di sekitar sarang.
Copyright © 2015 Bogor Agricultural University
Copyright are Protected By Law
It is prohibited to cite all or part of this thesis without referring to and mentioning the sources. Citation only permitted for the sake of education, research, scientific writing, report writing, critical writing or reviewing scientific problem. Citation does not infict the name and honour of Bogor Agricultural University
Thesis
As a partial fulfilment of the requirements for a Master Degree in Animal Biosciences Master Program of Graduate School of
Bogor Agricultural University
VARIATIONS IN THE DENSITY AND ULTRAMORPHOMETRIC
OF ANTENNAL SENSILLA BETWEEN
Apis andreniformis
AND
Apis cerana
IN THE THREE CASTES OF HONEY BEES
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
PREFACE
Praise and grateful to Allah the All Mighty, for the completion of this Master Thesis entitled Variations in the Density and Ultramorphometric of Antennal Sensilla between Apis andreniformis and Apis cerana in the Three
Castes of Honey Bees. The study was conducted from June 2014 to June 2015. This research was supported by Master Program Scholarship (BPPDN) from the Directorate of General Higher Education of Indonesia (DIKTI), grant no. 1094/E4.4/2013.
I thank profusely to Dr. Ir. Rika Raffiudin, M.Si and Dr. Berry Juliandi, S.Si, M.Si as the supervising commitee, which have supervised and put so much trust to me during my master study and the completion of the thesis. I sincerely thank to Dr. Ir. Sih Kahono, M.Sc as the thesis defense examiner and to Dr. Ir. R.R. Dyah Perwitasari, M.Sc as the Head of Animal Bioscience Postgraduate Master Program, Department of Biology, for insightful comments and correction of the thesis. My gratitude also to honey bee researcher Prof. Siti Salmah, Drs. Mochammad Chandra Widjaja, MM and Dr. Jasmi, for the knowledge and experience that you shared, that had raised my interest on honey bees.
I address my thankfulness also to staff Scanning Electron Microscope Laboratory, Division of Zoology, Research Center for Biology of Indonesian Institute of Sciences (LIPI) for their magnification technical assistance. I specially thank to staff of National Beekeeping Center (PUSBAHNAS), sampling team in Padang Pariaman (West Sumatra), and sampling team in Bogor (West Java) for the invaluable assistance during field data collection.
I also would like to thank to all the lectures in Animal Bioscience Master Program, who at the end shaping my knowledge up through their high dedication into the research and teaching. The endless gratitude is addressed to my parents, brothers and sister who have supported me by their own way during the study. The least, but very important, I would like to thank to all my friends; class of BSH 2013, BSH students, Desmina Kristiani Hutabarat, Febri Ayu, and Rosi Fitri Ramadani.
Hopefully this scientific work will be useful.
Bogor, October 2015
CONTENT
LIST
LIST OF TABLE xv
LIST OF FIGURES xv
LIST OF APPENDICES xv
1 INTRODUCTION 1
Background 1
Objectives 2
2 LITERATURE REVIEW 3
Honey Bees Evolution 3 Samples Collection 8 Antenna Preparation and SEM Analysis 9 Data Analysis 9 4 RESULTS AND DISCUSSION 10 Results 10 Antennal structure and sensilla types of A. andreniformis and A. cerana, and in the three castes 10 Densities of total antennal sensilla in bees exhibiting different type of nest in the three castes of A. andreniformis and A. cerana 12
Different distributions of antennal sensilla between flagellar sides, and between the upper and lower ends of flagella 16 Ultramorphometric variations of trichodea and placodea sensilla of three castes in two sides of antenna in A. andreniformis and A. cerana 17
Discussion 19 Density and distribution variations of sensilla types in open and cavity nesting bees suggests an environmental adaptation 19
Antennal sensilla density varies among castes 20
Ultramorphometric sensilla are different among the three castes and flagellar sides 21
5 CONCLUSSION 22
REFERENCES 22
LIST OF TABLES
1 The three castes of A. andreniformis and A. cerana 8
2 Ultramorphometric of trichodea and placodea sensilla of the three
castes of A. andreniformis and A. cerana 17
3 Comparison ultramorphometric of trichodea and placodea sensilla on anterior and posterior sides of the three castes of A. andreniformis and
A. cerana 18
LIST OF FIGURE
1 The type of honey bee nest 1
2 Bayesian consesus tree derived of Apis from the cox2 sequence 3
3 Polyagethism of A. cerana workers 4
4 Geographical distribution area of A. andreniformis and A. cerana 5
5 Sex determination of queen eggs 6
6 Reproduction system structures of three castes of honey bees 6
7 Schematic of sensilla anatomy 7
8 Morphology of honey bee antenna 9
9 Ultramorphometric measurement of honey bee sensilla 10
10 Structure of A. cerana worker antenna 11
11 Antennal sensilla types in several flagella of A. andreniformis and A.
cerana 11
12 Total densities of antennal sensilla of A. andreniformis and A. cerana 12
13 Sensilla distribution on the fl8 anterior side the three castes of A.
andreniformis and A. cerana 13
14 Density of antennal sensilla in A. andreniformis and A. cerana of queen,
drone, and worker 16
15 Density of antennal sensilla on the anterior and posterior side 16
LIST OF APPENDICES
1 Antennal sensilla of an A. andreniformis queen flagella 25
2 Antennal sensilla of an A. andreniformis drone flagella 27
3 Antennal sensilla of an A. andreniformis worker flagella 30
4 Antennal sensilla of an A. cerana queen flagella 32
5 Antennal sensilla of an A. cerana drone flagella 34
1
1
INTRODUCTION
Background
Honey bees (Hymenoptera: Apidae) live in two different types of nest, the single comb open air nests of Apis andreniformis (Figure 1a), A. florea and A. dorsata and multiple comb cavity nests of A. cerana (Figure 1b), A. koschevnikovi, A. nuluensis, A. nigrocincta, and A. mellifera nests (Smith 1991; Oldroyd and
Wongsiri 2006; Raffiudin and Crozier 2007). Honey bee in both nest types have different dance behaviors, which are A. andreniformis perform a horizontal dance
compare to vertical dance of A. cerana (Winston 1991; Dyer 2002). The different
dance behaviors might adapt different nest environment (Raffiudin and Crozier 2007; Hepburn and Radloff 2011).
