optimisins refined breached d"egdoriz.
end"P,"liH:T'JlJ:;,':'".'#iill,f,::ff'fJ?:',ffivarue
toprovide the stabre
Muhammad vusuf ditong
a
.
,,,--
4nirE
t6rt^n Departmentof chemicatEngineering,ru"rti'iiEiginiilrg,
u.niviiiiyotsumatera
utara' Medan 20155' lndonesia [email protected]
Vd.s
Nog
(SeP.2012)32S
lsst\t @7+6846 lndian Journal of Science and Technology
specJon 0-175 g/100 g'
: Distillation, lodine value, Bottom flash distiller standard, Distillate,
Feed ca
il
Tabte t. The composition of IRBDPSFA orhvd-rolv.zed RBDPS
and
ih;"'q';iitv
'
or
i:Ilut"
which
is
producHSRBDpSFA(Muhammad,2009)
--=-:-===^-
0'a]una'mmio,.
20.10;
YYlTTlg
,r?o'. Abstracton the commerciar scare, the quarity standard of erenoed siearic Acid Distiiled
(BS{D) courdn't be achieved by normal distilration. BSAD
iodine-;ilJ;;;stry
higher than maximu,qru'i',iv-"iliio*i1o
z
mg/100 g), with the same iodinevarue (0.80 s/100 s)
"t'i""i'Hvoios"nui"a
spiitt"Jiieois rutry'n.iioi
HiiraDFarA,ieed
ci'pacitv 5'5 ton/hour witt bottom frash dist,rer
t"r"p?riirIiEacning
zil;'c. rt
"ieparation
""oilor"ti"n
of chemical impurities (so sensitive tt oxidation/temp"r.trr"mJui "r,unging, and
ioi;;"rJ"r
*Ir"
0"r".
ir'"
"tr""t
of the reduction oi feed iodine value (lV) increasing of feed
"upu"ity ano uottom
t"rp"i"tu[?
tiair, aistitt"r
""rrrn
(BFD) w.ere ootimized to observeBSAD',t
iodine vatue trend by ,.in'g u factorial
q."tig;i3';?
*
sf'utntO
rn" tpiiti*"condition
was found on thefeed lV c
0.60 g/100 g, at tee^o"c-;p'"itv
o.o't"n/hou-r,'J"i
ero iemperature'iifd-
roai"e varue ofBSAD was stable and it
lntroduction
Brended stearic acid dist*rate (BSAD) contains -60
o/o
maximum-oi-
esnu
tv
specs)
which
is
producew/wof parmiticacid(c15H31cooHorc,,o)and
-4oo/owrw
"or*J*iurry
uy
using the. samelv
(0'80 9/100g)
<of stearic acid (c17H35cooH or c1s)'
'n'
*l'nil'"il;-t
H::I**i,
g::*"illfiI'fliil"
''i",,1:'-"i1?li"Ji
:*tt,;,::,j
!?;:'ii';'",8*,i:::'J;'Xii'ii'J'i:tp"J
I'..'"lJ'i
*;;n'tiioimur
and thJ iodine varue was unstabrachieved
using RBDps
as
raw
,.nri"riui-'rr.,"
bqr!" "r-
ti'"-
practicar
data,
the
increasing
rcomposirions
of
snsDpsrR
are
shownir"i"nr"
i.
HsRBDpt;Ris-tv'wtrich-is
higher
than 0'80
9/100 RBDps is highry suitabre to obrain oo "2"c.,u;+o'"7"-c.,ror
proau"li-tt"
r,ign"tBStq
lV (Yusuf' 2010)' Based c
BSAD by cutring a pario-t
i["
r"*",
and higrrer uoirerth-an
*',ur"
iiit.,'it'.
;rppo."d
that tire lower HSRBDPSFA
cro_ra
in
the
suitabre dist,ration process;;*Iti;";
;f
!v
toi.tirijil"
p-"!!i
t""d)
can reach, the rower BSAHSRBD,.FA.
distillatronprocess-
L-
rv on
ir,"
iing?t of it-quality standard will be. How mutThe
off
specsof
BSAD iodinevalue
(BSADlv)
.llouriin"
orl'l;'I:-*,lY o"'
lt
should be foundot
created lower competitive level in oleo
"n"rni"jt
,urfet.
li
Based
on
the
experience' practically
the
lowcourdn,t
be
producedin
tne
normar p,oorJiion"v"r".
nsne'op"-din]v
"un'ue-^obtainedthrough SRBDPS' producrion
wi,
be
i""*i"i""i
,no *ariing
""Ligv.'it
;
hydrosenition'
This was also
based
on
fatty
acovera, items of quarity specs courdn,t
ue.oiiainBt.
