Location
and
distribution
pattern
of
nitric
oxide synthesizing neuronal
cells
in
the digestive
tract
and
urinary
bladder of mice
Ahmad
Aulia
Jusuf
Abstrak
Dalam rangka menyelidiki lebih lanjut lokasi dan pola penyebaran sel sarafyang mengandung enzim "nitrtc oxide syûhase" (NOS) pada susuian saràf pertfer otonom saluran cerna dan lundung kemih, organ tersebut diwarnai dengan
teloik
histokimia untuk 'mendetelrsiNADPH diaforase. Sejumlah kelompokan sel yang positif lerhadap pewarnaan NADPH diaforase terdetelcsi
di
semua bagian saluran cerna, yaitu esofogus, lambung, usus halus, dah usus besar. Selain itu, sel tersebuliuga
terdetelesi pada kandung kelnih. Set-sel ini hadir dalam jumiah yang sangat sedikit pada lapisan submukosa (pleksus submukosa Meissner/ "submucous plexus of Meissner") dan dalam jumlah banyak pada daerah antara lapis dalam dan luar otot polos saluran cerna (plelcsus mienterilas Àuerbach/"myenturtc plexuses ofAuerbach"). Pada knndung kemih, sel tersebut terletak pada leher kandung kemih ("bladder neck"), dan pada lapisan otot polos sebelah dalam. Karena jumlah sel sarafyang mengandung NADPH diaphorase per milimeter persegitebti banyai terdapat di lambung dan usus besar, ada
kemu
Hal inimengesankan fungsi nitric oxide (NO) yang cukup
penting
,
ut pada daeiah leher ttorâung tremih ("bladder neck") mungkinmenuniu
sJingter vesila-uretra ( " vesico-uret hral sphincter mus cle ")'
Abstract
In
order to investigate further the location and distribution patternof
NOS containing neuronal cellsin
the peripheral autonomic nerve systemof
digestivetrac!
and urinary bladder, the digestive and bladder organs were stainedby
NADPH diaphorase' histochàmical staining. Some groups ofNADPH diaphorase positive neuronal cells were detected along all parts of lhe digestive tract, including esophagus, stomach, small and large inlestine, as well as urtnary bladder. In the digestive tract, these cells were exisled in a,"ry s^àil
number in the submucous layer (submucous plexuses of Meissner) and ubiquitousin
the area between lhe external and internal loyer ofsmooth muscle (myenleic plexuses ofAuerbach). In the bladder, those cells existed at the bladder neck, and in the inner layer of the muscle of urtnary bladder. Since the number of NADPH diaphorase containing neuronal cells per millimeler square is more numerous in stomach and large inlestine, lhere is a possibilily lhat the amounl of nitric oxide in those organs is also higher. Thesefacts suggest an importantrolefor
nitrict oxice (NO) in those organs. Furlhermore, lhe existence of those cells in the limited area of urinary bladder i.e. in the bladder neck may suggesl the role of nitic oxide (NO) in vesico-urethral sphincter muscle function. Kquords: NADPH diaphorase, nitric oxide synthase, submucous plexuses of Meissner, myenteric pluuses of Auerbach, bladder neckNitric
oxide (NO)
is
a simple
gas
with free
radical
chemical properties,
but
it
is often
confusedwith
thechemically distinct nitrous oxide
(N2O)which
is
used as an anesthetic andis chemically
stable.Nitric
oxide
is
ap
important
messenger
molecule produced
by
endothelial cells, neurons, skeletal muscle,
neutro-phils,
macrophages,
pancreatic islets, endometium,
Deportment of Histologt, Faculty of Medicine, University oJ Indonesia, J akart a, Indones i a
Inlenutional Center
for
Menical Research (ICMR)' School oJ Kobe, Japan 260, E-mail address : [email protected]epitheli
estinaltract,
andothers.r
diator
becauseit
is
a gaswith
unknown
storage mechanism.It
diffusesfreely
across membranes, and is extremelylabile
with
abiological
halflife
on the order ofseconds.No binds
to
and
activates
soluble guanylyl
cyclaseresulting
in
increased
cGMP
level.