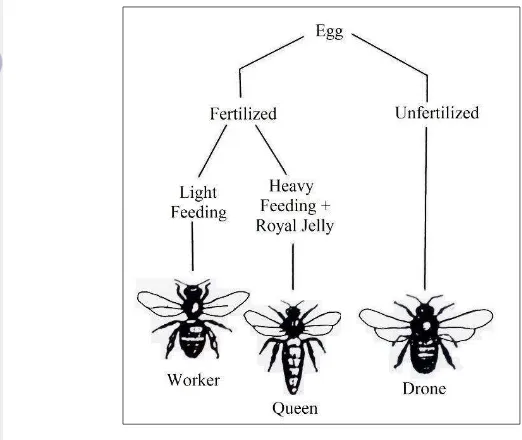
Social life is one of the characteristics of honey bee. A colony of honey bee consist of three castes based on their tasks (Ruttner 1988). However, the division of honey bee caste can also based on the reproductive ability. The reproductive caste consist of queen and drones, whereas the non-reproductive caste all workers (Winston 1991). Each caste of honey bees have spesific function or task in the colony. The role of queen honey bee has the role in produce eggs and control the colony using queen pheromone. Drones have the role to mate the queen.
The inside role of workers in the colony are cleaning the cell, tending the brood, receiving nectar, capping the cell, attending the queen, building the comb, food handling, and cleaning debris. The outside role of worker are ventilation, guarding, and foraging (Winston 1991). Therefore, honey bees need the spesific communication to carry out vary kind activity between or within castes.
Those communications use odor or pheromones and are received by the antennal sensilla. Antennal sensilla of the queen receive odor or pheromones that mediate communications with the workers in the hive and with the drones during mating flight. Drones use these sensilla to detect queen and workers pheromones
Figure 1 The type of honey bee nest. (a) open nesting A. andreniformis and (b)
cavity nesting A. cerana (Figure b from Suwannapong et al. 2011).
2
(Winston 1991). Workers use olfactory sensilla to detect odors inside the colony such as brood pheromone, queen pheromone, and outside the colony for detected floral odors (Chapman 1998). Besides chemoreceptor, the antennal sensilla facilitatesalso as mechanoreceptor, hygroreceptor, thermoreceptor, and gustatory receptor (Chapman 1998).
Four antennal sensilla types of open nesting A. andreniformis workers are
trichodea, bassiconica, placodea, and campaniform and 81.6% of the total sensilla is trichodea (Suwannapong et al. 2012). In cavity nesting A. mellifera there are
five types of sensilla, those are trichodea, bassiconica, placodea, ampulacea, and coeloconica (Dostal 1958). Placodea sensilla in the drones are higher than in the workers in A. mellifera (Esslen and Kaissling 1976). The total number of hair-like
sensilla (trichodea) per antenna in workers is higher than the queens and drones in (Fang et al. 2012).
Further examinations showed that the anterior side of A. mellifera worker
antennae has higher number of antennal sensilla compare to the posterior side (Esslen and Kaissling 1976). The ultramorphometric analysis of antennal sensilla are different between A. florea and A. mellifera worker. The average length of
bassiconica, area of placodea, and diameter of coeloconica of open nesting A. florea worker are higher than cavity nesting A. mellifera African race worker (Al
Ghamdi 2006). This suggest that to the length of antennal sensilla is adapt to maximize the effective to capture the odor molecules from the outside environment (Wyatt 2003).
There is no information on the variations in antennal sensilla among queen and drone castes of the open nesting A. andreniformis. This study
questioned other types of sensilla besides the four known types of sensilla found in the cavity nesting A. mellifera and open nesting A. andreniformis. Due to lack
of data for other cavity nesting bees, thus, this study focused on A. cerana. This is
due to A. cerana has the most widely distributed species of cavity nesting honey
bee (Ruttner 1998).
Other question is the variations in the distributions and densities of sensilla within the three castes of those species. There is no data between the anterior and posterior sides of antennae and between the lower and mostly for upper ends of each antenna within the three castes of both species. More over, there is no ultramorphometric of antennal sensilla within the three castes of open nesting A. andreniformis and cavity nesting A. cerana.
Objectives
The objectives of this research were to analyse: (1) the variations of the type and density of antennal sensilla within the three castes of the open nesting A. andreniformis and cavity nesting A. cerana, (2) the sensilla distribution between
the anterior and posterior sides of antenna and between lower and upper end of each flagellar antenna within the three castes of A. andreniformis and A. cerana,
3
2
LITERATURE REVIEW
Honey Bees Evolution
There are nine species of honey bees, those are clustered in the three groups based on the worker size and nesting type. There are two recognized species of dwarf bees, i.e. A. florea and A. andreniformis. Five species of cavity
nester bees with medium-sized workers are A. mellifera, A. koschevnikovi, A. nuluensis, A. nigrocincta, and A. cerana. At least two species of giant bees those
are A. dorsata and A. laboriosa (Oldroyd and Wogsiri 2006; Raffiudin and
Crozier 2007; Hepburn and Radloff 2011).
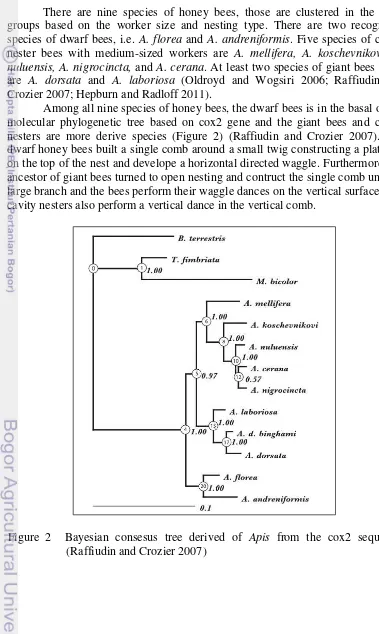
Among all nine species of honey bees, the dwarf bees is in the basal of the molecular phylogenetic tree based on cox2 gene and the giant bees and cavity nesters are more derive species (Figure 2) (Raffiudin and Crozier 2007). The dwarf honey bees built a single comb around a small twig constructing a platform on the top of the nest and develope a horizontal directed waggle. Furthermore, the ancestor of giant bees turned to open nesting and contruct the single comb under a large branch and the bees perform their waggle dances on the vertical surface. The cavity nesters also perform a vertical dance in the vertical comb.