Tlr"
rrva.i".aii"n-tneorylRobert'
1990)'Thiswasn'ttheor
off specs rV are tne
laule
of the rowert"i.i"iutrru
ot
*"v tJ.orr"
*re pro',otem- rodinevaiue of BSAD was al
BFD corumn. rt
o"crelll;;;;,"
rii;c.-iriii
affecteo
nigher wtrentre'feed
capailtv higher than 5'5 ton/hc
BSADcompositions,frombigger-lossof
G+ra;iBSii.t9
1;1Y:yf''ZOiol' So thequestibn "what will the optimt smailer weight ratio
of c,u-to
c,r.ol Bq4D"l"o
a5no
aistilraiion "ohdition, togetthe
inspecs BSAD
lvr
hcompositions tend to
L;+i
tp""t'
tt.i" u big effect totire
to be studied'rower production
yieriLr-s;;;;;
uis t,oudtu arso tothe
t;*,"ru1X#:i,.J,'..,1,15:?l
#i:!ELrr":J,':tl;
U9
No Fatty acid name
Symbols double
bond
SRBDPSFA,
o/o wlw
HSnaopsrn,
o/o w/w
1 Lauric acid Ce 0
0,1 0.1
2. Myristic Acid Crl 0 1.3 1.3
3 Palmitic Acid Cro 0 57,4 57.4
4. Stearic Acid Crpo 0 6.1 40.2
tr Oleic Acid Craa 1 27.9
6, Linoleic Acid C'ts-z 2 6.2
ii"g"iJint-ihit,
the
optimum^feed
lY^'
oiiifiation" conditions of HSRBDPSFA have to
t"t"ri"n"O
carefullyand
commerciallyin
Istudy (Peter et' a1.,2004)
ioitinu value
was one
of
the
qu€ oarametersof the
commercial premium BSt[".iA"t
its
color, heat stability and fatty zIJrpoiitions
(Pr.xxx,
2005)'99tting,th:lT
the'better
and
the
more stable
lV'
Heliiii"""r'"0
DeodorizedPalm
Stea.rin (T?? 10)' There.is:'ffi
i;
n"J*uv
for getti'ng the in specs of BSAD in thewas
used
as
raw
material; becausethe
cheni-.,,,riii"s
or impurities that's so sensitive to oxidationir"tin""o;u"un
i""t".t"d
from RBDPS to a cenaink
normal Production cYcle'
lMta*
bft r-Uytt
E,r rdin ad ExvirqnErf GSe)7t)
lodirBmlrc'
http/Arr,vw.in{st.og
llY.Rt
It
is
so
suitableto
produce BSAD through hydrolysis, hydrogenationand
distillation. lmpurities
reduction (includes unsaturatedbondl
of
the
crude
fatty
acid (SRBDPSFA) can reduce fatty acid color and lV through fatty acid hydrogenation (Robert, 1990). The experience shows on the specific conditions, distillation process also separatedthe
impuritiesto
a
certain levelso
that the amountof
unsaturated bond (indicatedby
lV)
will
be lowerand
better.So
hydrogenated SRBDPSFA feed could reduce BSAD'lt/
also through distillation and/or fractional distillation.By
using
of
this
HSRBDPSFA,reduction
of
HSRBDPSFAIVand
optimization
ofdistillation process conditions
which
is
done
in
this research can reduce unsaturated bondor
obtain lower and more stable arnount of unsaturated bond or BSAD lV according to BSAD lV quality standard.According
to
the
practical
experiencesin
oleo chemical industry,crude
fatty acid
of
HSRBDPSFA contains impuritiessuch
as;
a
trace
of
caproic acid (CsHllCOOH) or C6, caprylic acid (C7H15, COOH) or Csand
capricacid
(C1gH21COOH)or
C16.The light
end composition of the first fractionation column of distillation showedthis
fact. These lower fractionsare
easier to oxidize and have bigger potential to form double bond or increasethe
iodine valueof
fatty acid whichis
being purified (Nur&
Septin, 2006; Yusuf, 2010). The otherimpurities
in
crude
fatty acid
are
hydrocarbons,aldehydes, ketones, sterols, phosphatides, glycerol and un-hydrolyzed RBDPS which also have big potential to increase the lV of fatty acid according to their chemical properties (Ketaren, 2008). The lower
the
impurities infatty acid are, the better the fatty acid quality is or the bigger
the
impurities canbe
separated,lhe
better the fatty acid quality is.lmpurities
of
crude fatty acid feed (CFAF) can be separated through distillation processto
improvelV