The exact
mechanism
by
which cGMP
exerts
its
biological
Nitric
oxide
also bindsto
avariety
of iron- and
sulfur-containing
proteins.5Nitric
oxide
is
synthesized
via
catalytic actions
of
nitric
oxide
synthase (NOS). Severalhomologous but
separate
genes
code
for
neuronal
NOS
(nNOS),
endothelial
NOS
(eNOS), and macrophage-derived
NOS (also known
as
inducible
NOS/iNOS).5
Neuronal
NOS and endothelial NOS are produced
constitutively and
are
calmodulin
dependent; their
activity are stimulated
by
calcium. Those enzymes
also needed reducednicotinamide
adenindinucleotide
phosphate
(NADPH) as
an
electron donor
in
their
catalytic
activity.
Inducible
NOS
are
producedinducibly by
glucocorticoid
andcytokines
like gamma
interferon
and
lipopolysaccharide,
and
does
not
depend oncalcium
for
its
activity.6
All
forms of NOS
use arginine as
their
substrate,
and
form
NO
andcitnrlline
stoichometrically.
In neurons,
NOS
diaphorase.T'8Hope et ale
also
efor
the ideathat
NADPH
diaphorasemay correspond to
neuronalnitric
oxide
synthase.
This
NADPH-
and
CA2*/Calmodulin-dependent
eîT;yrl"ire(NO!)
forms NO by
conversion
of
arginine
to
citruline.lo
In
the
presenceof
NADPH
diaphorase,
the
reduced
form
of
nitric
oxide
qmthase
can
catalyze
the
conversion
of
a solubletetrazolium to
aninsoluble
visible
formazan.ll
This
multiple step
reaction
used
in
NADPH
diaphorasehistochemical staining
is
illustrated
in the
schematicfigure
below.'-This NADPH
diaphorase
histochemical staining
isresistant
to
formaldehyde fixation and displays the
cahracteristicsof
an
enzymatic reaction. I ao2,Ca2*
/caM
o2,NADpHArginine
----}
Arginine-OH-JCitrulline
+ NOA
ca2'lcaMI
NOS-flavin
-+
Nos-flavinH2NADPH
It
NBT
--+
NBT formazan
NADPH Diaphorase
CaM
:
calmodulinNADPH = nicotinamide adenin dinucleotide phosphate NO
:
nitric oxideNOS: nitric oxide synthase
NBT = Nitro blue tetrazolium
NOS
initially receives the electrons
from
NADPH in
acalcium/calmodulin-
and
arginine-independent
stepthat presumably involves reduction
of
oneor
both
of
the
associated
flavins.
The
reduced
NOS
will
berapidly
re-oxidized
in
the
presenceof
an
appropriateelectron acceptor, such
as
nitro
blue
tetrazoliutn
(NBT).
In
the
presence
of
calcium, calmodulin
andmolecular oxygen, the
reducedNOS
will
hydroxylate
theguanidino group
of
arginine
in
a P-450like
mono-oxygenase reaction.13 Thetightly associated
hydroxy-arginine is then
further
oxidized
by
NOS
to citrulline
andNO.
In
the
neryous system,
nNOS
is
located
to
discretepopulations
of
neurons
in
the
cerebellum, olfactory
bulb,
hippocampus,
cortex,
striatum, basal forebrain,
and
brainstem.
NOS
is
also
concentrated
in
theposterior
pituitary gland,
supra-optic
and
para-ventricular hypothalamic
nuclei, and
in
discreteganglion
cellsof
the adrenalmedulla.ls
The
localization
and
distribution pattern
of
NOS
containing neuronal
cells in
the peripheral
autonomicnervous system, especially
in
the
digestive
tract
andurinary
bladder are stillunclear.