Figure 2 Bayesian consesus tree derived of Apis from the cox2 sequence
4
Honey Bees Castes
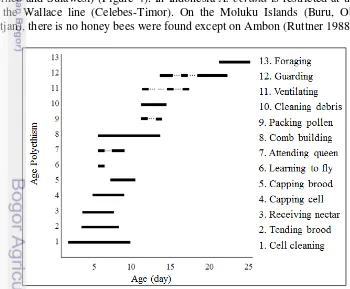
Honey bees are social life insect and consist of three castes (queen, drone, and worker). In one colony of A. cerana there are about 6,884 individuals (Ruttner 1988). Honey bees from different castes have different function in their colony. Queen has the role in producing eggs and controlling pheromone, while drones have the role as to mate the queen. The workers have the role based on age polyethism (Figure 3), those are inside and outside colony. Inside tasks include cell cleaning, cell capping, brood and queen tending, comb building, food handling. Whereas outside task are ventilating, guarding, orientation flights, foraging and any other activities (Winston 1991).
Distribution of Apis andreniformis and Apis cerana
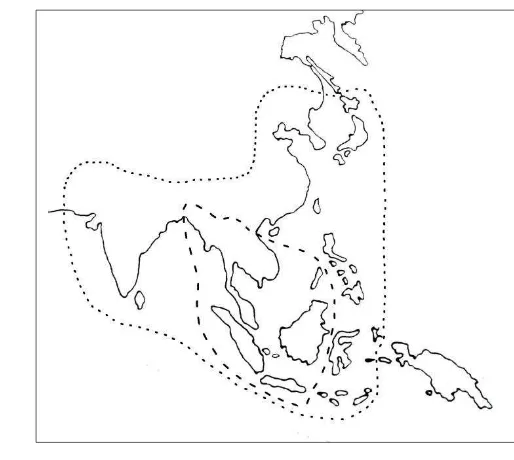
The geographical distributions of A. cerana is more wide compare to A. andreniformis. Open nesting A. andreniformis distribute in Southern China,
Thailand, Indonesia (Sumatera, Java, and Borneo), Malaysia, Philippines (Palawan), Burma, Laos, and Vietnam (Figure 4) (Wongsiri et al. 1996; Hepburn
and Radloff 2011). Furthermore, the distribution of A. cerana in China, India,
Malaysia, Burma, Laos, Vietnam, Philippines, Japan, Iran, Pakistan, Nepal, Bhutan, Afghanistan, North Korea, South Korea and Indonesia (Sumatera, Java, Borneo and Sulawesi) (Figure 4). In Indonesia A. cerana is restricted at the west
of the Wallace line (Celebes-Timor). On the Moluku Islands (Buru, Obi, and Batjan), there is no honey bees were found except on Ambon (Ruttner 1988).
5
Figure 4 Geographical distribution area of A. andreniformis (broken line) and A. cerana (dotted line) (Ruttner 1988 and Wongsiri et al. 1996)
Nest Types
Based on the nest structures, honey bee nests consist of the single comb open air nests and multiple comb cavity nests. The single comb open air nest encircle a small branch or twig, those type of nest are a typical nest of A. andreniformis (Figure 1a) and A. florea. In addition, the single comb open air
nests of A. dorsata builds the comb on the branch of a tree, an overhang on a tall
building, or on cliff faces. Meanwhile, multiple comb cavity nests honey bee contruct the nest inside closed cavities such as hollow trees trunks, caves, cleft in rocks and wall which are the characterization for A. cerana (Figure 1b), A. koschevnikovi, A. nuluensis, A. nigrocincta, and A. mellifera nests (Smith 1991;
Oldroyd and Wongsiri 2006; Raffiudin and Crozier 2007).
Nest homeostasis such as the nest temperature, humidity, carbon dioxide level, and other enviromental factors are maintenanced at relatively constant levels regardless of external conditions. Nest homeostasis regulation are the functions of honey bee worker, their were detected enviromental factors using antennal sensilla (Chapman 1998).
Sex Determination and Sexual Dimorphism
6
Figure 5 Sex determination of queen eggs (Winston 1991)
Drones, queen, and workers have unique specialization, particularly associated with reproduction. The reproduction organs of drone are designed for mating. Furthermore, the male structures of reproduction organ, the penis consists of an endophallus and copulatory claspers (Figure 6a). The sperm are transferred during mating that are produced in the testes and stored in the seminal vesicles until mating (Winston 1991).
a b c
7
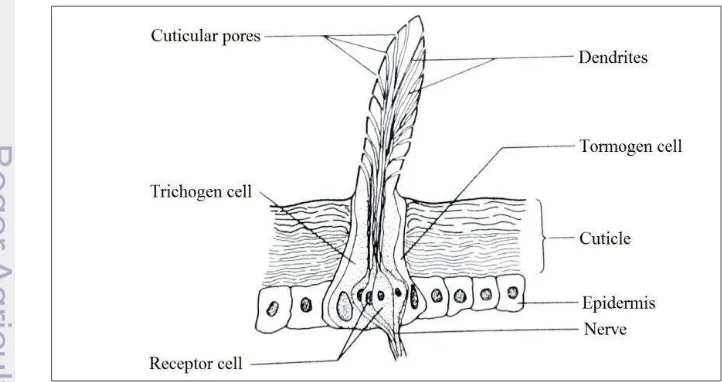
Structure and Functions of Sensilla
The antenna are the noses of insect, consist of scape, pedicle, and flagella. Antenna of worker A. mellifera consist of ten flagella (fl1-fl10) and cover by
sensilla (Chapman 1998). The structure of sensilla have external cuticular component within which neural processes lie in close to pores in cuticle (Figure 7). This organ is a developmental unit composed of neural cells and support cells that secrete the cuticular component of the sensilla (Chapman 1998).
Antennal sensilla of A. andreniformis have four different types, those are
bassiconica, campaniform, placodea, and trichodea type with total 4,892 sensilla per antenna (Gupta 1992; Suwannapong et al. 2012). Each of antennal sensilla
type have specific functions. These sensilla are important for perception of carbon dioxide, humidity, taste, and temperature (Winston 1991). Bassiconica and campaniform in A. mellifera workers are involved in hygroreception (Schneider
1964). The function of bassiconica sensilla is a tasting organ in A. mellifera
workers (Dostal 1958). Placodea are involved in detecting a range of sex pheromones released by A. florea and A. mellifera queens (Brockman and
Brückner 2001) and to receive odor in worker A. mellifera (Erickson 1982). The
hair sensilla trichodea as touch receptor or mechanoreceptor in A. mellifera and
bassiconica are possibly as taste organ (Dostal 1958).
