AND pH CONTROL
CUCU SUKMAYA
DEPARTMENT OF PHYSICS
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
AND pH CONTROL
CUCU SUKMAYA
DEPARTMENT OF PHYSICS
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
And say:
“Work (righteousness): Soon will Allah observe your work, and
His Messenger, and the Believers: Soon will ye be brought back
to the knower of what is hidden and what is open: then will He
show you the truth of all that ye did
.”
CUCU SUKMAYA. Synthesis of Apatite-Chitosan Composite Using Duck Eggshell and Chitosan with Temperature and pH Control. Supervised by AKHIRUDDIN MADDU and SETYANTO TRI W.
Composite of apatite-chitosan was prepared by using chitosan as polymer matrix. Calcium phosphate was prepared from CaO and (NH4)2HPO4, which the ratio Ca : P had to be 1.67. Chitosan is produced by deacetilase from biopolymer chitin. Duck eggshell (95% CaCO3) as starting material was converted to be calcium oxide (CaO) through calcinations with temperature 1000oC for 5 hours. In-situ and ex-situ methods were prepared from apatite and chitosan (dissolved with acetate acid 2%). Calcium phosphate prepared for bone implantation, but it is too fragile and brittle to be implant into the body. So addition of chitosan can decrease crystalinity and increase mechanic characteristic (tougher). Composite apatite-chitosan was resulted by fast precipitation. Fast precipitation was held in a breaker glass with nitrogen atmosphere, under controlled temperature at 37oC and pH 7, in order to increase amount of hydroxyapatite. Precipitation with CaO which was dropped by (NH4)2HPO4. Hydroxyapatite and chitosan were appearing at samples with in-situ and ex-situ methods which indicating by peak matching of XRD (closed lattice parameter, crystal size), overlapping at FTIR spectra and supporting by SEM picture (homogenous and agglomerated particle). However, it still needs further research to examine biocompatibility osteoconductive degree of sample by in-vivo and in-vitro test.
Approved by:
1
stSupervisor,
(Dr. Akhiruddin Maddu)
NIP : 19660907 199802 1 006
2
ndSupervisor,
(Setyanto Tri W, M.Si.)
NIP : 19760731 200501 1 003
Known by:
Head of Physics Department
(Dr. Ir. Irzaman, M.Si)
NIP : 19630708 199512 1 001
Graduation date:
Name :
Cucu Sukmaya
AND pH CONTROL
Thesis
a paper submitted in partial fulfillment
of requirement for bachelor degree
Faculty of Mathematics and Natural Sciences
Bogor Agricultural University
CUCU SUKMAYA
DEPARTMENT OF PHYSICS
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
Alhamdulillah, I could finish this thesis successfully, which is one of biophysics project of Physics Department funded by Hibah A2 Bersaing Dikti 2008. This research synthesizes biocomposite of
hydroxyapatite-chitosan for using in medical application as material of bone implantation.
The realization of this project would not have been possible without the dedicated helping of Dr. Akhiruddin Maddu, and Mr. Setyanto Tri W, M.Si as my research supervisors. To all of these people, I owe its
whole-hearted gratitude that impossible to describe. Nonetheless, I also would like thank to Mr. Sulistyoso Giat MT and Mr. Wisnu from BATAN for XRD analysis, Mr. Wikanda and Mr. Wawan from Geology Laboratories Bandung for SEM-EDXA characterization.
I am also thankful for Ummi, Abi (alm), Ayu, Ende, Ujang, Mr. Henky, Leti and whole families, Hydroxyapatite team work, for the pray and support. To my entire lecturer thank you for all guidance and dedication. For all of Physics 42 and for my entire friend who stand beside me, it is pleasure to be here with you all. Thank you so much.
Cucu Sukmaya was born in Ciamis on 1 September 1988 as the first child from the couple of Elon Sunardi (Alm) and Lilis Rosmiati.
He was a student in TK Miftahul Huda at 1991-1993, SD Negeri 1 Gereba at 1993-1999, SMP Negeri 1 Kawali at 1999-2002, and SMA Negeri 1 Kawali at 2002-2005.
CONTENTS
Page
FIGURE LIST ... viii
TABLE LIST ... ix
APPENDIX LIST ... x
INTRODUCTION Background... 1
Objective of Research ... 1
Hypothesis ... 1
Time and Place of Research ... 1
THEORY Human Bone Structure ... 1
Apatite ... 2
Hydroxyapatite ... 3
Chitin and Chitosan ... 3
Duck Eggshell ... 4
Apatite-Chitosan Composite ... 4
Sample Characterization ... 5
Atomic Absorption Spectroscopy (AAS) ... 5
X-RAY Diffraction (XRD) ... 5
Scanning Electron Microscopy (SEM) ... 5
Fourier Transform-Infra Red (FT-IR)... 6
MATERIALS AND METHODS Materials and Equipments ... 6
Experimental Methods ... 6
Duck Eggshell Calcinations ... 6
Precipitation and Composite Synthesis ... 6
RESULT AND DISCUSSION Characteristic of Calcined Duck Eggshell ... 7
Characteristic of Apatite-Chitosan Composite ... 9
X-Ray Diffraction (XRD) ... 9
Fourier Transform-Infra Red (FTIR) ... 10
Scanning Electron Microscopy (SEM) ... 11
CONCLUSION ... 11
REFERENCES ... 12
FIGURE LIST
Page
1. The crystal structure of hydroxyapatite ... 3
2. Shrimps as one of the chitin resource ... 3
3. Structure of chitosan ... 4
4. Ducks and Duck Eggshell ... 4
5. Atomic Absorption Spectroscopy processes ... 5
6. X-Ray Diffraction processes ... 5
7. Scanning Electron Microscopy processes ... 6
8. Fourier Transform Infra-Red processes ... 6
9. FTIR spectra of heated duck eggshell at 1000oC within (a) 5 hours (b) 10 hours... 8
10.XRD pattern of heated duck eggshell at 1000oC within (a) 5 hours (b) 10 hours ... 8
11.XRD patterns of A1, A2 and A3 ... 9
12.XRD patterns of B1, B2 and B3... 10
13.FTIR spectra sample A1, A2, A3 and Chitosan ... 10
TABLE LIST
Page
1. Contain of bone minerals ... 2
2. Composite material for use in the body ... 2
3. Duck eggshell major composition ... 4
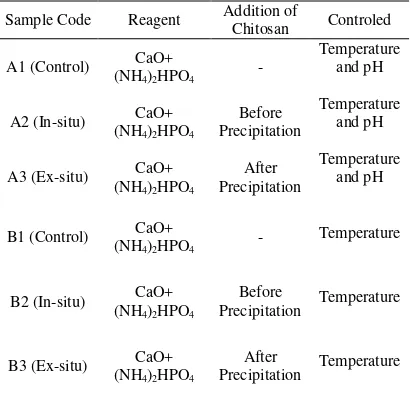
4. Samples code ... 7
5. Percent of calcium calcined duck eggshell by AAS... 8
6. Percent transmission of carbonate groups ... 8
7. Crystallinity of sample ... 9
8. Lattice parameter of sample ... 9
APPENDIX LIST
Page
1. Experimental Flow Chart ... 14
2. XRD pattern of calcined duck eggshell ... 15
3. Characterization Devices ... 16
4. Calculation of Chemical Reaction ... 18
5. Calculation of maximum XRD peak of samples ... 19
6. JCPDS Reference ... 25
7. Lattice parameter calculation match to hidroxyapatite ... 28
8. Crystal size of samples ... 40
INTRODUCTION
Background
A biomaterial is a synthetic material used to replace part of a living system or in function to intimate contact with living tissue [1]. A biomaterial is different from a biological material such as bone that is produced by a biological system, but it has resembled structure and function. Bones are rigid organs that form part of the endoskeleton of vertebrates. They function to move, support, and protect the various organs of the body, produce red and white blood cells and store minerals. Damage in bone caused by accidental case and suffering of diseases, can be heal with implant of hard tissue. Fractured bone case has different effect depending on age levels. Bone Specialists have researched about precise biomaterial referred to biocompatibility degree and prices. Biocompatibility is a material characteristic that non-toxic and compatible for the body, which has been proved by in vivo and in vitro test [2].
Clinical research in producing bone material has produced interesting result, for example auto graft, allograft and xenograft.
An ideal biomaterial precise selection for implantation process must be biocompatible, bioactive and overflow. Based on ways to make it, substitution biomaterial classified to synthetic biomaterial and natural biomaterial. Synthetic biomaterials are biomaterial which use synthetic reagents, for example CaCl2 and Ca(OH)2 whereas natural biomaterials are biomaterial which use natural reagents, for example eggshell, coral reef and algae.
One of ways to increase biocompatibility degree of biomaterial is using natural reagents, such as Duck Eggshell as source of calcium precursor. Beside to increase biocompatibility degree, using duck eggshell also has economic value (in some area, eggshell included as organic waste). The other reason in using duck eggshell is that highest percentage than eggshell chicken. Moreover, as we knew that calcium is the important compound in bone minerals.