Gabella et al.16failed
to
detect intramural ganglia
in
the
urinary
bladder
of
the mouse.However, Grozdanovic
et allT claimed thatthey
had succesfully
demonstratedthe occurrence
of
intramural
neurons
in
the lower urinary tract
of
the mouse.The knowledge
of
localization
anddistribution
pattern
of
NOS
containing neuronal
cells
in
thedigestive
tract
and urinary
bladder
will
give
implication
andmore stimulation
to
study the
role
of
nitric
oxide on the
function of
the digestive tract
andurinary
bladder.In
order
to
investigate further
the
localization
anddistribution pattern
of NOS
containing neuronal
cellsin
the
peripheral autonomic nervous system
of
digestive
tract
and
urinary
bladder,
we
stained
bothwhole mount and
sections
of those organs with
NADPH
diaphorase histochemical
staining
andcounted
the number (density)
of
positive cells
per-millimeter
squarein
the
digestive
tract
and
the
total
number
of those
cells
in the urinary
bladder. We
also discussed thepossible
function of NO in those organs
and the possible correlation between
the
localization
and distribution pattern
of
those positive cells
andtheir
naturalfunctions.
METHODS
Animal
maintained
in the
animal
centerin a
specific
pathogen freecondition. In this experiment
3 male and 3 femalemice
were used
in both group
(the experimental
andcontrol
group).Sectioned NADPH
diaphorase histochemical staining
Using a wing
needle,
mice were cardiacly
perfusedwith
0.1
M
phosphatebuffer @H7.4)
containing4%oparaformaldehyde.
The isolated tissues were
thenpost-fixed
with
4yo
paraformaldehyde equilibrated
with
20oÂsucrose,
for
12 hours.
Tissue
blocks for
cryostat
sections
were
prepared
by
embedding
each tissueinto
a tissuecompound
andallowed them to
beharden
in
the
cryostat
chamber.
Using
a
cryostat, these blocks weresectioned
at
10
pm,
and
mountedon the
glass slides.The
slideswere
then washedwith
0.1 M phosphate buffer (pH7.4)
for
3-5 minutes,
andput
on the rackunderlied
with wet paper
towel.
In the
experimental
group, after
washing,
the slides
were
incubated
in 0.1
M
phosphate
buffer
OH
7.4)containing
1.0
mg/ml p-NADPH (Sigma, St.
Louis,
MO),
0.1 mg/ml
nitro
blue tetrazolium
(NBT)
and0.3% Triton X-100,
for
60
minutes,
at 37oC.In the
control group, the
same procedure
were
performed,
except
the last
step. Afterwashing, the
control slides
were
incubated
in 0.1
M
phosphatebuffer (pH 7.4)
containing
only
0.1
mg/ml NBT
and0.3%
Triton
X-100,for
60minutes, at37oC.
lVhole
mount
NADPH
diaphorase histo-chemical
staining
Digestive and urinary bladder
tissues
were isolated
from
cardiacly
perfusedmice
as described above, andpost
fixed with
4%oparaformaldehyde
for
12 hours.In
the experimental group,
after
washing
with
PBS,whole
tissueswere
incubated
with
0.1 M phosphate
buffer G,H 7.4)
containing
1.0 mg/ml
B-NADPH
(Sigma, St.
Louis,
MO), 0.1 mg/ml nitro blue
tetra-zolium
and0.3%oTriton
X-100,
for 90-120 minutes,
at37oC. In control group,
after
washing
with
PBS,whole
tissuesv/ere incubated
with
0.1
M
phosphatebuffer (pH 7.4)
containing
only
0.1
mg/ml
NBT and
0.3%Triton X-100, for
90-120minutes, at37oC.
The Number
of
NADPH
diaphorase
positive
neuronal cells
The number
of NADPH
diaphorasepositive neuronal
cells
in the
urinary bladder is very
little (only
a singlecell
or a cluster
of
ganglion cells) while they were
particularly numerous
in
the bladder neck
regions.Therefore,
in
the
whole
mount
samples, total numberof those
cells was counted
directly
in all
microscopic
fields, under
light
microscope.
The number
per-millimeter square
(density) of
those
cells
in
severaldifferent
parts
of the
digestive
tract
were
countedby
using
enlargement
of
microphotographs
of
defined
organs.