Several function of antenna are to locate the host or prey and to block chemicals odor for the insect survival. This was shown by the study of fig pollinating wasps Ceratosolen solmsi marchali (Hymenoptera: Agaonidae) (Li et al. 2009). This phytophagous wasps mainly use sensilla to locate their fig Ficus
(Moraceae) hosts. Encarsia sophia (Hymenoptera: Aphelinidae) is a parasitoid use
for biological control of Bemisia tabaci. It select the prey aided by the sensilla
(chemoreception organs) (Zhang et al. (2014). Further, the sensilla of the fleshfly Neobellieria bullata (Diptera: Sarcophagidae) is important structure to block the
nitric oxide synthase in plant derived odorants (Wasserman and Itagaki 2003).
8
3 METHODS
Study Site and Time
This research was conducted at Division of Animal Function or Behavior, Department of Biology, Bogor Agricultural University and at Scanning Electron Microscope (SEM) Laboratory, Division of Zoology, Research Center for Biology of Indonesian Institute of Sciences (LIPI) from June 2014 until June 2015.
Samples Collection
The honey bee specimens of A. andreniformis and A. cerana were
obtained from Padang Pariaman, West Sumatera (S 00° 32’ 75.0” and E 100° 09’ 77.2”) in 104 m altitude and from Bogor, West Java (S 06° 33’ 24.9” and E 106° 43’ 29.7”) in 116 m altitude, respectively. Ten individuals of workers and drones
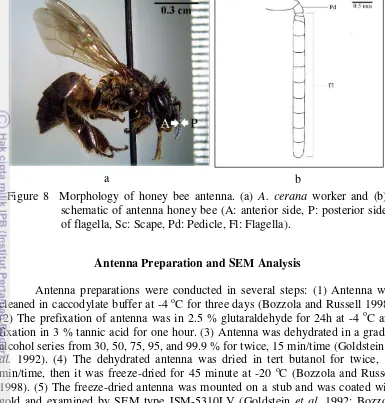
as well as one individual of queen of both species (Table 1) were taken and all preserved in 70% ethanol (Goldstein et al. 1992). The antennae of individual
honey bees (Figure 8) were taken using insect pins under a stereomicroscope. Table 1 The three castes of A. andreniformis and A. cerana
Species Queen Castes Drone Worker
A.a
A.c
9
Antenna Preparation and SEM Analysis
Antenna Preparation and SEM Analysis
Antenna preparations were conducted in several steps: (1) Antenna was cleaned in caccodylate buffer at -4 oC for three days (Bozzola and Russell 1998). (2) The prefixation of antenna was in 2.5 % glutaraldehyde for 24h at -4 oC and fixation in 3 % tannic acid for one hour. (3) Antenna was dehydrated in a graded alcohol series from 30, 50, 75, 95, and 99.9 % for twice, 15 min/time (Goldstein et al. 1992). (4) The dehydrated antenna was dried in tert butanol for twice, 10
min/time, then it was freeze-dried for 45 minute at -20 oC (Bozzola and Russell 1998). (5) The freeze-dried antenna was mounted on a stub and was coated with gold and examined by SEM type JSM-5310LV (Goldstein et al. 1992; Bozzola
and Russell 1998). The coated and examined was operation by SEM laboratory tecnicians.
Each antenna of A. andreniformis and A. cerana caste was placed on six
stubs. (1) Queen: one of the left and right antenna of queen was placed at two stubs. (2) Drones and (3) workers: four of the right part antenna of each caste was put on two stubs. Antennal sensilla of three castes antenna of A. andreniformis
and A. cerana taken by using SEM were recorded using a computer-controller
microscope apparatus.
One of antenna per caste in both species was observed the anterior side or the posterior to analysis type, density, and distribution of antennal sensilla. The anterior side of antenna is the side in the same direction to the head and the opposite direction of side antenna to the head as the posterior side (Figure 8a).
Data Analysis
The antennal sensilla types were characterized by ultramorphology with 2,000-3,500X and the density of antennal sensilla were analyzed with 350-750X
b a
0.5 mm
Figure 8 Morphology of honey bee antenna. (a) A. cerana worker and (b)
10
magnification of SEM. Numbers of antennal sensilla was calculated using a manual counter and ImageJ program (http://www.rsbweb.nih.gov/ij) in areas varied between 14,000-63,000 µm2 and the values are shown as density of sensilla per 0.01 mm2 following Ågren and Hallberg (1996). Density and distribution of antennal sensilla was counted on the flagella of anterior and posterior sides of queen, drone, and worker A. andreniformis and A. cerana.
The ultramorphometric of trichodea and placodea sensilla were analysed due to the most abundance in each flagella (Esslen and Kaissling 1976; Fang et al.
2012). Those were measured with the same magnification of SEM. Ultramorphometric data are measurement of the length, width, and area of this sensilla as examined using an imageJ program (Figure 9a-c). The data was analyzed within three castes using ANOVA and data of sensilla in both flagella sides with t-test was performed using R program version 3.1.3 (CRAN.R-project.org).
4 RESULTS AND DISCUSSION
Results
Antennal structure and sensilla types of A. andreniformis and A. cerana, and in the three castes
This study observed the antenna of A. andreniformis and A. cerana that
consist of scape, pedicel and flagella (Figure 10). In both bees species, the antenna consist of 10 flagella (segments) in the queen and the worker, but 11 flagella in the drone. The antennal sensilla only found in flagella and not in other part of antenna (Appendix 2–7).