of fatty acid distillate. The impurities such as: fatty acid (< C12) and C14, hydrocarbons, aldehydes and ketones are separated as the light end fraction (low boilers) in the first fractionation column andthe
other impurities such as:sterols,
phosphatides,glycerol
and
un-hydrolyzed triglyceride is separated as heavy fraction (high boilers) or residue (Brocmann et al., 1981).lt can be also applied to purify CFAF of HSRBDPSFA.Providing the BSAD with the same quality standard as before had been done by increasing light fraction until
5
% of
feed capacity, togetherwith
increasing precut column temperature unde;' vacuum pressureto
remove more the impurities, such as color bodies, fatty acids that are lower than lauric acid (CrrHzaCCOH) or C12, myristicacid
(CrsHzTCOOH)or
C1a, hydrocarbons, aldehydes and/or ketonesof
HSRBDPSFA (thatare
sensitive to color changing which cause it to be darker and increase the lV of fatty acid). lt's follorred by reducing BSAD yielduntil 85%
(desired higher).The
increasingof
precut column temperature affectedthe
increasingof
BFD temperature andso
it
was
potentialto
increaselV
of lndian Journal of Science and TechnologyR€s€ardlatide
olrdian Soddyfor mucatim ard BNiro-rrert (See) 71
w7
Vd.
5
r.b.9
(Sep.2012)
lSSilt Cs74 6846BSAD relatively. The temperature had to be reduced so that lV will be relatively low. This was also based on the down grade acceleration of fatty acid that was affected by
temperature (Brocmann et al., 1987; Ketaren, 2008).
The
providingwas
done
by
recyclinglhe
rubber grade distillare to the drier as the other big potential to increase BSADlV,
besides CFAFlV
and
distillation procqss conditions (the present distillation facility or Feld and Hanh style) (PT. XXX, 2005). ln this research, it will be separated as a byproduct of rubber grade distillate to separate bigger the impurities of BSAD to obtain lower lV. See Fig. 1 (the modified Feld and Hanh style).Based on this fact, it's supposed that feed lV wasn't suitable yet to produce BSAD as its quality standard of lV (0.2 9i100 g) and
itwas
stable. lt has to be studied. The distillation operating conditions weren't optimum also. For this, light end yield will be kept constant on the range 1.5-3% so that BSAD yield will be higher and closer to 93.0 %
(as
a
normal specificyield
of
BSAD which could be obtained practically). To study, the feed capacity will be optimized above 5 ton/hour and BFD temperature will be set until finding new optimum point on the new optimum operating conditions of distillation.Method
The raw
material HSRBDPSFAwas
produced byhydrogenation
of
SRBDPS
from
Splitting
Plant (SRBDPFA was producedby
hydrolysisof
RBDPS) inPT. XXX's facility. The raw material HSRBDPSFA was used as crude fatty acid feed. lt was obtained and will be
purified
on the
same plant
site
of
the
same manufacturer's facility. Medium pressure steam (11 bars) was usedto
perform vacuum pressure and oil thermal heater was used to heat up fatty acid in order to purify it and to produce BSAD.The research was done by factorial method with 3
independent variables: feed rate (F) at level of 5.5; 5'75 and 6.0 ton/hour, feed lodine Value (lV) at level
of
0.7and 0.6 g/100 g and bottom temperature of flash distiller (B FD) at 218, 220 and222'C (5 mbar). Factorial method was designed
by
constant modet3 x 2 x 3
and was repeated 2 times for each factor and BSAD lV was one c'the
quality parameter whichwas
observed.Statistj-i
analysis was performed by Minitab Release -14 soft"'a'e (Nur
&
Septin, 2006). Samples were analyzed e'ie"..:
hours which amounts were 100 ml of liglttfract:cr
:.:':
-of BSAD and 100 ml of residue acid. Analysis!'::s:--=
lodirevdLE'
lfipl/lvvrwinq$.sg
Table 2. Steps of the research
Variables al (F=s,50) a2 ( F= 5,75 ) a3(F=6,0)
cr (zraoc)
br1o,z1 bz(o,o) br1o,z1 bz1o,o1 br 10,21 bz1o,o)
X X X X
x
Xcz (zzooc)
x
X X X Xx
X X X X X X
cg {zzzoc)
X X X X X X
X X X X X
x
-7
\r,
l---.]-r -:: =mf,@
,"f-*\
W
-,3,an Joumal of Science and Technology
Tab/e 3. h|teans of the BSAD lV as e{fect of three optimized
No F, ton/
hour lV,g/1009 BFD, CO BSAD IV, g/1 00 g
1 5,5 0.7 218 220 222 0.150
0.1 60
0,1 70
0.6
218
220 222
0.145
0.1 55
0.1 60
2 5,75
0.7
218 220 222
0.1 60
0,1 70
0,1 70
0.6
218 220 222
0.155
0.1 65 0.16s 3 6,00 0.7 218 220 222 0.165
0.1 75 0,180
0.6
218 220
222
0,1 69 0,170
0.1 75
?98
Vd.s
M.9
(Sep.2012)
lssr\t 09746846symbol
of
*
betweentwo
symbolsof
this
process variable statesthe
interaction betweentwo
or
more variables. So F*lV is the interaction levels of F with lV and so on for the others, dk is degrees of freedom. uk is sumof
square,kt is
central-squ-are,f*"rr-anO
p4n6yuofe distribution test and significance tbstZnJlyiis of variance.
Effe_ct of process
varilutei-is-iii.rtn"j"i,'
tt bono,,.pq
_0.05.
f0y
significanceof
the
optimbed process variables effect on the BSAD iodine vaiueThe results of Factorial anatysis and its significance (Table
4)
describe the..influenc6-otipti-ired
process variables on the BSAD lV. Statistical
,nliyri"
shows tttat feed rrate,-F (p"no,u= 0,000) ano reeo-lJo-ine value-,lV
(P9"o^,=1 0,010), B FD (p,nova= 0,0p0)
*"r"
to*"r. than (po = 0.05), the effects were so significint
tolhl
changinj oi BSAD iV. lnteraction of F and iV 1e",*, = ii,gzOl, F and B FD (Pano,a = 0,67g), lV and
g
fD
ti;"*"
='O,]ZS),f
analV
also with
B
FD
(pano,a=
0,di"di
diin,r
affected significant (Puno,n> po = O.OSJ changing'of SSAD lV.The
iglT!9f
,"j.chmain factor toihe eS-AdM is shown in theI
L-l
it
-t,
f--Il:
:
iIN.
i-r
lz
I I ILr
lndii impur condi had gT and tl
can b
The e
BSAt TI CAUSE reduc Separi same CFAF condit lV car lower
Table
was done
oasea
riril""o
Fig.3.method Tg 1- 64 to anatyse.BsAD tV
(David,2006).
Eifect
of
O-ptimrlftrr'Iros f
g
r-o4loanalyseBSADlV(David,2006).
lffect
of
Optimizationof
the main
variablesand
lts The correct results will be obtained by keepingtop
lnteraction on BSADlv
temperature of pre-cut column
at
190oc"no
pr"i"u"r"
Jt
The increasingorgseo
v
was affected significantly21
mbar;
Flash
Distiller
pressureit-
b
'moar
;"d
by the optimizatio-n or ttre main variables (increasing of B temperature (B FD) as per the steps shown inrior"');
rb,
reeo'flow rater
uno o""r"asing of feed iodine value), residue distiller pressure
at
1 mbar andtemperai;;;;i
because significanceiest-anatysis
of
variance p,no,"2S0"C.
shows,pun*]_olBFD
E",_""
of F
and punouuof
lV wereThe
research stepswere
basedon
the
factorial
lower tnan-olstpol.
5i"
f'"'ir"
4. The trends can be seen method by 3 x 2 x 3 and were repeated 2 times.f;b|",
in Fig.2. [image:3.612.30.298.57.338.2]shows steps of research' The symboli
ire
useo intre
ih.e impact of the optimized main variables on BSADtable such
asi
o1,
a.z,3s
=
variationof
CFAFrate
lV is shown inig
,6;using
tuinita b 14 software).This (ton/hour); br, bz, bs = varii-tion of feedlvlg/l00
g)
and
picture showsthe-'sig;;i";;;effect
of the optimized main c1' G2' c3 = variation of BFD("c)'
variableso,
gsADiV.'il-"'.ur"
effect is also snown oy
Results and
discussions
the averagevalues of the increment
of
BSAD lV within The results are providedin
Table3.