The
averageand
standarddeviation
valuesof
the
density
or total
number
of
thosepositive cells in
the different parts
of
the digestive tract
or
urinary
bladder
were statistically
calculated
from
6
different
observed animals.
RESULT
To investigate the
localization
anddistribution
patternof
nitric
oxide synthesizing neuronarl cells
in
thedigestive
tract and urinary
bladder,
histochemical
staining method
to
detect
NADPH
diaphorase
wasperformed.
The
diaphorase
positive
neuronalperikarya, neuronal fibers and epithelium
cells of the
mucosallayer
showedblue precipitates
obtainedfrom
the
conversion
of
nitro
blue tetrazolium
into
NBT
formazan
(Figure
I
and
2).
These
blue precipitates
wereclearly
locatedonly
in the
cytoplasm of
neuronal cells andtheir
nerve fibers.Digestive
tract
The numerous groups
of NADPH
diaphorasepositive
neuronal cells were
detected
along
all
part
of
thedigestive
tract,
including
esophagus, stomach,
andsmall
and large intestine
(Figurel
and
2A,C).
Thesecells
existed invery
limited
number
in the submucous
plexusesof
Meissner
locatedin
the
submucosal layer.Furthermore,
they
were
ubiquitous
in myenteric
plexusesof
Auerbach located
in
the
area between theexternal
and intemallayer,
of
the
smooth muscle coat(Figure
I
square
box areasrand Figure 2C
arrow
marked).
The epithelial cells
in
the mucosal
layer
along
all parts
of
digestive tract were
alsoreactive
for
this NADPH
diaphorase
histochemical
staining.
No
NADPH
diaphorase
positive
cell
was found
in
the digestive tissuesof
thecontrol
samples.In
the
large
intestine
(Figure
2A and
C)
many
diaphorase
positive
neuronal
perikarya were located
within
the primarymeshwork
of the
myenteric
plexusof Auerbach,
and in the area between the external andgave
the
innervation
to
the
muscle
layer
of
thedigestive
tract through the
neurites
and also
to
the submucousplexus
of
Meissner.
The
neurpnal fibers
which
connected theneuronal cells
one anothercould
be
detected
more clearly
in
the large
intestine
thanthose in
the
other parts
of
the
digestive
tract.
TheseNADPH
diaphorasepositive
neuronal cells belong to
bipolar
andmulti-polar
neuronal cells
(Figure
I
and,2A,
C).Urinary bladder
Neuronal
perikarya staining
for
NADPH
diaphoraseparticularly existed in
the
urinary
bladder
at
thebladder base and bladder
neck region (around
the ureteralorifices
andin
thefloor
of
the bladder). Thesecells were
located
inside
of
the
muscle
coat
of
thebladder
wall
(Figure
28
and
D). As
in
the
digestivetract,
theseNADPH
diaphorasepositive cells
had alsofibers
that connected the neuronalcells
oneto
another.Epithelial cells
of
urinary bladder (urothelium)
alsoexhibited positive reaction
for
NADPH
diaphorasestaining.
All
of
theconfiol
samplesexhibited
negativereaction for
NADPH
diaphorase staining.The number
of
NADPH diaphorase reactive
cellsin
the digestive
tract
and urinary
bladder
The number
of NADPH
diaphorasepositive
neuronalcells varied
among
parts
of
the
digestive
tract.
Thedensity
of
thosepositive
cells was higher
in
the
largeintestine and was
very
low in
the
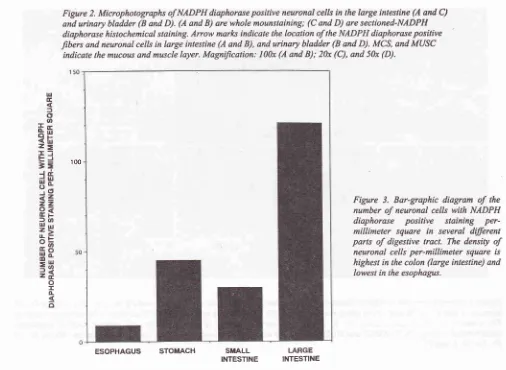
esophagus(Figure
3).