In the recent time has developed a new kind of natural biomaterial that hydroxyapatite
with formula Ca10(PO4)6(OH)2, it has good characteristic such as pores, resorption, bioactive, non-corrosion, inert and worn-out endure, but in other side hydroxyapatite have weakness likes brittle and fracture, that become constraint in design. To solve that constraint, added strongest material, elastic,
biocompatible and cheap and in this research, we use chitosan as added material for apatite
be apatite-chitosan composite. As we knew that chitosan is a kind of natural polymers as product of processing shrimp shell waste, beside it cheap, strong and elastic, chitosan has characteristic non-toxic and overflow [3].
Objective of Research
This research is conducted in order to synthesis and characterize apatite-chitosan composite based on calcium from duck eggshell and chitosan from shrimp shell waste, so that relevant in implantation process. Synthesis is based on in-situ and ex-situ methods, while characterization is performed through X-Ray Diffraction (XRD), Fourier Transform-Infra Red (FTIR) and Scanning Electron Microscopy (SEM).
Hypothesis
Precipitation of apatite-chitosan composite in the body normal condition, body temperature (~37oC) and body pH (~ 7) are able to increase quantity of hydroxyapatite compound that appear.
Time and Place of Research
This research was conducted from August 2008 through January 2009, which took place in IPB Biophysics Laboratory. The sample analysis performed in Pusat Pengembangan
Ilmu Pengetahuan dan Teknologi
(PUSPITEK) BATAN Serpong Tanggerang,
Pusat Penelitian dan Pengembangan
(PUSLITBANG) Kehutanan Bogor and Geology Laboratory Bandung.
THEORY
Human bone structure
vessels and cartilage. There are 206 bones in the adult human body and about 270 in an infant.
Bones have some main functions: Protection — Bones can serve to protect internal organs, such as the skull protecting the brain or the ribs protecting the heart and lungs; Mineral storage — Bones act as reserves of minerals important for the body, most notably calcium and phosphorus; Movement — Bones, skeletal muscles, tendons, ligaments and joints function together to generate and transfer forces so that individual body parts or the whole body can be manipulated in three-dimensional space. The interaction between bone and muscle is studied in biomechanics, etc [4].
The primary tissue of bone, osseous tissue, is a relatively hard and lightweight composite material, formed mostly of calcium phosphate in the chemical arrangement termed calcium hydroxyapatite (this is the osseous tissue that gives bones their rigidity). It has relatively high compressive strength but poor tensile strength of 104-121 MPa, meaning it resists pushing forces well, but not pulling forces. While bone is essentially brittle, it does have a significant degree of elasticity, contributed chiefly by collagen. All bones consist of living cells embedded in the mineralized organic matrix that makes up the osseous tissue [5].
The matrix is the major constituent of bone, surrounding the cells. It has inorganic and organic parts. The inorganic is mainly crystalline mineral salts and calcium, which is present in the form of hydroxyapatite. The matrix is initially laid down as unmineralised osteoid (manufactured by osteoblasts).
Mineralization involves osteoblasts secreting vesicles containing alkaline phosphates. This cleaves the phosphate groups and acts as the foci for calcium and phosphate deposition. The vesicles then rupture and act as a centre for crystals to grow on.
Table 1 Contain of bone minerals
Element Contain (wt. %)
Ca 34
P 15
Mg 0.5
Na 0.8
K 0.2
C 1.6
Other elements 47.9
The organic part of matrix is mainly composed of collagen. This is synthesized intracellular as tropocollagen and then
exported, forming fibrils. The organic part is also composed of various growth factors, the functions of which are not fully known. These factors present include glycosaminoglycans, osteocalcin, osteonectin, bone seal protein and Cell Attachment Factor. One of the main things that distinguish the matrix of a bone from that of another cell is that the matrix in bone is hard.
Table 2 Composite material for use in the body [5]
Material Advantage Disadvantage Application Polymers Nylon PTFE Polyester Silicone Ductile, light, easy to fabricate
Not strong, prone to creep, degradable
Suture, vascular prosthesis, accetabular cup, artificial ligament Metals
Ti and its alloys Co-Cr alloys Stainless steels Au, Ag, Pt Ductile, strong, tough
Prone to corrosion, unwanted ion release
Artificial joint, bone plate and screw, dental root implant, pacer, suture wire Ceramics Carbon Aluminum oxide Hydroxyapa tite Biocompati ble, inert or bioactive, strong in compressio n, stiff
Brittle, weak in tension, sometimes fragile
Cardiovascular device, dental prosthesis, joint prosthesis, orthopedic implant Composite Carbon-carbon Metal-PMMA HA-HDPE Strong, stiff, tailor-made, distinctive properties
Difficult to make, high production cost
Joint implant, heart valve, bone cement
Apatite
Apatite is a group of phosphate minerals, usually referring to hydroxyapatite, fluorapatite, and chlorapatite, named for high concentrations of OH−, F−, or
Cl
− ions, respectively, in the crystal. The formula of the admixture of the three most common end members is written as Ca5(PO4)3(OH, F, Cl), and the formulae of the individual minerals are written as Ca5(PO4)3(OH), Ca5(PO4)3F and Ca5(PO4)3Cl, respectively [20].Apatite is one of few minerals that are produced and used by biological micro-environmental systems. Apatite has a Moh's Scale hardness of five. Hydroxyapatite is the major component of tooth enamel. A relatively rare form of apatite in which most of the OH groups are absent and containing many carbonate and acid phosphate substitutions is a large component of bone material.
For this reason, toothpaste typically contains a source of fluoride anions (e.g. sodium fluoride, sodium monofluorophosphate). Similarly, fluoridated water allows exchange in the teeth of fluoride ions for hydroxyl groups in apatite. Too much fluoride results in dental fluorosis and or skeletal fluorosis. [6]
Phosphorite is a phosphate-rich
sedimentary rock, which contains between 18% and 40% P2O5. The apatite in phosphorite is present as cryptocrystalline masses referred to as cellophane [7].
Hydroxyapatite
Hydroxyapatite belongs to the apatite family. Apatite is a general term crystalline calcium phosphate mineral. There are many apatite compound, including flouroapatite, chloroapatite, carbonate-apatite, and hydroxyapatite. Hydroxyapatite chemical formula is Ca10(PO4)6(OH)2. Hydroxyapatite is a calcium phosphate including hydroxide
and has Ca/P (molar ratio) 1.67.
Hydroxyapatite is one of few material that is classed as bioactive material, so that it will support bone ingrowths without breaking down or dissolving when be used for implantation in human body [8].
Figure 1 The crystal structure of hydroxyapatite
(Hanson, Bob. 2005. 150000000:1 Model of Hydroxyapatite.
www.stolaf.edu/people/hanson) Hydroxyapatite is the most stable calcium phosphate phase at normal temperature and pH between 4.2 and 12. Hydroxyapatite crystal unit has hexagonal structure which lattice parameter a = b = 9.432 Å and c = 6.881 Å (Fig. 1). It does not have the mechanical strength to enable it to succeed in long-term load bearing application. It is the most commonly used calcium phosphate in the medical field, as it possesses excellent biocompatibility and is osteoconductive.
Hydroxyapatite in human body usually named biological hydroxyapatite. It found mainly in human or animal teeth and bones. In medical application, hydroxyapatite is used
for bone implantation. Hydroxyapatite has been utilized as a fertilizer, a fluorescent substance, an absorbent, a catalyst, and many kind of biomaterial. Biomaterial based on hydroxyapatite has been applied to dental, orthopedic, and other medical uses [2].
The various methods for preparing hydroxyapatite have been wet method that use solution reaction (from solution to solid), dry method that use solid reaction (from solid to solid), hydrothermal method that use hydrothermal reaction (from solution to solid), alkoxide method that use hydrolysis reaction (from solution to solid), and flux method that use fused salt reaction (from melt to solid).
In hydroxyapatite structure, carbonate can substitute OH- ion, and form carbonate apatite type A, and if substitute PO43- ion will form carbonate apatite type B. Generally, precipitation at low temperature will form carbonate apatite type B, while apatite from dry reaction at high temperature will produce carbonate apatite type A. Biological apatite is predominance of carbonate apatite type B and a few of carbonate apatite type A [9].
Chitin and Chitosan
Chitin and chitosan are
aminoglucopyranans composed of GlcNAc and GlcN residues. These polysaccharides are renewable resources which are currently being explored intensively by an increasing number of academic and industrial research groups [10].
Figure 2 shrimps as one of the chitin resource It is usually understood that chitin (Chemical Abstracts Registry (CAS)
whereas soluble polymers are named chitosan. Chitosan is prepared from suitable chitinous raw materials, mostly by a sequence of deproteinization, demineralization, and chemical deacetylation procedures. The molecular weight of chitosan depends on the source of the biological material, as well as on the condition of the acetylation process [11].
Figure 3 structure of chitosan
Low toxicity, antimicrobial activity and physic-chemical functionality of chitosan offer a great potential for medical applications, as documented in several review (Muzzarelli, 1997b; Muzzarelli et al., 1997a; Paul and Sharma, 2000). Chitosan is currently not registered as a drug for the treatment of disease, but GlcN – either as the hydrochloride or the sulfate – is used widely for the relief of pain in musculoskeletal rheumatoid disease, including arthrosis, arthritis or osteoporosis [14].