The effectof
Tabte 5u,iJr.oru
ofi.riig.
zl.process variables were analyzed
with
MinitabRelease
The effecton
increasingof F
is
significantto
the14, where, F is feed rare, lV is feed iooine vaiue ano e
iD
in"rurrinj
oGs"AD';V.Ai"i
of s.5 ron/hour BSAD tV is is the bottom temperature of flashaisittLiiotumn,
*o u
0.15666/91199g,
;i i ;
5.75 ton/hour BSADIV
isTahta,
rT..An^/t/^-:^--_- ,
0.164167 g/100g
and
at F
6.0 ton/howgiAD
rV
i.
Tab/e,4. BSAD tV Variance analysis and significaiion
Source
DK JK kt I tr
anova I banova
Remark Variation
F a 0,0001256
0,0006028 18,08 0.000 Significant
IV 1 0,000278 0,0002778
8,33 0.01 0 Significant
BFD a 0,0012722
0,00063G1 19,08 0.000 Significant
F-IV
2 0,00000s6 0,0000028 0,08 0.920 Not significantF*BFD 4 0,0000778 0,00001 94 0,58 0.679 Not significant
IV-BFD 2 0,0000056 0,0000028 0,08 0.920 Not significant
F*IV-BFD 4 0,0000111 0,0000028 0,08 0.986
Not significant
Error 18 0,0006000 0,0000333
Total ,tr
Table4.
BSADtvVarianceanalrysandsrgnificaiion
0.170g33
9/100
nl
,H:
source
;;;l
i":?:x'.?*lJrfr?t?;irii
ffi
-"""
t
i
-"
I
""
I
'
from F of 5.5 kg/hour to 5.75I
r
i r
ks/hour ana O.6OOOO69/100 g from F S.75 kg/hour to 6.0
i
BFD
i ,
i_o,oorrrz
I
o,o
kg/hour. The averaoe vatue1,r",J3i,.J""i3,,
i8fi*jx
g/1 00 g.I
IV. BFD
| 2
i
0,0000056o,oooA
This is easy
toI
t
j
Hl,"J;,S,.r,"?u"",,."",'..,tI
impurities
which
were,ora,
r
t35i
I
r_l;l:ffi:::r:I,",35?,",Y;,,iT
Research artide2
J
1
R*eth
a lrdan S
73 @lrdian Societyfcr E L€atimand Fvirryrrerd
(isee)
frtto,ffiffiT*
tuly.RitorgalndianJ.$i.Td"nd.
lndian Journal of Science and Technology
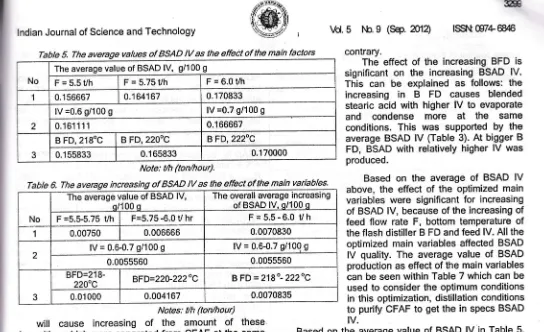
Table 5. The average vatues of BSAD lV as the e{fect of the main factors
-7
gg
Vd.s
hlo.g (Sep.2012)
lSSiltg/46846
contrary.
w.
1€ ESS ate )ce. d= lt.-)te> AD his ain by hin nce ESS l-rat lDa
lof
dB lnd ted -he the Its rtly ,fB re), nova ere )enTabte 6. The average increasing of BSAD lV as the effect of the main variables.
Notes: t/h (ton/hour)
will
cause
increasingof
the
amount
of
these impurities which were separated from CFAF at the same conditions. This increasing caused the produced BSAD had greater lV.The effect of reducing feed lV on BSAD lV reduction and the effect of increasing B FD on increasing BSAD lV can be seen on the same tables, Table 5 and Tables 6.
The effect of feed iodine value reduction is significant on BSAD lV reduction.
The average
of
BSADlV
reduction happened and causedthe
reductionof
feed
iodinevalue (lV).