Since thenumber
of
thosepositive
cells
wasvery
little in
the bladder, we
counted
all of
the
positive
cells
presentedin
the bladder
directly from
thewhole
mount
samples underlight
microscopy.
The densityof
thosecells
per-millimeter
squarein
thedifferent
partsof digestive
tract were 9
+
0.54
(esophagus);45 +
3.89 (stomach);3l
+
l.14
(small intestine); and
l2l
+
12.63(large intestine).
Mean-while,
the
total
number [image:4.595.83.562.353.662.2]of
those cellsin
theurinary
bladder was 59+
14.35.Figure
I.
Microphotographs ofNADPH diaphorase positive neuronal cells in the esophagus (A and D), stomach (B and E), and small inlestine (C and F). (A, B, and C) are whole mount staining; (D, E, and F) are sectioned-NADPH diaphorase histochemical staining. The areasin
the small square-boxes(D,
E,
andF)
indicate the locationof
neuronal cellswith
positive NADPH diaphorase histochemical staining. MCS, SbMCS, and MUSC indicate lhe mucous, submucous, and muscle layers. Magnilication: 6Ax(i,
B, andFigure 3. Bar-graphic diagram
of
thenumber of neuronal cells with NADPH
diaphorase
positive staining
per-millimeter squarein
several dilferent partsof
digestive tract. The densityof
neuronal cells per-millimeter square is
highesl in the colon (arge intestine) and lowest in lhe esophagus.
Fig;ure 2. Microphotographs of NADPH diaphorase positive neuronal cells in the large inlestine (A and C) and urinary bladder (B and D). (A and B) are whole mounstaining; (C and D) are sectioned-NADPH diaphorase histochemical staining.
Atow
marlcs indicale the location of the NADPH diaphorase positive fibers and neuronal cells in large intestine (A and B), and urinary bladder (B and D). MCS, and MUSCindicate the mucous and muscle layer. MagniJication: l00x (A and B); 20x (C), and 50x (D).
150
r00 ut .E l
oo
Ëu
<lx
z=
If
EJ==
l.i
qË
iiz
zz
P3
)'an
ïg
3E
HE
=H
iÉ
o
T À E
o
SiIALL
I.ARGE [image:5.595.81.572.90.364.2] [image:5.595.69.575.373.743.2]DISCUSSION
The location
of
theblue precipitates
in
the cytoplasm
and nerve fibers indicate that
nitric
oxide
synthase(NOS)
andNADPH
diaphorase belongsto
cytoplasmicproteins. However, not
only
the
neuronal
cells,
theepithelium cells
of
mucosallayer
in
thedigestive tract
and
urinary
bladder
were also
positively
stained.These results
were similar
to
the
other
previous
reports.lT So
far,
thereis
noreport describing whether
these
cells
really
contain
nitric
oxide
synthaseor
not.The
correlation between
the
existence
of
positive
NADPH
diaphorase
staining
in
the epithelium
layer
and
their firnction is
still
poorly
understood.
In
their
histochemical
studies,
Bredt
et
all5 could
not
find
nitric oxide
synthasein
parenchymal
or
stromal cells
of
theliver,
heart,lung,
spleen,kidney,
thymus, testis,or
salivary glands. Despite
theseobservations,
it
cannot be
excluded
that
mucosal
NADPH
diaphorase (that represents anisoform
ofnitric
oxide
synthase) isnot readily
detectablewith
the
antiseraraised
againstthe
neuronal
nitric
oxide
synthase.
In
fact,
theseantisera
did
not
react
with nitric
oxide
synthaseof
activated macrophages.Furthermore,
if
theepithelium
cells
really
containnitric
oxide,
it
is supposed thatNO
is
involved in
thecellular
processof epithelium
cells.Although
NADPH
diaphoraseactivity
was detectedin
processes
along
thegreatest
density
of
indicated
as the
number
or
NiTi,n-Hl*::
positive
neurona
(Figure
3),
was
found
in
by
that
in
stomach.The
entericneuronal
cells
in
the
sub-mucous
layer were
also easierto
be
identified in
the large intestine (Figure
2A
and
C).