Duck Eggshell
The generalized eggshell structure, which varies widely among species, is a protein matrix lined with mineral crystals, usually of a calcium compound such as calcium carbonate. It is calcium build-up and is not made of cells. Harder eggs are more mineralized than softer eggs.
Calcium (Ca) as precursor in apatite mineral synthesis consists in Duck Eggshell in a large scale. Duck Eggshell is kind of bio-mineral composite ceramic which contained 95 % calcium carbonate (CaCO3), and for last 5 % are calcium phosphate, magnesium carbonate, and solutes protein.
Figure 4 Ducks and Duck Eggshells
Without protein, crystal structure is unstable to keep that shape. Matrix compound has control functions to mineralization, crystallographic texture and biomechanical characterization [12].
Table 3 Duck Eggshell major composition [What are eggshell made of? http://FredSenese senese@antoine. frostburg.edu]
Major
composition Contents (%) Melting point
Water 29 - 35 -
Protein 1.4 - 4 -
Calcium
carbonate 95 828°C
Calcium - 837 - 841oC
Magnesium 0.37 - 0.4 648 - 649.3 oC Eggshell color is caused by pigment deposition during egg formation in the oviduct and can vary according to species and breed, from the more common white or brown to pink or speckled blue-green. Although there is no significant link between shell color and nutritional value, there is often a cultural preference for one color over another [13].
Apatite-chitosan Composite
Composite material is combination two or more material phase, either macro or different microform or it is the chemical composition to obtain equilibrium of character applied in wide application. In general, expansion of composite technology is to increase structural efficiency and material character characteristic significant, like for the application of light material but very strong [5].
Ceramics, polymer, metal and composite material, advantage and disadvantage owned by it, developed to overcome bone problems. Polymer haves the power of low mechanic compared to bone, metal haves the power of big mechanic but very corrosive, than ceramics are brittle and it is hardness is low come easy break. Best approach is when producing all the character from polymer, metal and ceramic in the form of composite materials.
Nature composite formed from most of ceramics (hydroxyapatite) and polymer
(collagen), with level of complex
microstructure enables to be imitated causing gives mechanical property at high bone. Many researches which has been done substitution of bone to from composite material formed
from hydroxyapatite and polymer.
and hardly easy to dissolve in dilution of acid. Some studies at composite apatite-chitosan that partially is biodegradable become an advantage. When matrix polymer is reabsorbed, new bone can grow around of hydroxyapatite particles.
Atomic Absorption Spectroscopy (AAS)
Atomic Absorption Spectroscopy is a technique for determining the concentration of a particular metal element in a sample [1]. Atomic absorption spectroscopy can be used to analyze the concentration of over 62 different metals in a solution.
Figure 5 Atomic Absorption Spectroscopy processes
The technique makes use of absorption spectrometry to assess the concentration of an analyte in a sample. It relies therefore heavily on Beer-Lambert law.
In short, the electrons of the atoms in the atomizer can be promoted to higher orbitals for an instant by absorbing a set quantity of energy (i.e. light of a given wavelength). This amount of energy (or wavelength) is specific to a particular electron transition in a particular element, and in general, each wavelength corresponds to only one element. This gives the technique its elemental selectivity.
As the quantity of energy (the power) put into the flame is known, and the quantity remaining at the other side (at the detector) can be measured, it is possible, from Beer-Lambert law, to calculate how many of these transitions took place, and thus get a signal that is proportional to the concentration of the element being measured [15].
X-Ray Diffraction (XRD)
ray Diffraction analysis makes use of X-ray emission resulting from collision between electron and target that can be Cr, Fe, Co, Cu, Mo or W. X-ray emission is continuously specific distributed for each certain wavelength of target. This process has side effect that is the change of kinetic energy of electron become heat, therefore the X-ray quantities influenced by melt point and thermal conductivity of target. XRD analysis
could inform us the structure of sample, such as crystal system, lattice parameter, and preferred orientation. XRD result also can inform volume fraction and crystalline of sample. It is also useful to identify a mixture that is referred to as semi quantitative identification of sample phase. Then X-ray is transmitted through sample that will be characterized, so x-ray will be transformed into varied type of energy and absorbed some.
Figure 6 X-Ray Diffraction processes
Interaction of X-rays with sample creates secondary diffracted beams of X-rays related to inter-planar spacing in the crystalline powder according to a mathematical relation
called Bragg’s Law below [13]:
nλ=2 d sin θ
n is an integer, λ is the wavelength of the X
-rays, d is the interplanar spacing generating
the diffraction, and θis the diffraction angle. λ
and d are measured in the same units, usually
angstrom [15].
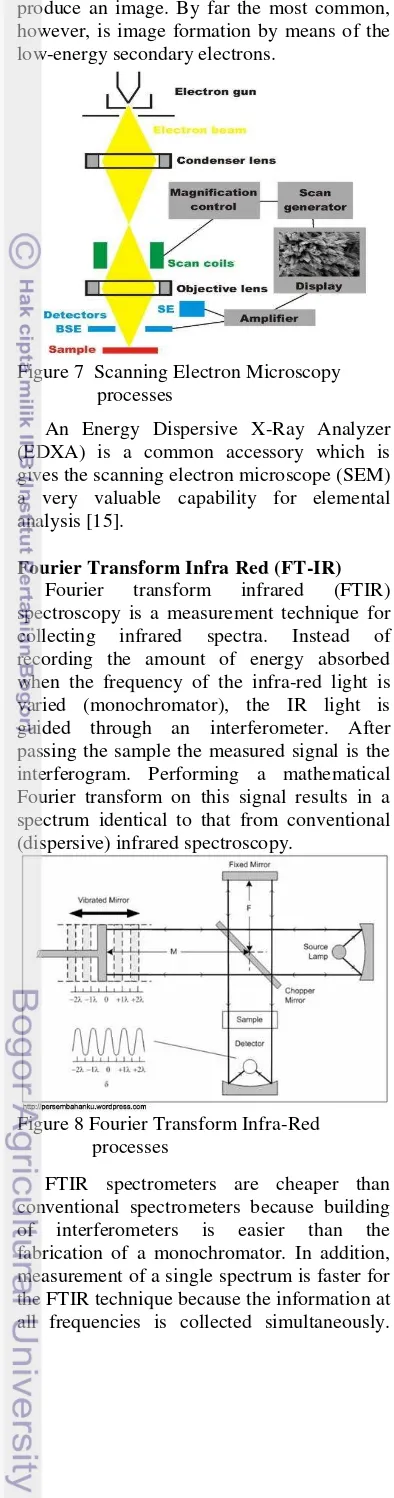
Scanning Electron Microscopy (SEM)
The image in scanning electron
microscope is formed and displayed by making use of electrons. In scanning electron microscope, the surface of a solid sample is scanned in raster pattern with a beam of energetic electrons [14].
The column of an SEM contains an electron gun for producing electrons and electromagnetic lenses corresponding to the condenser system. However, these lenses are operated in such a way as to produce a very fine electron beam, which is focused on the surface of the sample [15]. At any given moment, the sample is bombarded with electrons over a very small area. They may be elastically reflected from the sample or absorbed by the sample and give rise to secondary electrons of very low energy, together with X- rays.
produce an image. By far the most common, however, is image formation by means of the low-energy secondary electrons.
Figure 7 Scanning Electron Microscopy processes
An Energy Dispersive X-Ray Analyzer (EDXA) is a common accessory which is gives the scanning electron microscope (SEM) a very valuable capability for elemental analysis [15].
Fourier Transform Infra Red (FT-IR)
Fourier transform infrared (FTIR) spectroscopy is a measurement technique for collecting infrared spectra. Instead of recording the amount of energy absorbed when the frequency of the infra-red light is varied (monochromator), the IR light is guided through an interferometer. After passing the sample the measured signal is the interferogram. Performing a mathematical Fourier transform on this signal results in a spectrum identical to that from conventional (dispersive) infrared spectroscopy.
Figure 8 Fourier Transform Infra-Red processes
FTIR spectrometers are cheaper than conventional spectrometers because building of interferometers is easier than the fabrication of a monochromator. In addition, measurement of a single spectrum is faster for the FTIR technique because the information at all frequencies is collected simultaneously.
This allows multiple samples to be collected and averaged together resulting in an improvement in sensitivity. Because of its various advantages, virtually all modern infrared spectrometers are FTIR instruments [16].
MATERIALS AND METHODS
Materials and Equipments
The materials which use in this research are CaO, pro-analyze (NH4)2HPO4.2H2O, Chitosan, aquades, and aquabides.
The equipments are beaker glass, mortar, crucible, aluminum foil, pipette Mohr, magnetic stirrer, centrifuge, hotplate, analytical scales, furnace, incubator, digital thermometer, pH meter, whatman filter paper, X-Ray Diffraction (XRD), Fourier Transform Infra Red (FT-IR) and Scanning Electron Microscopy (SEM).