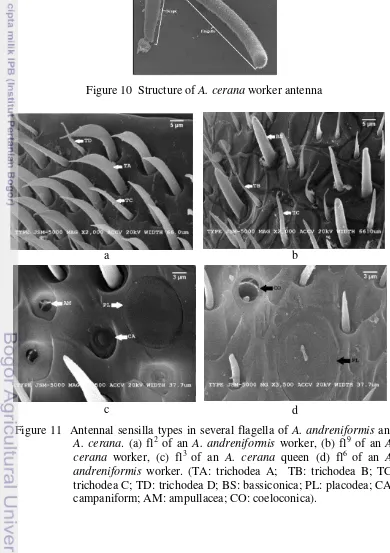
There are six sensilla types, trichodea, bassiconica, placodea, campaniform, ampullacea and coeloconica, in the three castes of the open nesting A. andreniformis (Figure 11). The first four types are in agreement with
Suwannapong et al. (2012). Therefore, ampullacea and coeloconica are the two
types of antennal sensilla that are new recorded in A. andreniformis from this
study. Accordingly, the three castes of A. cerana have six sensilla types the same
a b c
11
with those of A. andreniformis, except coeloconica and bassiconica, which is
absent on queen and drones, respectively (Appendix 1).
Further, this study found four variants (A, B, C, and D) of trichodea sensilla, which is in agreement with Esslen and Kaissling (1976) and Suwannapong et al. (2012). Trichodea A are hair-like sensilla with a saber-shape
and a pointed tip (Figure 11a), whereas trichodea B are long hair sensilla that are slightly curved with sharpened tips (Figure 11b). Trichodea C and D are both hair sensilla with nearly tapered tips and latter has an S-shape (Figure 11a).
b
Figure 10 Structure of A. cerana worker antenna
Figure 11 Antennal sensilla types in several flagella of A. andreniformis and A. cerana. (a) fl2 of an A. andreniformis worker, (b) fl9 of an A. cerana worker, (c) fl3 of an A. cerana queen (d) fl6 of an A. andreniformis worker. (TA: trichodea A; TB: trichodea B; TC:
trichodea C; TD: trichodea D; BS: bassiconica; PL: placodea; CA: campaniform; AM: ampullacea; CO: coeloconica).
a b
12
This study observed the existence of bassiconica that are straight pegs with a blunt tip of a relatively shorter length than trichodea (Figure 11b), and placodea (plate-like sensilla) that were characterized by a radially striated oval plate surrounded by a narrow membranous ring (Figure 11c-d). Several sensilla also existed as campaniform (peg sunken in pit sensilla), which are characterized as emerging from oval pits and having a bell-shape with no pores or openings. Ampullacea (deep flask sensilla) and coeloconica (sensory pit peg) are pit organ-like, with the ampullacea having a hole with a small opening in (Figure 11c) and the coeloconica having a large opening (Figure 11d). These classifications followed those of Esslen and Kaissling (1976) and Suwannapong et al. (2012).
Densities of total antennal sensilla in bees exhibiting different type of nest in the three castes of A. andreniformis and A. cerana
The total densities of the six sensilla types in the cavity nesting A. cerana
are higher compare to the open nesting A. andreniformis (Figure 12). The density
of bassiconica in workers is the highest compare to the other castes in both species, and the density of placodea in the drones is the highest as well (Figure 13). However, the densities of certain sensilla such as bassiconica, campaniform, ampullacea, and coeloconica are higher in A. andreniformis than those in A. cerana (Figure 14). Several similar and different density patterns of each sensilla
type existed within castes of honey bees exhibiting both nesting behaviors. The sensilla are varied among the three castes of bees (Figure 14).
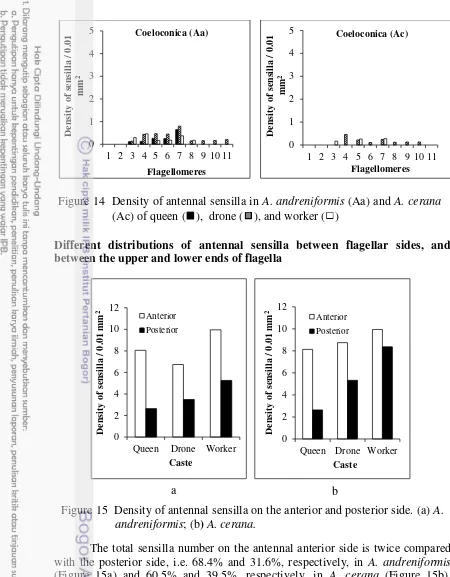
Sensilla density of trichodea C, trichodea D, and bassiconica are more abundant in worker flagella among other castes in both species, while placodea, ampulacea, coeloconica sensilla are more abundant in drone among other castes (Figure 14). Interestingly, this study did not find coeloconica in the queen of A. cerana. Density of trichodea A has the highest in A. cerana queen and trichodea B
are more higher on in A. cerana worker. Furthermore, bassiconica were not found
in A. cerana drone (Figure 14; Appendix 1, 5, and 7).
13
a b
c d
e f
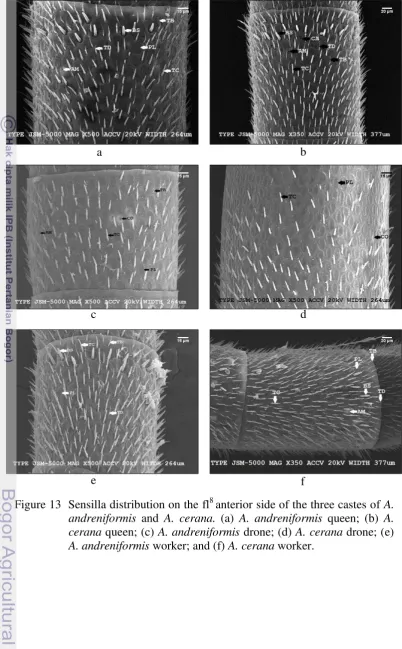
16
Different distributions of antennal sensilla between flagellar sides, and between the upper and lower ends of flagella
The total sensilla number on the antennal anterior side is twice compared with the posterior side, i.e. 68.4% and 31.6%, respectively, in A. andreniformis
(Figure 15a) and 60.5% and 39.5%, respectively, in A. cerana (Figure 15b).
However, there is no distribution pattern similarity of each sensilla type between
A. andreniformis and A. cerana (Figure 14; Appendix 2-7).
The distribution pattern of sensilla trichodea A are more abundant in lower end of the antenna compare to upper end of antenna for both species (Figure 15).