Since
the
number
of
NADpH
diaphorasepositive
neuronalcell
washigher
in
stomach and largeintestine than
in
other parts
of
the
digestive
nait
(Figure
3),
it
is
supposedthat
nitric oxide (NO)
as aneurofransmitter is required
in
alarger
amountby
thesmooth muscle
of
the
stomach andlarge intestine
for
their
adequate
movement
or
peristalsis during
thedigestion
process
of
food
in
the
stomach
anà
the excretion processof
fecesfrom
the large intestine.Several
studiesrevealed that
nitric
oxide,
which
wasreleased
ftom
inhibitory
non-adrenergic non-cholinergicnerve,
served
as an inhibitory non-adrrnergic
non-cholinergic
(NANC)
ne*ohànrrnitterls
in
severalauûonomic
functions.
Niûic
oxide
is involved
in
mediating relaxation
of
smooth muscles
of
thedigestive
tract,re2owhich
is
necessaryfor facilitating
the passage
of
material, changing food
to
abolus
aridgenerating peristalsis
in
the
digestive
tract.rs-20Mice
lacking
thenitric
oxide
synthase(NOS)
geneexhibited
grossly
enlarged stomachs,
with
hyperhophy
of
thepyloric
sphincter
and
the
circular
muscle
layer.leMeanwhile, mice
with
hyperinnervation
of
NADPH
diaphorase
positive enteric neuronal cells
exhibited
megacolon phenomena
with
prolonged
tansit time
of
barium throughout
the
gastrointestinal
tract
during
barium
enema (ourunpublished
data).The
NADPH
diaphorasepositive
neuronalcells
in
the*itrary
bladder consisted
of
single
cell
or
group
of
cells,
which
were
particularly
numerousin
theregion
of
bladder
baseor
bladder
neck
(around the
ureteralorifices
and
in
the
floor
of
the bladder).
Those cells,which
are located
in
the
wall of
urinary
bladder
areconsidered
to
be the
source
of
the
parasympatheticpost-ganglionic
nervesupply to this
organ."
Since thevesico-urethral
sphincter muscle is also located almostin
the
same area
as NADPH
diaphorase positive
neuronarl
cells,
nitric
oxide
is
thought
to be
aneurotansmitter necessary
for
facilitating
therelaxation
of
vesico-urethral sphincter.ls'le However,
its
exact
function in vivo
remains
ill-defined.
Studiesin
fetal
sheep demonstrated thatnitric
oxide
inhibition
caused
bladder hyperactivity and
increased
bladder capacity, perhapsby preventing
sphincter relaxation.2lln
rat, inhibitors
of
the
NO
system
caused bladderhyperactivity
and decreasedbladder
capacity.22Inour
observations,
mice
with
hyper-innenration of
NADpH
diaphorase
positive
neuronal
cells
showed
decreasedbladder capacity and
decreased
threshold
pressure, perhapsby hypenelaxation of
vesico-urethral sphinctermuscle
(our unpublished
data).The exact
function
androle of nitric
oxide
(NO)
as anonadrenergic noncholinergic
(NANC)
neurotransmitterin
various
tissuesand organs
of
the body
is still
far
from
understood.
The
pharmacological effects
of
nitrates
areused
in
clinical
conditions, e.g.
ischemicheart
diseases,hypertension, and
motor
disorders
of
esophagus.