Experimental Methods
Duck Eggshell Calcinations
Duck Eggshell as precursor has to be washed at first, than removing its inner membrane would be easier, before heating it by using furnace in order to remove organic composition of its content and decompose calcium carbonate into calcium oxide at 1000oC for 5 hours. This treatment has the best duration using furnace indicated the largest amount calcium oxide or the least calcium carbonate and efficiently electricity [18]. So after calcinations of Duck Eggshell was finished, powder of eggshell (CaO) will be characterized by X-Ray Diffraction (XRD) to know amount of calcium oxide and calcium
carbonate and Atomic Absorption
Spectroscopy (AAS) to know presentation of calcium in sample.
Precipitation and Composite Synthesis
Control Preparation of Composite
Apatite is obtained by dissolving Duck Eggshell (CaO) which has been calcination in 100 ml aquabides in a beaker glass is continued with addition of (NH4)2HPO4 dissolved in 100 ml aquabides is done with dropper from burette with speed 60ml/minute. Calculation of number of Duck Eggshell and (NH4)2HPO4 based on result from ratio of concentration Ca/P 1.67. Calcium Content from Duck Eggshell follows result of AAS.
with or without pH controlled at about 7). Aging sample during 24 hours at incubator with temperature 37oC. Precipitate then is filtered using centrifuge. Draining of precipitate is done by using incubator at temperature 50oC during 45 hours.
In-situ Preparation of Composite
Treatment of in-situ same as control but at making of sample in-situ Duck Eggshell (CaO) which has been dissolved in 100 ml aquabides is added by chitosan which has been dissolved applies CH3COOH 2%. Amount of chitosan applied through comparison with control result which has been obtained before all 55:35 (55 is result of apatite from control and 35 is amount of chitosan used). CH3COOH 2% which in adding as according to the many chitosan which will be dissolved (applies comparison of volume). Continued by addition of (NH4)2HPO4 dissolved in 100 ml aquabides with dropper from burette with speed 60ml/minute.
Precipitation done at specific situation (nitrogen atmosphere, temperature about 37oC with or without pH controlled at about 7). Aging sample during 24 hours at incubator with temperature 37oC. Precipitate then is filtered using centrifuge. Draining of precipitate is done by using incubator at temperature 50oC during 45 hours.
Ex-situ Preparation of Composite
Treatment of ex-situ same as control, dissolves Duck Eggshell (CaO) which has been calcination in 100 ml aquabides in a beaker glass is continued with addition of (NH4)2HPO4 which dissolved in 100 ml aquabides is done with dropper from burette, with speed 60ml/minute. Addition of chitosan which has been dissolved using CH3COOH 2% is done by after precipitation completed before precipitate in aging, dropped by using pipette. Amount of chitosan applied through comparison with control result which has been obtained before all 55:35 (55 is result of apatite from control and 35 is amount of chitosan used). CH3COOH 2% which in adding as according to amount of chitosan which will be dissolved (used comparison of volume).
Precipitation done at specific situation (nitrogen atmosphere, temperature about 37oC with or without pH controlled at about 7). Aging sample during 24 hours at incubator with temperature 37oC. Precipitate then is filtered applies centrifuge. Draining of
precipitate is done by using incubator at temperature 50oC during 45 hours.
Tabel 4 Samples code
Sample Code Reagent Addition of Chitosan Controled
A1 (Control) (NH4)2HPO4 CaO+ - Temperature and pH
A2 (In-situ) (NH4)2HPO4 CaO+ Precipitation Before Temperature and pH
A3 (Ex-situ) (NH4)2HPO4 CaO+ Precipitation After Temperature and pH
B1 (Control) (NH4)2HPO4 CaO+ - Temperature
B2 (In-situ) (NH4)2HPO4 CaO+ Precipitation Before Temperature
B3 (Ex-situ) (NH4)2HPO4 CaO+ Precipitation After Temperature
XRD Characterization
Equipment XRD used is Shimidzu XRD 7000, source of target of CuKα (λ= 1.54056
Angstroms). Before characterized, sample blended until become powder, and then about 1 gram then is packed into holder which is fairish 2x2 cm2 at diffract meter
.
SEM/EDXA Characterization
Sample is put down in aluminum which has plate two sides then is arranged in layers with auriferous formation as thick 48 nm. Sample which has been arranged in layers observed to applies SEM (Scanning Electron Microscopy) with strain 22 kVs and magnification 5000x, 10000x and 20000x. Characterization with Energy Dispersive X-Ray Analysis (EDXA) is a set with SEM.
FTIR Characterization
Precipitate which has been dried and blended becomes powder characterized by
FTIR spectroscopy. Two milligrams
precipitate mixed with 100 magnesium’s KBr, made infrared pellet (IR) then is tested with wave number reach 4000-400 cm-1, KBr always is figured in each gauging to eliminate adsorption of background.
RESULT AND DISCUSSION
Characteristic of Calcined Duck Eggshell
samples were characterized by AAS, XRD and FTIR.
Duck eggshell contain calcium carbonate (CaCO3). When it is heated, CO2 release into the air to form calcium oxide.
Table 5 Percent of calcium calcined duck eggshell by AAS
Sample Calcium (%)
1000ºC, 5 hours 83.96
1000ºC, 10 hours 85.90
Result of FTIR characterization show compound of calcium carbonate.
Table 6 Percent transmission of carbonate groups
Sample 1450 cmPercent transmission -1 875 cm-1
1000ºC, 5 hours 6 7
1000ºC, 10 hours 7 8
According to FTIR spectra (Figure 10), still there is carbonate content in all samples which is indicated by the presence of IR
carbonate’s group bands around wave number of 1450 cm-1 and 875 cm-1. Although all samples have carbonates bands, each of them diverse in their transmission (Table 7).
The higher percent transmission means the lower carbonates content that exist in the samples. Among two samples, sample B of which heated at 1000oC for 10 hours has the least content of carbonate, 8% at 875cm-1 and 7% at 1450 cm-1.
Figure 10 FTIR spectra of heated duck Eggshell at 1000oC within (a) 5 hours, (b) 10 hours
Although sample B has the least content of carbonate than sample A, but in this research, we use sample A of which heated at 1000oC for 5 hours, because of electricity efficiency of sample A better than sample B. Besides that, the difference percent transmission between sample A and sample B is not significant.
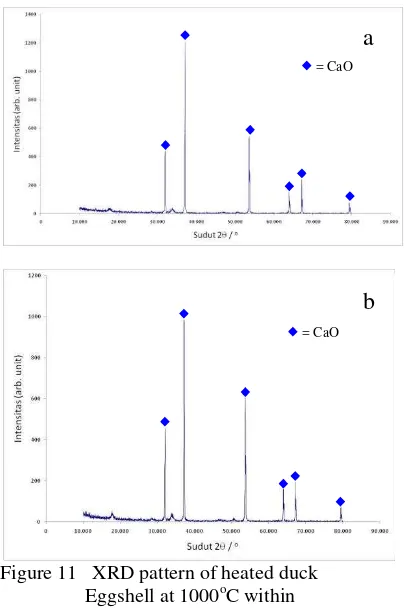
Figure 11 XRD pattern of heated duck Eggshell at 1000oC within
(a) 5 hours, (b) 10 hours
This finding is emphasizing by XRD result (figure 11).
According to JCPDS data base, sample A is dominated by CaO (JCPDS 82-1691), although there is also minor appearance of CaCO3 peak (JCPDS 47-1743).
Figure 11 shown sample A (1200) has intensity (arb. unit) higher than sample B (1000). it is mean that increasing of absorption value of CaO, followed by increasing amount of CaO at the sample.
Experimentally, cooling process after calcinations is conducted rapidly, as the increasing duration of heating treatment was indeed result much more of reconverted calcium carbonate. Therefore, for further process, 1000oC for 5 hours (sample A) set up is chosen.
Characteristic of Apatite-chitosan Composite
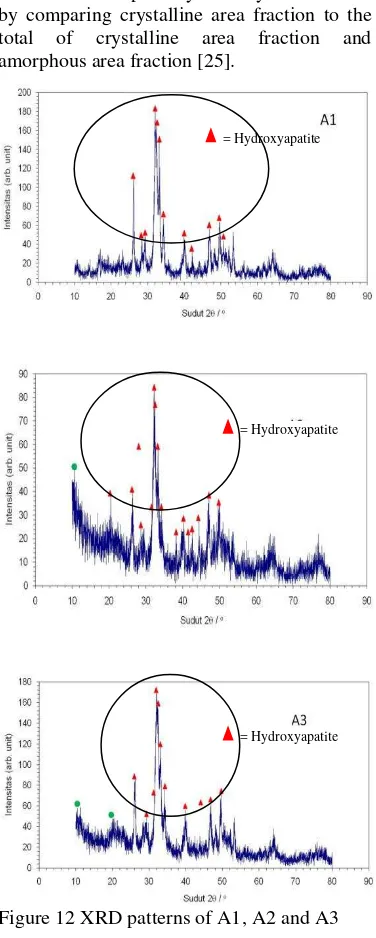
X-Ray Diffraction (XRD)
X-Ray Diffraction pattern (Figure 12) shows that the majority of precipitates correspond to hydroxyapatite (JCPDS 09-0432). Lattice parameter determination using
Cohen’s methods is calculated from the value of d for hexagonal structure, the distance
between adjacent planes in the set (hkl), as the
following equation [24].