Whereas, sensilla trichodea B, trichodea D, and bassiconica show the opposite
Figure 14 Density of antennal sensilla in A. andreniformis (Aa)and A. cerana
(Ac) of queen ( ), drone ( ), and worker ( )
17
study found sensilla trichodea C, placodea, and ampullacea are distribute evenly along the flagella of three caste in both species, except sparsely in fl1 and fl2 for trichodea C and placodea and ampullaceal are not found on fl1and fl2 for. Otherwise, sensilla campaniform and coeloconica do not show distribution pattern (Figure 14).
Ultramorphometric variations of trichodea and placodea sensilla of three castes in two sides of antenna in A. andreniformis and A. cerana
Ultramorphometric measurement of the length, width, and area of sensilla of trichodea and placodea are significantly different (p < 0.05) among the three caste of A. andreniformis, except trichodea C (Table 2). Significantly different of
all sensilla types measurements among the three castes are also found in A. cerana
(p < 0.05), except the length (p = 0.065) and area (p = 0.097) of trichodea A and
placodea (p = 0.079) (Table 2).
Table 2 Ultramorphometric of trichodea and placodea sensilla of the three castes of A. andreniformis and A. cerana
Measure Sensilla Type Queen Ultramorphometric (µm) ± SE Drone Worker Sig. 0.05
Apis andreniformis
Different letter in the same row indicate significant different (ANOVA, p < 0.05)
18
Ultramorphometric of trichodea B of queen in both species are the largest among other castes. On the contrary, the measurement of trichodea C and trichodea D of A. cerana drone are the smallest than other castes (Table 2). Thus,
ultramorphometrics sensilla show variation between the castes.
Among the three castes of A. andreniformis the areas measurement of
placodea between anterior and posterior are significantly different (p < 0.05). A. andreniformis worker show ultramorphometric significantly different between
anterior and posterior (p < 0.05) of all sensilla types, except the length (t = 0.2277) and area (t = 0.7083) of trichodea A. The measurement of length and width of all sensilla types of Apis. cerana queen on two sides of flagella are
significantly different (p < 0.05) (Table 3).
Table 3 Comparison ultramorphometric of trichodea and placodea sensilla on anterior and posterior sides of the three castes of A. andreniformis and A. cerana
Measure Sensilla Type Ultramorphometric of sensilla on anterior : posterior side t value
19
Asterisk (*) in the same row indicate significant different (t-test, p < 0.05)
ns indicate not significant (-) indicate no data
Discussion
Density and distribution variations of sensilla types in open and cavity nesting bees suggests an environmental adaptation
Previously, Suwannapong et al. (2012) found four sensilla types trichodea,
bassiconica, placodea, and campaniform in workers of A. andreniformis, was
collected in Thailand. In agreement with previous studies, the four described types were found, along with two new sensilla types, ampullacea and coeloconica, in the three castes of the open nesting A. andreniformis. These data are suggest because
inadvertence the types of sensilla in the previous studies.
Our findings corroborated that the cavity nesting honey bee A. cerana has
a higher total density of sensilla compared with the open air nesting A. andreniformis. This is in agreement with the higher density of sensilla on cavity
nesting drones of A. melliferra than on the open nesting A. florea (Brockmann and
Table 3 (Continued)
Measure Sensilla Type
Ultramorphometric of sensilla on anterior : posterior side
20
Brückner 2001). This phenomena suggests that cavity nesting honey bees, which live in the dark (Oldroyd and Wongsiri 2006), have adapted to use antennal sensilla for communication inside hives. The suggest there are do not useful the visual communication. Otherwise, there are useful as possible to chemoreception and mechanoreception communication the inside colony using the antennal sensilla. The other side, cavity nesting type of A. cerana was evolution from open
nesting A. andreniformis (dwarf bees) which are the ancient honey bees (Raffiudin
and Crozier 2007; Hepburn and Radloff 2011). This phenomena suggest as it related to variations of densities and distributions of antennal sensilla in both species. There are cavity nesting of A. cerana have densities of antennal sensilla
more than open nesting A. andreniformis.
However, the adaptation to an open air environment, which increased the density of bassiconica, campaniform, and coeloconica compared with A. cerana,
was shown for all castes of the open nesting A. andreniformis. Schneider (1964)
noted that in A. mellifera workers, bassiconica and campaniform are involved in
hygroreception, and coeloconica in hygrothermoreception (Yokohari et al. 1982)
and perceiving carbon dioxide (Dostal 1958). Open air nesting A. andreniformis
that live in exposed combs (Winston 1991) may require higher numbers of hygrothermoreceptor sensilla to detect fluctuations in temperature and humidity compared with the more stable environmental conditions in cavity nesting. Those are important for regulated the nest temperature and humidity, by fanning their wings, producing water droplet or clustering in swarm (Wilson 1974).
The ratio of total density of sensilla on the anterior and posterior antennal side in all castes in open and cavity nesting honey bees are almost twice that is in accordance with the sensilla of worker and drone castes of A. mellifera (Esslen
and Kaissling 1976). These phenomena might be suggest an environmental adaptation in which the anterior areas are more prominent as chemoreceptor, mechanoreceptor, thermohygroreceptor, and other receptor that originate from the frontal head area.
In addition, the sensilla distribution (except trichodea A) is also greater at the upper end of the flagella compared with at the lower end that is close. These findings are in accordance with Wilson (1974) in that sensilla of A. mellifera are
mostly located on the upper flagellar ends where they are more effective in sensing the surfaces of the honeycomb cell walls to receive odors and vibrations. Furthermore, Dostal (1958) mentioned that trichodea as touch receptor or mechanoreceptor in A. mellifera and bassiconica are possibly as taste organ.
Antennal sensilla density varies among castes
Honey bees perform the most elaborate social behavior among the corbiculate bees. Here found several sensilla types that could be used for caste characterization. First, drones have the highest density of placodea among the castes in both open nesting A. andreniformis and cavity nesting A. cerana. Similar
data was reported for the flagella, which are covered by placodea, of drones in A. mellifera (Esslen and Kaissling 1976). Brockman and Brückner (2001) mentioned
that placodea are involved in detecting a range of sex pheromones released by A. florea and A. mellifera queens. Congregation behavior of drone A. cerana japonica occured at relative humidity 89% (Fujiwara et al. 1994), beside tree
21
of A. andreniformis drone is unknown (Oldroyd and Wongsiri 2006). Thus, drones
might be detected humidity and sex pheromones released by queen use the sensilla placodea. Other general function of sensilla placodea is to receive odor receptor in worker A. mellifera (Erickson 1982).