Furthermore,
nihoglycerin
is
used in
also
in
other
places)
as
anatient complaining
of biliary
are interesting because recent
animal
studies suggest
that the
cholexystokinin-induced relaxation
of
the
sphincter
of
Oddi
ismediated
by
the
NANC
neural pathway
operatedby
an
unidentified hansmitter
substance.zrBurnett
et alzareported
that
NO
\ila{i
a
physiologio mediator
of
in
rat
penile newons
innervating
the
corpora cavernosaand
in
neuronal
plexusesin
the adventitial
layer
of
penile arteries. Small
doseof NO
synthetaseinhibiton
abolished electrophysiologically
inducedpenile
erections. Ferrante
et
al.tt
reported
thatNADPH
diaphorasepositive
neuronswere selectively
spared
in
neurodegenerative diseases
such
asÉuntington's
diseaseÉ.Huang
et
alre reported
that
mice
lacking
nitric oxide
synthase
(NOS)
genedeveloped grossly enlarged
stomachs
with
hyper-fiophy
of
thepyloric
sphincter and thecircular
musclelayer, resembling
infantile pyloric
stenosisin
human,in
which
gastric
outlet obstruction was
associatedwith
the lack
of
NADPH
diaphorase neurons
in
thepylôrus. Hatano
et
al26and
Shirasawaet
al6 reportedthat
mice
lacking NcxÆIox
lll.l
gene
developedmegacolon
with
hlryerinnervated
enteric
ganglia.Those
mice
can
be
used
as a
model
for
humanneuronal intestinal
dysplasia(MD)
diseases,in which
myenteric neuronal hyperplasia and
megacolon
are seen.Finally
theimpact
of
the resultsof
researchesin
nitric
oxide
on clinical
aspectof
diseases,in
which
nitric
oxide
(NO)
mayplay
a specialrole
as the cause,is
very
important and might
be
useful
to find
the therapyfor
such diseases.CONCLUSION
The
numerous groupsof NADPH
diaphorasepositive
neuronal cells were
detected
along
all
parts
of
thedigestive
tract, including
esophagus,stomach, small
and large intestine
aswell
asurinary
bladder.
Thesecells
existedin
avery small
numberin
the submucous plexusesof
Meissner located
in
the
submucouslayer,
and ubiquitous
in
myenteric
plexuses
of
Auerbach
located
in the
areabetween
the
external and internal
layer
of
the
smooth muscle of
the
digestive tract.
In
the
bladder, those
cells
existed
at the
bladder
neck, insideof
the muscle coatof urinary
bladder.Since
the
number
of
NADPH
diaphorase positive
neuronal cells per
millimeter
square is more numerousin
stomach and large intestine, there is
a possibility
that
theamount
of ninic
oxide
in
those organsis
alsohigher.
This fact
suggeststhe important
role of nitric
oxide (NO)
in
those
organs. Furthermore,
the existenceof
thosecells in
ttre
limited
areaof
urinary
bladder
i.e. in
the bladder neck may be correlatedwith
the function
of nitric
oxide (NO)
for vesico-urethral
sphincter muscle.
Acknowledgements
The author
would like to
express'theappreciation
andspecial thank
to Dr.
Masahiko Hatano
MD,
Division
of
Developmental
Genetics,
Chiba
University,
Graduate School
of
Medicine, for his kind
technical
guidance,
and the supply
of
animals and
materials;and
to Mrs.
W.
Indarty
Aulia
for
herhelp in preparing
andtyping this manuscript.
REFERENCES
l.
SynderSH.
Nitic
oxide:
first
in a
new
classof
neurofansmitter? Science 1992;257 :494-6.2.
Dimmeler S, Lottspeich F, BruneB. Nitric
oxide causeADP-ribosylation
and
inhibition
of
glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem 1992; 267:167714'3.
Kots
AY,
SkuratAV,
SergienkoEA,
Bulargina TV,Severin ES. Nitroprusside stimulated the cysteine-spesific mono (ADP-ribosylation) of glyceraldehyde-3-phosphaæ dehydrogenase
from
human erythrocytes.FEBS
Lett 1992;300:9-12.4.
Mc Donald LJ, Moss J. Stimulation by nitric oxide of anNAD
linkageto
glyceraldehyde-3-dehydrogenase. Proc Natl Acad Sci USA 1993; 90:623841.5.
Marletta
MA.
Niric
oxide
synthase structure and mechanism. J Biol Chem 1993;268:122314.6.