Table 7 Crystallinity of sample
Sample Crystallinity (%)
A1 83.22
A2 65.00
A3 62.33
B1 85.13
B2 67.19
B3 65.32
Lattice parameter obtained by calculation is in assumption that those peaks are correspondence to hydroxyapatite as the tendency shows that sample consist of this compound. Peak of XRD pattern is determined by calculation of 2θ accuracy (appendix 7).
Table 8 Lattice parameter of sample
Sample a (Å) c (Å)
Reference 9.418 6.884
A1 9.445 6.885
A2 9.653 6.998
A3 9.835 7.035
B1 9.518 6.985
B2 9.916 7.068
B3 10.011 7.123
The presence of chitosan in sample influences lattice parameter.
Phase that has the highest accuracy is determined as the sample phase. However, there still a probability for that peak be used by more than one phase. Characterization was performed in order to get crystallinity degree that explains the fraction of crystalline phase
that exist in sample. Crystallinity is calculated by comparing crystalline area fraction to the total of crystalline area fraction and amorphous area fraction [25].
Figure 12 XRD patterns of A1, A2 and A3 Lattice parameter of samples (table 8) influence by pH. Sample A1, A2 and A3 has high accuracy of lattice parameter than sample B1, B2 and B3. Lattice parameter accuracy obtained by calculation in assumption that those peak correspond to hydroxyapatite emphasize the tendency that samples are composed of hydroxyapatite, detailed calculation as shown on appendix 7.
Crystallinity of samples (Table 10) is less than 85% but more than 60% and decrease as the addition of chitosan into sample. Crystallinity of sample A1, which normal process (without addition of chitosan) is the highest than sample A2 and A3.
= Hydroxyapatite
= Hydroxyapatite
The data gives information that chitosan as natural polymer; addition into calcium phosphate will reduce its crystallinity. X-Ray Diffraction pattern shown the presence of chitosan at sample A2 at 2θ = 10.57, A3 at 2θ
=10.42 and 19.72. And then sample B2 at 2θ =10.64 and B3 at 2θ =10.40.
Figure 13 XRD patterns of B1, B2 and B3
Based on XRD pattern, A1, A2, and A3 has the higher amount of hydroxyapatite than B1, B2, and B3 which shown with high intensity peak in every sample.
Table 9 Crystal size of sample
Sample code d002 (nm)
A1 29.7133
A2 27.9246
A3 27.4797
B1 25.9294
B2 29.1900
B3 26.5918
Crystallite size is calculated as demonstrated by Scherer’s equation below at 002 planes (appendix 8).
β is FWHM (full width at half maximum) of
the broadened diffraction line on the 2θ scale (radians), λ is wavelength, which is 1.54060x10-10 m and
k is constant, which is
0.94 for biological material. Crystal size varied among addition of chitosan and pH controlled. Comparing to the crystal size of human bone, that in range of 18-23 nm, so the closest crystal size are sample A1, A2, and A3. So, the analysis considering amount of hydroxyapatite, lattice parameter, crystal size and crystallinity, indicated sample A1, A2, and A3 are better, since it is closest crystal size to that human bone and lattice parameter to hydroxyapatite, low crystallinity, and high amount of hydroxyapatite.
Fourier Transform Infra Red (FTIR)
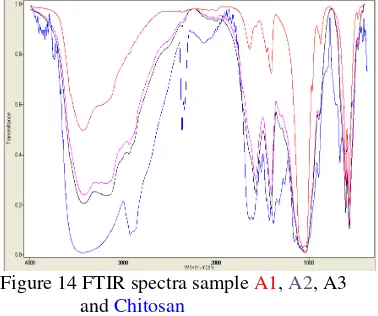
These FTIR spectra below record wavelength versus percent transmission, as shown in detailed on figure 14. The size of the peak or through in the spectrum is a direct indication of the amount of material presence. All IR spectra show the stretching and vibration modes of OH- groups appear at 3565 cm-1 and 635 cm-1, whereas the bands derived from 635 cm-1 is indicated groups of hydroxyapatite [15].
Figure 14 FTIR spectra sample A1, A2, A3 and Chitosan
Analysis of FTIR shows that formed of apatite at sample A and B with functional group appearance PO4, OH and CO3. Functional Group NH2, C-H and amide I and amide II is characteristic from chitosan which appear at sample A2 and A3, it is mean that at sample A2 and A3 has been formed apatite-chitosan composite.
At this sample also seen happened overlapping some wavelength like functional group NH2 that overlap with functional group OH. Happened overlapping at some wavelength numbers owned by chitosan and apatite, show already happened bond between chitosan with hydroxyapatite.
Identification of functional group NH2 and C-H, amide I and amide II at sample A2 and A3, proves that bond chitosan and calcium phosphate were happen. It is mean apatite-chitosan have successfully is formed. Method in-situ and ex-situ show the difference at appear of chitosan. However, functional groups appear in both methods applied same, only different from its transmittance and wavelength.
Scanning Electron Microscopy (SEM)
As seen in figure 15, there are SEM picture of all sample with 20.000 times magnification. It is shown that there is microcrystalline particle as figured of arranged particle orderly. As figured as like a huge number of small ball densely, it is indicating apatite is formed in the samples.
From the sample, we can see that there is indicating hydroxyapatite in spherical phase at samples. Almost all samples have its agglomerated particles, which can be seen on sample A3. Moreover, it can be seen that sample A1 and A2 have more homogenous and densely arrange particles, indicated by the least of agglomerated and differences element on sample surface.
Particle hydroxyapatite in composite disseminates uniform, it can see through chitosan matrix, which has interacted between cells. Pores has different form compared to hydroxyapatite its self, in pure chitosan sample of pore more flatly and when hydroxyapatite joins pore seen more integers compared to be flat.
Figure 15 SEM picture of sample A1, A2, A3 and chitosan
CONCLUSION
In-situ and ex-situ methods results hydroxyapatite, indicated by peak matching of XRD result and supporting by closed lattice parameter to pure hydroxyapatite. pH controlled has significant influence to hydroxyapatite result (quantity), shown by XRD pattern.
Apatite-chitosan composite appear in in-situ and ex-in-situ sample, proved by chitosan peak at XRD pattern, overlapping functional group at FTIR spectra and image from SEM. At least brittle characteristic from ceramic can be overcome with addition of natural polymer (chitosan). Chitosan usage is intended to improve the biocompatibility of samples.
A3
A1
A2
REFERENCES
1. Yildirim, Oktay.2004. Preparation and Characterization of Chitosan/Calcium Phosphate Based Composite Biomaterials.[disertasi].
Turki: Department Materials Science and Engineering, Mayor Materials Science and Engineering. Izmir Institute of Technology.
2. Aoki, Hidaki. Science and medical applications of Hydroxyapatite.
Institute for Medical and Dental Engineering. Tokyo Medical and Dental University. 1991.
3. Yamaguchi.L, Tokuchi.K,
Fukuzaki.H, Koyama.Y,
Takakudaka.K, Monma.J,
Tanaka.H.2001.Preparation and
Microstructure Analysis of Chitosan/HA Nano-Composite.
pp.20-27, Vol.55.Journal of Biomedical Material Research.
4. Steele, D. Gentry; Claud A. Bramblett (1988). The Anatomy and Biology of the Human Skeleton. Texas A&M University Press. p. 4.
5. Baht, Sujata
V.2002.Biomaterials.Pangbone England:Alpha Science International Ltd.
6. Pusateri, A. E., S. J. McCarthy, K. W. Gregory, R. A. Harris, L. Cardenas, A. T. McManus & C. W. Goodwin Jr. (2003). Effect of a chitosan-based
hemostatic dressing on blood loss and survival in a model of severe venous hemorrhage and hepatic injury in swine. Journal of Trauma 4 (1): 177-182.
7. Epple, M., K. Schwarz. Biomimetic Crystallization of Apatite in a Porous
Polymer Matrix. Chem Eur J
1998;4(10):1898-1903.
8. Dasgupta P, A.Singh, S. Adak and KM Purohit.2004.Synthesis and Characterization of hydroxyapatite Produced from Eggshell. International
Symposium of Research on Material Science and Angineering;20-22. 9. Boulpaep, Emile L.; Boron, Walter F.
(2005). Medical physiology: a cellular and molecular approach. Philadelphia: Saunders. pp. p.1089– 1091. ISBN 1416023283.
10. Zhang. Y, Zhang.M.2001. Micro-structural and Mechanical
Characterization of Chitosan Scaffolds Reinforced by Calcium Phosphate. Journal of Non-Crystalline Solids.pp.159-164.Vol.282.
11. Kumar.MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb Aj.2004.Chitosan Chemistry and Pharmaceutical Perspective. Chem Kev.104 (12):60.17-84.
12. Ramakrishna. S, Mayer. J,
Wintermantel. E,
Leong.K.W.2001.Biomedical
Application of polymer-Composite Materials: A review.pp.1189-1224, Vol.61.Composite Science Technology.