However, coeloconica, which are involved in perceiving carbon dioxide and in hygrothermoreception (Dostal 1958), were absent in A. cerana queen. It is
presumably due to this cavity nesting queen always stay in the hive, thus it is not expose to carbon dioxide, humidity, or great temperature fluctuations.
Our studies found that workers have a great abundance of bassiconica, therefore that sensilla can be determined as the characterized of the workers. This sensilla if an tasting organ (Dostal 1958), that might serve serve as chemoreceptor for the pollen and nectar during foraging. Bassiconica were not found in A. cerana
drones and were sparsely found in A. andreniformis drones.
Ultramorphometric sensilla are different among the three castes and flagellar sides
Almost all of ultramorphometrics (length, width, and area) of sensilla type were differ within three castes of open nesting A. andreniformis andcavity nesting A. cerana. This is in agreement with Al Ghamdi (2006) that worker of A.mellifera
and A. florea showed the ultramorphometric differ of each sensilla type.
Ultramorphometric of placodea of A. andreniformis drone are the highest
compare to other caste which is in contratry with the drone of Melipona quadrifasciata (Ravaiano et al. 2004). The function of placodea on A. florea and A. mellifera drone is to detect the sex pheromones (Brockmann and Brückner
2001) and to receive odor in worker A. mellifera (Erickson 1982).
In addition, ultramorphometric of trichodea B of A. andreniformis and A. cerana queen are the largest than other castes, meanwhile trichodea C and D of A. cerana drone are the smallest than other castes. The hair sensilla (trichodea) in A. mellifera worker involved as mechanoreceptor (Dostal 1958).
The comparison of ultramorphometric sensilla on anterior and posterior sides are significantly different, in particularly A. cerana worker and A. andreniformis drone caste. Therefore, the sensilla measurement correlate to the
function of sensilla. Olfactory organ of honey bee must effective capture the odor molecules exposure of the olfactory sensory from the outside environment (Wyatt 2003). This study investigated variations in sensilla distribution, size, and density within three castes of the open A. andreniformis and cavity nesting A. cerana to
elevate our understanding of the division of labor and the mechanisms of communication in honey bees.
The implication of antennal sensilla study can be use for key identification of honey bee species between A. andreniformis or A. cerana based on densities
and distribution of sensilla type. Moreover, the existence of sensilla types can be use as the characterization of A. cerana castes, such asthe absent of coeloconica
22
5 CONCLUSSION
There are six types of sensilla in open nesting A. andreniformis and cavity
nesting A. cerana: trichodea, bassiconica, placodea, campaniform, ampullacea,
and coeloconica. Sensilla trichodea consist of four variants those are trichodea A, B, C, and D. This research found, two new sensilla types, ampullacea and coeloconica, in the three castes of the open nesting A. andreniformis. Total
densities of sensilla cavity nesting A. cerana are higher than open nesting A. andreniformis. Sensilla densities of workers are the highest among other castes
and the higher anterior side. All types of sensilla are prominent on the upper end flagella, except trichodea A. Sensilla types is unequally distribute between castes in A. cerana and between flagellar side in A. andreniformis. Antennal sensilla
density is vary between castes. Ultramorphometric sensilla are different on flagella sides and within castes. Ultramorphometric measurement of placodea of A. andreniformis drone is the highest among other castes, thus might have a fuction
to detect queen pheromone. However, the trichodea of all caste from the two species show no clear pattern of the measurents.
REFERENCES
Ågren L, Hallberg E. 1996. Flagellar sensilla of bumble bee males (Hymenoptera, Apidae, Bombus). Apidologie. 27:433-444.
Al Ghamdi AA. 2006. Scanning electron microscopic studies on antennal organs of adult honey bee workers in genus Apis (Hymenoptera: Apidae). Bull. Entomol Soc Egypt. 83:1-11.
Bozzola JJ, Russell LD. 1998. Electron Microscopy. 2nd ed. New York (US):
Jones and Bartlett Publishers.
Brockmann A, Brückner D. 2001. Structural differences in the drone olfactory system of two phylogenetically distant Apis species, A. florea and A. mellifera. Naturwissenschaften. 88:78-81.
Chapman RF. 1998. The Insect Structure and Function. 5th ed. New York (US):
Cmabridge University Pr.
Dostal B. 1958. Riechfähigkeit und zahl der riechsinneselemente bei der honigbiene. Zeitschrift für vergleichende Phys. 41:179-203.
Dyer FC. 2002. The biology of the dance language. Annu Rev Entomol.
47:917-949.
Erickson EH. 1982. Evidence for electrostatic enhancement of odor receptor function by worker honeybee antennae. Bioelectromagnetics. 4:413-420.
Esslen J, Kaissling KE. 1976. Zahl und verteilung antennaler sensillen bei der honigbiene (Apis mellifera L.). Zoomorphologie. 83:227-251.
23
Fujiwara S, Miura H, Kumagai T, Sawaghuci T, Naya S, Goto T, Asanuma H, Suzuki K. 1994. Drone congregation of Apis cerana japonica
(Radoszkowski, 1877) above large trees. Apidologie. 25:331-337.
Goldstein JI, Newbury DE, Echlin P, Joy DC, Romig AD, Lyman CE, Fiori C, Lifshin E. 1992. Scanning Electron Microscopy and X-Ray Microanalysis A Text for Biologists, Materials Scientists, and Geologiests. 2nd ed. New
York (US): Plenum Pr.
Gupta M. 1992. Scanning electron microscopic studies of antennal sensilla of adult worker Apis florea F (Hymenoptera: Apidae). Apidologie. 23:47-56.
Hepburn HR, Radloff SE. 2011. Honeybees of Asia. New York (US): Springer.
Li Z, Yang P, Peng Y, Yang D. 2009. Ultrastructure of antennal sensilla of female
Ceratosolen solmsi marchali (Hymenoptera: Chalcidoidea: Agaonidae: A
gaoninae). Can Entomol. 141:463-477.
Oldroyd BP and Wongsiri S. 2006. Asian Honey Bees: Biology, Conservation, and Human Interactions. Cambridge (UK): Harvard University Pr.
Rafiudin R, Crozier RH. 2007. Phylogenetic analysis of honeybee behavioral evolution. Mol Phylogen Evol. 43:543-552.