ShirasawaS,
Yunker
AM&
Roth
KA, Brown
GA,Horning
S,
Korsmeyer SJ.Enx
(Hox lll.l)-deficient
mice
develop myenteric neuronal
hyperplasia and megacolon. Nature Medicin e 1997 ; 3 (6): 646-50.7.
DawsonTM,
Bredt DS, FotuhiM,
Hwang PM, SnyderSH.
Nitric
oxide
synthaseand
neuronal NADPH diaphorase are identicalin
brain and peripheral tissues. Proc Natl Acad Sci USAl99l;
88:7797-801.8.
Bredt DS, Glatt CE, Hwang PM, Foohi M, Dawson TM, Snyder SH.Nific
oxide synthase protein and mRNA arediscretely
localized
in
neuronal populationsof
the mammalianCNS
togetherwith NADPH diaphorase.
Neuron 199l;7:615-24.
9.
Hope
BT, Michael
GJ,
Knigge
KM,
Vincent
SR. Neuronal NADPH diaphoraseis
a nitric oxide synthase. Proc Natl Acad Sci USAl99l;
88:2811'4.10.
KnowlesRG,
PalaciosM,
Palmer RMJ, Moncada S. Formationof
nitric oxide from L-argininein
the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA1989;86:5159-62.
I
l.
Scherer-Singler U, VincentS&
Kimura H, McGeer EG. J Neuroscience Methods 1983; 9:229-34.12.
BredtDS,
Snyder SH.Nitric
oxide,a
novel neuronal messenger (Review). Neuron 1992; 8:3-ll.
16.
17.
2t
t4.
15.
23.
24.
25.
18.
l9
Hope BT, Vincent SR. Histochemical characterization
of
neuronal NADPH-diaphorase.J
Histochem Cyochem1989;37:653-61.
Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role
for nitric
oxide. Nature 1990; 347 :7 68-70.Gabella G. Intra mural neurons in the urinary bladder
of
Guinea pig. Cell Tiss Res 1990; 261:231-7.
Grozdanovic
Z,
BaumgartenHG,
Bruning
G.
Histo-chemistry of NADPH-diaphorase, a marker for neuronal nitric oxide synthase, in the peripheral autonomic neryoussystem of the mouse. Neuroscience 1992;48(l):225-35.
Bult H, Boeclocstaens GE, Pelckmans PA, Jordaens FH,
Van
MaerckeYM,
HermanAG. Nitric
oxide as
aninhibitory non-adrenergic non-cholinergic neuro-transmitter.
Nature 1990; 345:346-7.
Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman
MC.
Target disruptionof
the
neuronalnitric
oxidesynthase gene. Cell 1993;75:1273.
Keef
KD,
ShuttleworthCW, Xue
C,
Bayquinov O, Publicover NG, SanderKM.
Relationship between nitricoxide and vasoactive intestinal polypeptide
in
entericinhibitory neurotransmission. Neuropharmacology 1994;
33: I 303.
Mevorach
RA,
BogaertGA,
KoganBA.
Roleof nitic
oxidein
fetal lower urinary tract function. JUrol
1994; 152:5 10.Persson
K,
Igawa Y, MattiasonA,
Andersson KE. Effectof inhibition of the L-arginine/nitric oxide pathway in the
rat lower urinary tract in vivo and invitro. Br J Pharmacol
1992;107:78.
Behar J, Biancani P. Effect
of
cholecystokinin and theoctapeptide
of
cholecystokinin on the feline sphincterof
Oddi and gallbladder. J Clin Invest 1980; 66:1231-9.Burnett
AL,
LowensteinCJ, Bredt DS,
Chang TSK,Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science 1992; 257 :401-3.
Ferrante
RI,
KowallNW,
BealMF,
Richardson EP Jr,Bird ED, Martin JB. Selective sparing of a class of shiatal
neurons in Huntington's diseases. Science 1985: 230:561-3.
Hatano M, Aoki T, Dezawa M, Yusa S, Iitsuka Y, Koseki
H, et al.
A
novel pathogenesis of megacolon in NcxÆIox