13. Kilner, R. M. (2006). "The evolution
of egg colour and patterning in birds". Biological Reviews 81: 383-406.
doi:10.1017/S1464793106007044.
http://journals.cambridge.org/action/di splayAbstract;jsessionid=9512583989 170F5153AF39F18ECDFF8A.tomcat 1?fromPage=online&aid=455543.
14. Rezwan K, QZ Chen, JJ Blaker, AR Boccaccini.. Biodegradable and bioactive porous polymer inorganic composite Scaffolds for Bone tissue Engineering. Biomaterials 2006; 27:
3413-3431.
15. Douglass A Skoog, F James Holler, and Timothy A Nieman. Principles of instrumental analysis, fifth edition.
United States of America: Thomson Learning, Inc; 1998.
16. Wang, M. Composite Scaffolds for Bone Tissue Engineering. American
Journal of Biochemistry and Biotechnology 2006; 2 (2): 80-84.
17. R.L. Smith & G.E. Sandland, "An Accurate Method of Determining the Hardness of Metals, with Particular Reference to Those of a High Degree of Hardness," Proceedings of the Institution of Mechanical Engineers,
Vol. I, 1922, p 623–641.
18. Dasgupta P, A.Singh, S. Adak and KM Purohit.2004.Synthesis and Characterization of hydroxyapatite Produced from Eggshell. International
Symposium of Research on Material Science and Angineering;20-22.
19. Chen.F, Wang.ZN,
Lin.CJ.2002.Preparation and
Nano-composite for use Biomedical Materials.pp.858-861, Vol.57.
Materials Leters.
20. Salvi S, Williams‐Jones A. 2004.
Alkaline granite‐syenite deposits. In
Linnen RL, Samson IM, editors. Rare element geochemistry and mineral deposits. St. Catharines (ON): Geological Association of Canada. pp. 315‐341.
21. Steinbuchel. A, Rhee.
K.S.2005.Polysaccharides and
Polyamides in The Food Industry Properties, Production, and Patents.Wiley-VCH Verlag
GmbH&KGaA, Weinheim.
22. Berth, G. Dautzenberg, H. Peter, M.G.1998.Physicochemical
characterization of chitosans varying in degree of acetylation. Carbohydr. Polym.36, 205-216.
23. Tipler, Paul.A.1991.Fisika untuk Sains dan Teknik.Erlangga:Jakarta.
24. Hankins W, Hankins M. 1974. Introduction to Chemistry. C. V. Mosby Company. London.
Appendix 1 Experimental Flow Chart
No
Deproteinzation of Duck eggshell
Materials and equipments preparation
Characterization of Apatite-Chitosan Composite with FTIR, XRD and SEM
Data analysis and report arrangement
Yes
Precipitations
Aging and Samples Drying
XRD and AAS of CaO’s powder
Appendix 2 XRD pattern of calcined duck eggshell
A
1000oC, 5 hours
B
Appendix 3 Characterization Devices
Fourier Transform Infra Red Device
Scanning Electron Microscopy Device
Appendix 4 Calculation of Chemical Reaction
Ca/P Mr (NH4)2HPO4 Mr Ca [Ca] [P] V1 aqubides (liter)
1.67 132.05 40.08 0.5 0.299 0.10
Gram EggShell Gram Ca Gram (NH4)2HPO4 Asam Asetat
Appendix 5 Calculation of maximum XRD peak of samples
A1
Peak Control HAp OKF AKA AKB Phase
2θ int int-f 2θ int %Δ2θ 2θ int %Δ2θ 2θ int %Δ2θ 2θ int %Δ2θ
10.42 42 12.5749 10.820 12 96.3031 9.744 40 93.0624 10.723 14 97.1743 AKA
13.30 36 10.7784 12.919 1 97.0509 AKA
15.18 38 11.3772 16.841 6 90.1372 16.043 90 94.6207 16.801 5 90.3518 OKF
16.78 42 12.5749 16.841 6 99.6378 16.043 90 95.4061 16.801 5 99.8750 AKA
22.76 52 15.5689 22.902 10 99.3800 22.723 80 99.8372 22.673 7 99.6163 OKF
25.86 174 52.0958 25.879 40 99.9266 25.955 100 99.6340 25.951 35 99.6493 25.726 25 99.4791 HAp
28.30 74 22.1557 28.126 12 99.3814 28.126 80 99.3814 28.511 17 99.2599 28.126 <2 99.3814 HAp
28.76 72 21.5569 28.966 18 99.2888 28.126 80 97.7459 28.511 17 99.1267 28.126 <2 97.7459 HAp
31.74 334 100.0000 31.773 100 99.8961 31.589 100 99.5220 31.503 100 99.2477 32.172 100 98.6572 HAp
31.92 322 96.4072 31.773 100 99.5373 31.589 100 98.9522 31.503 100 98.6763 32.172 100 99.2167 HAp
32.84 216 64.6707 32.902 60 99.8116 32.533 90 99.0563 32.569 50 99.1679 32.172 100 97.9237 HAp
34.00 124 37.1257 34.048 25 99.8590 33.889 19 99.6725 34.168 10 99.5083 HAp
41.86 44 13.1737 42.029 10 99.5979 42.527 2b 98.4316 HAp
43.72 40 11.9760 43.804 8 99.8082 43.715 2b 99.9886 AKB
46.68 110 32.9341 46.711 30 99.9336 47.071 16 99.1693 HAp
48.20 76 22.7545 48.103 16 99.7983 47.071 16 97.6015 HAp
49.44 124 37.1257 49.468 40 99.9434 49.554 16 99.7699 HAp
A2
Peak Insitu HAp OKF AKA AKB Chitosan Phase
2θ int int-f 2θ int %Δ2θ 2θ int %Δ2θ 2θ int %Δ2θ 2θ int %Δ2θ 2θ int % Δ2θ
10.57 38 3.7750 10.82 12 97.6895 9.744 40 91.5230 10.723 14 98.5732 10.44 361 98.7548 Chitosan
15.20 40 5.4286 16.841 6 90.2559 16.043 90 94.7454 16.801 5 90.4708 OKF
16.76 42 5.9857 16.841 6 99.5190 16.043 90 95.5308 16.801 5 99.7560 AKA
17.90 46 6.3929 18.785 4 95.2888 17.443 80 97.3800 18.583 1 96.3246 OKF
18.62 44 6.6500 18.785 4 99.1216 17.443 80 93.2523 18.583 1 99.8009 19.46 497 95.6835 AKA
21.76 52 7.7714 21.819 10 99.7296 20.783 70 95.2990 21.511 2 98.8425 HAp
23.08 66 8.2429 22.902 10 99.2228 23.082 80 99.9913 23.357 40 98.8141 OKF
25.84 154 9.2286 25.879 40 99.8493 25.955 100 99.5569 25.951 35 99.5723 25.726 25 99.5569 HAp
28.80 82 10.2857 28.966 18 99.4269 29.267 90 98.4043 28.511 17 98.9864 29.355 10 98.1094 HAp
31.86 280 11.3786 31.773 100 99.7262 31.589 100 99.1421 31.503 100 98.8668 32.172 100 99.0302 HAp
31.88 278 11.3857 31.773 100 99.6632 31.589 100 99.0788 31.503 100 98.8033 32.172 100 99.0924 HAp
34.10 146 12.1786 34.048 25 99.8473 33.889 19 99.3774 34.168 10 99.8010 HAp
39.90 80 14.2500 39.818 20 99.7941 39.76 13 99.6479 39.401 6b 98.7335 HAp
41.34 46 14.7643 42.029 10 98.3607 40.396 16 97.6631 HAp
43.78 46 15.6357 43.804 8 99.9452 43.715 2b 99.8513 HAp
45.24 52 16.1571 45.305 6 99.8565 45.209 2b 99.9314 AKB
46.58 88 16.6357 46.711 30 99.7196 47.071 16 98.9569 HAp
48.16 74 17.2000 48.103 16 99.8815 48.985 10 98.3158 HAp
A3