Ravaiano SV, Ferreire RP, Campos LAO, Martins GF. 2014. The antennal sensilla of Melipona quadrifasciata (Hymenoptera: Apidae: Meliponini): a study
of different sexes and castes. Naturwissenschaften. DOI
10.1007/s00114-014-1184-0.
Roux O, Baaren JV, Gers C, Arvanitakis L, Legal L. 2005. Antennal structure and oviposition behavior of the Plutella xylostella specialist parasitoid: Cotesia plutellae. Microsc Res Tech. 68:36-44.
Ruttner F. 1988. Biogeography and Taxonomy of Honeybees. Berlyn (DE):
Spinger-Verlag
Schneider D. 1964. Insect Antenna. Annu Rev Entomol.9:103-122.
Smith DR. 1991. Diversity in the genus Apis. New Delhi (IN): Westview Pr.
Suwannapong G, Benbow ME, Nieh JC. 2011. Biology of Thai Honeybees: Natural History and Threats. Florio RM, editor. Bangkok (TH): Nova
Science Publishers Inc.
Suwannapong G, Noiphrom J, Benbow ME. 2012. Ultramorphology of antennal sensilla in Thai single open nest honeybees (Hymenoptera: Apidae). J Trop Asian Entomol. 01:1-12.
Wasserman SL, Itagaki H. 2003. The olfactory responses of the antenna and maxillary palp of fleshfly, Neobellieria bullata (Diptera: Sarcophagidae),
and their sensitivity to blockge of nitric oxide synthase. J Insect Phys.
49:271-280.
Wilson EO. 1974. The Insect Societies. Cambridge (US): Harvard University Pr.
Winston ML. 1991. The Biology of the Honey Bee. Cambridge (US): Harvard
University Pr.
Wongsiri S, Lekprayoon C, Thapa R, Thirakupt K, Rinderer TE, Sylvester HA, Oldroyd BP, Booncham U. 1996. Comparative biology of A. andreniformis and Apis florea in Thailand. Bee World. 77:23-35 .
Wyatt TD. 2003. Pheromones and Animal Behaviour Communication by Smell and Taste. Cambridge (UK): Cambridge University Pr.
24
Zhang X, Zhang F, Luo C, Wang S. 2014. Ultrastructure of antennal sensilla of an autoparasitoid Encarsia shopia (Hymenoptera: Aphelinidae). Micron.
25
APPENDICES
Appendix 1 Antennal sensilla of an A. andreniformis queen flagella
Flagella
Anterior side Posterior side
26
Appendix 1 (Continued)
Flagella
Anterior side Posterior side
fl5 fl5
27
Appendix 2 Antennal sensilla of an A. andreniformis drone flagella
Flagella
Anterior side Posterior side
28
Appendix 2 (Continued)
Flagella
Anterior side Posterior side
29
Appendix 2 (Continued)
Flagella
Anterior side Posterior side
fl1 fl1
TA TC
TC
30
Appendix 3 Antennal sensilla of an A. andreniformis workerflagella
Flagella
Anterior side Posterior side
31
Appendix 3 (Continued)
Flagella
Anterior side Posterior side
fl5 fl5
32
Appendix 4 Antennal sensilla of an A. cerana queen flagella
Flagella
Anterior side Posterior side
33
Appendix 4 (Continued)
Flagella
Anterior side Posterior side
fl5 fl5
34
Appendix 5 Antennal sensilla of an A. cerana drone flagella
Flagella
Anterior side Posterior side
35
Appendix 5 (Continued)
Flagella
Anterior side Posterior side
36
Appendix 5 (Continued)
Flagella
Anterior side Posterior sde
fl2 fl2
fl1 fl1
TA TC
TA
TC
TA
TC
TC
37
Appendix 6 Antennal sensilla of an A. cerana worker flagella
Flagella
Anterior side Posterior side
38
Appendix 6 (Continued)
Flagella
Anterior side Posterior side
fl5 fl5
fl4 fl4
fl3 fl3
fl2 fl2
fl1 fl1
PL TC BS
TC
TB
PL
TC
BS
PL PL
TC
TC
PL
CO
TC
PL
TC TC
TC
TA
TA
TC
39
BIOGRAPHY
The author was born in Lhokseumawe, Aceh Province on February 28, 1990 as the fourth child of Teuku Ibnu Hajar and Nuriah. Authors pursued the Bachelor degree from the Department of Biology, Faculty of Mathematics and Natural Sciences, Syiah Kuala University, graduated in 2012. In 2015, the author graduated from Master Program of Animal Biosciences, Department of Biology, Bogor Agricultural University. The master program scholarship and research grant were obtained from Directorate of General Higher Education of Indonesia (DIKTI), grant no. 1094/E4.4/2013.
The author participanted in several scientific events, those are: (1) the commitee in International Conference on Biosciences (IcoBio) on August 5-7, 2015 at Bogor Agricultural University. (2) Studium Generale presented by Dr. Ir. H. Suswono, MMA (Ministry of Agriculture of Indonesia 2009–2014 Period) the
title “Peningkatan Daya Saing Produk Pertanian dalam Era Globalisasi” on
November 15, 2014 at Bogor Agricultural University. (3) Mini Workshop Elsivier Publishing Campus on September 9, 2014 at Bogor Agricultural University. (4) The seminar of B09 CRC project of “Palynology: Material, Methods and Apllilcations in Biological Science” presented by Siria Biagioni (Georg-August University Goettingen, Germany) on August 28, 2014 at Bogor Agricultural University. (5) Mini Workshop DNA Barcoding, Faculty of Forestry cooperated with Efforts on November 29, 2013 at Bogor Agricultural University. (6) Training in intensive English Language Training at Syiah Kuala University Language Center from March 4 to May 3, 2013. (7) Trainer Assistant of “Basic Science
Training for SMA and SMK teachers Aceh-wide” cooperated with Education Department and kfw entwicklungsbank PEM, on May 16-20, 2011 at Syiah Kuala University, Banda Aceh.
The author presented entitle “Antennal sensilla distribution of different caste of honey bee Apis andreniformis and Apis cerana (Hymenoptera: Apidae)”
in The International Kasetsart University Science and Technology Annual Research Symposium (I-KUSTARS) on 28-29 May, 2015 in Bangkok, Thailand. The author submitted the article with title “Differences in the density and distribution of antennal sensilla between open and cavity nesting in the three