Peak Eksitu HAp OKF AKA AKB Chitosan Phase
2θ int int-f 2θ int %Δ2θ 2θ int %Δ2θ 2θ int %Δ2θ 2θ int %Δ2θ 2θ int % Δ2θ
10.72 50 16.1290 10.82 12 99.0758 10.723 14 98.9720 10.44 361 99.1180 Chitosan
16.56 58 18.7097 16.841 6 98.3315 16.801 5 98.5656 AKA
19.42 40 12.9032 18.785 4 96.6196 19.891 80 97.6321 19.347 10 99.6227 19.46 497 99.7945 Chitosan
21.44 66 21.2903 21.819 10 98.2630 20.785 70 96.8487 21.511 2 99.6699 AKA
22.76 74 23.8710 22.902 10 99.3800 22.723 80 99.8372 22.673 7 99.6163 OKF
25.94 186 60.0000 25.879 40 99.7643 25.955 100 99.9422 25.951 35 99.9576 25.726 25 99.1682 HAp
28.20 92 29.6774 28.126 12 99.7369 28.126 80 99.7369 28.511 17 98.9092 28.126 <2 99.7369 HAp
28.96 102 32.9032 28.966 18 99.9793 29.257 90 98.9849 28.511 17 98.4252 29.355 10 98.6544 HAp
30.26 76 24.5161 28.966 18 95.5327 30.272 70 99.9604 30.485 10 99.2619 29.355 10 96.9170 OKF
31.82 310 100.0000 31.773 100 99.8521 31.589 100 99.2687 31.503 100 98.9937 32.172 100 98.9059 HAp
34.00 128 41.2903 34.048 25 99.8590 33.889 19 99.6725 34.168 10 99.5083 HAp
39.68 92 29.6774 39.818 20 99.8534 39.76 13 99.7988 39.401 6b 99.2919 HAp
41.88 54 17.4194 42.029 10 99.6455 42.527 2b 98.4786 HAp
43.92 58 18.7097 43.804 8 99.7352 43.715 2b 99.5311 HAp
46.74 114 36.7742 46.711 30 99.9379 47.071 16 99.2968 HAp
47.84 74 23.8710 48.103 16 99.4533 47.071 16 98.3663 HAp
B1
2θ Int Int-f HAp AKA AKB OKF Phase
% Δ2θ Int % Δ2θ Int % Δ2θ Int % Δ2θ Int
10.48 62 34.44 96.858 12 97.734 14 40.737 25 92.447 40 AKA
22.90 30 16.67 99.991 10 98.999 7 89.015 25 99.221 80 Hap
25.66 98 54.44 98.793 2 98.879 35 99.743 25 99.672 80 OKF
28.72 44 24.44 99.151 18 99.267 17 97.888 <2 97.888 80 AKA
31.60 180 100.00 99.456 100 99.692 100 98.222 100 99.965 100 OKF
32.66 110 61.11 99.264 60 99.721 50 98.483 100 99.610 90 OKF
35.12 30 16.67 98.985 6 99.620 19 97.610 6 92.048 90 AKA
38.98 35 19.44 99.429 8 99.676 2 98.931 6b 80.183 90 AKA
39.58 40 22.22 99.041 8 99.547 13 99.546 6b 78.339 90 AKB
41.82 22 12.22 99.503 10 94.819 13 98.338 2b 71.454 90 Hap
43.78 22 12.22 99.945 8 89.889 13 99.851 2b 65.429 90 Hap
46.42 62 34.44 99.377 30 83.249 13 98.617 16 57.314 90 Hap
47.88 46 25.56 99.536 16 79.577 13 98.281 16 52.826 90 Hap
49.16 69 38.33 99.377 40 76.358 13 99.205 16 Hap
50.72 38 21.11 99.550 20 72.435 13 99.080 10 Hap
B2
2θ Int Int-f HAp AKA AKB OKF Chitosan Phase
% Δ2θ Int % Δ2θ Int % Δ2θ Int % Δ2θ Int % Δ2θ Int
10.64 71 40.11 98.336 12 99.226 14 41.359 25 90.805 40 99.484 346 Chitosan
13.76 49 27.68 81.705 6 93.490 1 53.487 25 85.769 90 AKA
18.60 53 29.94 99.015 4 99.909 1 72.300 25 93.367 80 95.581 497 AKA
25.62 121 68.36 98.999 40 98.725 35 99.588 25 99.828 80 OKF
28.66 59 33.33 98.944 18 99.477 17 98.101 <2 98.101 80 AKA
31.56 177 100.00 99.330 100 99.819 100 98.098 100 99.908 100 OKF
31.84 149 84.18 99.789 100 98.930 100 98.968 100 99.205 100 HAp
32.62 117 66.10 99.143 60 99.843 50 98.607 100 99.733 90 OKF
33.70 62 35.03 99.578 25 99.442 19 99.123 40 96.413 90 HAp
39.60 48 27.12 99.553 20 99.598 13 99.495 6b 78.277 90 HAp
46.34 66 37.29 99.206 30 83.451 13 98.447 16 57.560 90 HAp
49.24 64 36.16 99.539 40 76.157 13 99.366 16 48.646 90 HAp
50.10 42 23.73 99.222 20 73.994 13 98.898 16 HAp
51.32 34 19.21 98.362 20 70.926 13 99.748 10 AKB
B3
2θ Int Int-f HAp AKA AKB OKF Chitosan Phase
% Δ2θ Int % Δ2θ Int % Δ2θ Int % Δ2θ Int % Δ2θ Int
10.40 77 45.56 96.118 12 96.988 14 40.426 25 93.268 40 99.617 346 Chitosan
19.92 55 32.54 93.958 4 97.038 10 77.431 25 99.854 80 99.600 552 OKF
25.38 121 71.60 99.897 2 98.977 3 98.655 25 99.234 80 HAp
27.72 53 31.36 98.556 12 97.226 17 98.556 <2 99.859 80 OKF
28.54 56 33.14 98.529 18 99.898 17 98.528 <2 98.528 80 AKA
31.36 169 100.00 98.700 100 99.546 100 97.476 100 99.997 80 OKF
31.84 142 84.02 99.789 100 98.930 100 98.968 100 99.205 100 HAp
32.56 100 59.17 98.961 60 99.972 50 98.794 100 99.167 90 AKA
33.54 69 40.83 98.508 25 98.970 19 99.602 40 96.905 90 AKB
35.22 31 18.34 99.287 6 99.270 1 97.888 6 91.741 90 HAp
39.36 44 26.04 99.602 8 99.766 19 99.896 6b 79.015 90 AKB
41.46 30 17.75 98.646 10 95.724 13 97.366 16 72.560 90 HAp
43.30 32 18.93 99.149 8 91.097 13 99.051 2b 66.904 90 HAp
46.22 59 34.91 98.949 30 83.753 13 97.764 2b 57.929 90 HAp
47.66 41 24.26 99.079 16 80.131 13 98.749 16 53.503 90 HAp
50.12 44 26.04 99.261 20 73.944 13 98.858 16 HAp
Appendix 6 JCPDS Reference
AKA
AKB
CaO (Calcium Oxide)
Appendix 7 Lattice parameter calculation match to hidroxyapatite
A1
2θ h k l α 2θ (rad) θ δ sin2θ αsin2θ sin2θ δsin2θ α2 2 δ2 α δ αδ
10.42 1 0 0 1 0 0.1819 0.0909 0.3271 0.0082 0.0082 0.0000 0.0027 1 0 0.1070 0 0.0000 0.3271
13.30 1 0 0 1 0 0.2321 0.1161 0.5292 0.0134 0.0134 0.0000 0.0071 1 0 0.2801 0 0.0000 0.5292
15.18 1 0 1 1 1 0.2649 0.1325 0.6857 0.0174 0.0174 0.0174 0.0120 1 1 0.4701 1 0.6857 0.6857
16.78 1 0 1 1 1 0.2929 0.1464 0.8335 0.0213 0.0213 0.0213 0.0177 1 1 0.6947 1 0.8335 0.8335
22.76 1 1 1 3 1 0.3972 0.1986 1.4967 0.0389 0.1168 0.0389 0.0583 9 1 2.2401 3 1.4967 4.4901
25.86 0 0 2 0 4 0.4513 0.2257 1.9025 0.0501 0.0000 0.2003 0.0953 0 16 3.6194 0 7.6099 0.0000
28.30 1 0 2 1 4 0.4939 0.2470 2.2476 0.0598 0.0598 0.2390 0.1343 1 16 5.0517 4 8.9904 2.2476
28.76 2 1 0 7 0 0.5020 0.2510 2.3150 0.0617 0.4318 0.0000 0.1428 49 0 5.3591 0 0.0000 16.2048
31.74 2 1 1 7 1 0.5540 0.2770 2.7674 0.0748 0.5234 0.0748 0.2069 49 1 7.6588 7 2.7674 19.3721
31.92 1 1 2 3 4 0.5571 0.2786 2.7956 0.0756 0.2268 0.3024 0.2114 9 16 7.8154 12 11.1824 8.3868
32.84 3 0 0 9 0 0.5732 0.2866 2.9408 0.0799 0.7192 0.0000 0.2350 81 0 8.6485 0 0.0000 26.4675
34.00 2 0 2 4 4 0.5934 0.2967 3.1270 0.0855 0.3419 0.3419 0.2673 16 16 9.7779 16 12.5079 12.5079
39.76 3 1 0 13 0 0.6939 0.3470 4.0905 0.1156 1.5033 0.0000 0.4730 169 0 16.7325 0 0.0000 53.1770
41.86 3 1 1 13 1 0.7306 0.3653 4.4531 0.1276 1.6589 0.1276 0.5683 169 1 19.8298 13 4.4531 57.8898
43.72 1 1 3 3 9 0.7631 0.3815 4.7767 0.1386 0.4159 1.2477 0.6622 9 81 22.8166 27 42.9900 14.3300
45.14 2 0 3 4 9 0.7878 0.3939 5.0244 0.1473 0.5892 1.3258 0.7402 16 81 25.2449 36 45.2199 20.0977
46.68 2 2 2 12 4 0.8147 0.4074 5.2930 0.1570 1.8836 0.6279 0.8308 144 16 28.0163 48 21.1722 63.5166
48.20 3 1 2 13 4 0.8412 0.4206 5.5573 0.1667 2.1675 0.6669 0.9266 169 16 30.8841 52 22.2294 72.2455
49.44 2 1 3 7 9 0.8629 0.4314 5.7718 0.1749 1.2241 1.5739 1.0094 49 81 33.3140 63 51.9464 40.4028
50.54 3 2 1 19 1 0.8821 0.4410 5.9609 0.1822 3.4624 0.1822 1.0863 361 1 35.5323 19 5.9609 113.2570
15.3850 6.9882 7.6874 1304 345 264.0934 302 240.0458 526.9688
D 183696.9 1304 302 526.9688 A : 0.001298
302 345 240.0458 B : 0.011895
526.9688 240.0458 264.0934 C : 0.008519
DA 238.525 1304 302 15.3850
302 345 6.9882 a : 9.445583
526.9688 240.0458 7.6874 c : 6.885799
DB 2185.109 1304 15.3850 526.9688
302 6.9882 240.0458
526.9688 7.6874 264.0934
DC 1564.865 15.3850 302 526.9688
6.9882 345 240.0458
7.6874 240.0458 264.0934
2 2
2 2
2 2
sin
,
sin
,
,
sin
,
C
B
A
C
B
A
C
B
A
A2
2θ h k l α 2θ (rad) θ δ sin2θ αsin2θ sin2θ δsin2θ α2 2 δ2 α δ αδ
10.57 1 0 0 1 0 0.1845 0.0922 0.3365 0.0085 0.0085 0.0000 0.0029 1 0 0.1132 0 0.0000 0.3365
15.20 1 0 1 1 1 0.2653 0.1326 0.6874 0.0175 0.0175 0.0175 0.0120 1 1 0.4726 1 0.6874 0.6874
16.76 1 0 1 1 1 0.2925 0.1463 0.8315 0.0212 0.0212 0.0212 0.0177 1 1 0.6914 1 0.8315 0.8315
17.90 1 0 1 1 1 0.3124 0.1562 0.9447 0.0242 0.0242 0.0242 0.0229 1 1 0.8924 1 0.9447 0.9447
18.62 1 1 0 3 0 0.3250 0.1625 1.0195 0.0262 0.0785 0.0000 0.0267 9 0 1.0393 0 0.0000 3.0584
21.76 2 0 0 4 0 0.3798 0.1899 1.3743 0.0356 0.1425 0.0000 0.0490 16 0 1.8888 0 0.0000 5.4973
23.08 1 1 1 3 1 0.4028 0.2014 1.5368 0.0400 0.1201 0.0400 0.0615 9 1 2.3616 3 1.5368 4.6103
25.84 0 0 2 0 4 0.4510 0.2255 1.8997 0.0500 0.0000 0.2000 0.0950 0 16 3.6090 0 7.5989 0.0000
28.32 1 0 2 1 4 0.4943 0.2471 2.2505 0.0598 0.0598 0.2394 0.1347 1 16 5.0648 4 9.0020 2.2505
28.80 2 1 0 7 0 0.5027 0.2513 2.3209 0.0618 0.4329 0.0000 0.1435 49 0 5.3864 0 0.0000 16.2461
31.86 2 1 1 7 1 0.5561 0.2780 2.7862 0.0753 0.5273 0.0753 0.2099 49 1 7.7630 7 2.7862 19.5035
31.88 2 1 1 7 1 0.5564 0.2782 2.7893 0.0754 0.5280 0.0754 0.2104 49 1 7.7804 7 2.7893 19.5254
34.10 2 0 2 4 4 0.5952 0.2976 3.1432 0.0860 0.3439 0.3439 0.2702 16 16 9.8795 16 12.5726 12.5726
39.90 3 1 0 13 0 0.6964 0.3482 4.1146 0.1164 1.5134 0.0000 0.4790 169 0 16.9297 0 0.0000 53.4895
41.34 3 1 1 13 1 0.7215 0.3608 4.3629 0.1246 1.6198 0.1246 0.5436 169 1 19.0353 13 4.3629 56.7183
43.78 1 1 3 3 9 0.7641 0.3821 4.7871 0.1390 0.4170 1.2510 0.6654 9 81 22.9167 27 43.0842 14.3614
45.24 2 0 3 4 9 0.7896 0.3948 5.0419 0.1479 0.5917 1.3314 0.7458 16 81 25.4206 36 45.3770 20.1675
46.58 2 2 2 12 4 0.8130 0.4065 5.2756 0.1563 1.8760 0.6253 0.8247 144 16 27.8322 48 21.1025 63.3075
48.16 3 1 2 13 4 0.8406 0.4203 5.5504 0.1665 2.1642 0.6659 0.9240 169 16 30.8070 52 22.2016 72.1553
49.50 2 1 3 7 9 0.8639 0.4320 5.7822 0.1753 1.2269 1.5775 1.0135 49 81 33.4335 63 52.0396 40.4752
11.7134 6.6126 6.4523 927 330 223.3175 279 226.9174 406.7389
D 106356 927 279 406.739 A : 0.003321
279 330 226.917 B : 0.011138
406.7389 226.917 223.317 C : 0.007827
DA 353.1566 927 279 11.7134
279 330 6.6126 a : 9.6537
406.7389 226.917 6.45231 c : 6.9988
DB 1184.566 927 11.7134 406.739
279 6.6126 226.917
406.7389 6.45231 223.317
DC 832.4186 11.71339 279 406.739
6.612595 330 226.917
A3
2θ h k l α 2θ (rad) θ δ sin2θ αsin2θ sin2θ δsin2θ α2 2 δ2 α δ αδ
10.72 1 0 0 1 0 0.1871 0.0935 0.3460 0.0087 0.0087 0.0000 0.0030 1 0 0.1197 0 0.0000 0.3460
16.56 1 0 1 1 1 0.2890 0.1445 0.8124 0.0207 0.0207 0.0207 0.0168 1 1 0.6599 1 0.8124 0.8124
19.42 1 1 0 3 0 0.3389 0.1695 1.1055 0.0284 0.0853 0.0000 0.0314 9 0 1.2221 0 0.0000 3.3165
21.44 2 0 0 4 0 0.3742 0.1871 1.3361 0.0346 0.1384 0.0000 0.0462 16 0 1.7852 0 0.0000 5.3444
22.76 1 1 1 3 1 0.3972 0.1986 1.4967 0.0389 0.1168 0.0389 0.0583 9 1 2.2401 3 1.4967 4.4901
25.94 0 0 2 0 4 0.4527 0.2264 1.9134 0.0504 0.0000 0.2015 0.0964 0 16 3.6613 0 7.6538 0.0000
28.20 1 0 2 1 4 0.4922 0.2461 2.2330 0.0593 0.0593 0.2374 0.1325 1 16 4.9865 4 8.9322 2.2330
28.96 2 1 0 7 0 0.5054 0.2527 2.3445 0.0625 0.4376 0.0000 0.1466 49 0 5.4966 0 0.0000 16.4114
30.26 2 1 0 7 0 0.5281 0.2641 2.5394 0.0681 0.4769 0.0000 0.1730 49 0 6.4486 0 0.0000 17.7758
31.82 2 1 1 7 1 0.5554 0.2777 2.7800 0.0751 0.5260 0.0751 0.2089 49 1 7.7281 7 2.7800 19.4597
32.84 3 0 0 9 0 0.5732 0.2866 2.9408 0.0799 0.7192 0.0000 0.2350 81 0 8.6485 0 0.0000 26.4675
34.00 2 0 2 4 4 0.5934 0.2967 3.1270 0.0855 0.3419 0.3419 0.2673 16 16 9.7779 16 12.5079 12.5079
39.68 3 1 0 13 0 0.6925 0.3463 4.0768 0.1152 1.4975 0.0000 0.4696 169 0 16.6204 0 0.0000 52.9986
41.88 3 1 1 13 1 0.7309 0.3655 4.4565 0.1277 1.6605 0.1277 0.5692 169 1 19.8607 13 4.4565 57.9349
43.92 1 1 3 3 9 0.7665 0.3833 4.8115 0.1398 0.4195 1.2586 0.6729 9 81 23.1510 27 43.3039 14.4346
46.74 2 2 2 12 4 0.8158 0.4079 5.3035 0.1573 1.8881 0.6294 0.8345 144 16 28.1271 48 21.2140 63.6420
47.84 3 1 2 13 4 0.8350 0.4175 5.4949 0.1644 2.1372 0.6576 0.9033 169 16 30.1935 52 21.9794 71.4332
49.50 2 1 3 7 9 0.8639 0.4320 5.7822 0.1753 1.2269 1.5775 1.0135 49 81 33.4335 63 52.0396 40.4752
11.7607 5.1664 5.8785 990 246 204.1608 234 177.1763 410.0832

![Table 2 Composite material for use in the body [5]](https://thumb-ap.123doks.com/thumbv2/123dok/693956.359015/14.595.321.514.209.480/table-composite-material-use-body.webp)