This reductionof
iodinevalue
(lV)
caused more
difficult separation of the iodine value together with BSAD at the same conditions. So the purifying that had been done on CFAF with lower lV produced lower BSAD lV at the same conditions. These data proved that the reduction of feed lV can improve quality of BSAD lV. So raw material with lowerlV
will
produce BSADwith
lowerlV or
on
theTabte 7. The average BSAD production as the effect of the main variables.
No The average value of BSAD production, kg/hour
1 F=5.5Uh F = 5.75 Vh F=6.0Uh
4.943.7 5,168.12 5,400.09
2 lV =0.6 g/100 g lV =0.7 g/100 g
5,171.92 5,169.'13
3 B FD, 218" C B FD,22O. C BFD,222,C
5,148.77 5,161.53 5,201.28
Note: tth =ton/hour
The effect
of
the increasing BFD issignificant
on the
increasing BSAD lV.This can
be
explainedas
follows: the increasingin B
FD
causes
blended stearic acid with higher lVto
evaporateand
condense
more
at
the
sameconditions.
This was
supportedby
the average BSAD lV (Table 3). At bigger BFD, BSAD with relatively higher lV was produced.
Based
on the
averageof
BSAD lV above, the effect of the optimized main variables were significantfor
increasing of BSAD lV, because of the increasing of feed ftow rate F, bottom temperature of the flash distiller B FD and feed lV. All the optimized main variables affected BSADlV
quality. The average valueof
BSAD production as effect of the main variables can be seen within Table 7 which can be used to consider the optimum conditions in this optimization, distillation conditions to purify CFAF to get the in specs BSADtv. :he
'is
is is he IV. Based on the average value of BSAD lV in Table 5,
the best BSAD lV is 0.161111 g/100 g. Based on Table 7 above the best BSAD production is obtained on the feed flow rate (F) is 6.0 ton/hour, feed lV is 0.6 g/100 g
and
BFDis222"C.
The effect of feed flow rate and iodine value interaction on BSAD lV.
The results of one way ANOVA statistical analysis on
the interaction
of F
with lVto
the BSADlV
are shown within the Table8.
Based on the data, significance test analysis of variance Punoru = 0.048 for the interaction ofincreasing
F
at the samelV
(0.7 9/100 g) and Puno,, =0.015 at the same lV (0.6 g/100 g). Both values of Panoua
are tbwer than 0.05 (Po). That's why the interaction of increasing
F
with
the
same
lV,
shows
significant increasingon
BSADlV,
also
basedon the
factorial analysis within Table 4. See also Table 9 as supportive of such explanation. These values explained that the etfect of the interaction of the increasing F factor with the samelV
on the
increasingof
BSADlV is
significanton
the same feed lV. The interaction trend of these 2 factors can be seen on the Fig. 3 and 3D surface plot on the Fig. 4'It
canbe
understood that the factof
this result is according to the mass and heat balance principles. The increasing of the CFAF mass, which was purified, needed more heator
proportional heatto
maintainthe
same temperature. This was caused by more evaporation and condensationof
the
BSAD
which
was
purified
or separated, includethe
impuritiesof lV in
BSAD' The increasing on F was caused by BSAD that was produced had more BSAD lV on the same feed lV')g
75 00 i.0 UE IV 30 to \F he rfe nd F F d. Researdt artideolrdian Socjetyfor klucdiot ard EUiffin'Ert ($e)
73
lodrcwlue' tttp/
^,rw.inq$.sg
ltv=---l#r i- -r
No
The average value of BSAD lV, g/1 00 g
F=5.5Vh F = 5.75 Vh F=6.0Uh
1 0.1 56667 0.164167 0"170833
2
lV =0.6 g/100 g lV =0.7 g/100 g
0.161 1 1 1 0.1 66667
3
B FD, 218"C B FD,22OOC BFD,222"C
0.155833 0.1 65833 0.1 70000
Note: t/h (ton/hour).
No
The average value of BSAD lV,
o/1 00 q
The overall average increasing of BSAD lV, g/100 s
F =5.5-5.75 Vh F=5.75 -6.0 V hr F=5.5-6.0 Vh
1 0.00750 0.006666 0.0070830
2 lV = 0.6-0.7 g/100
g lV = 0.6-0.7 g/10Q g
0.0055560 0.0055560
3
BFD=21
8-2200C BFD=220-222'C B FD = 2180-2220C
[image:4.612.35.579.33.365.2]uF
lndian Journal of Science and Technology
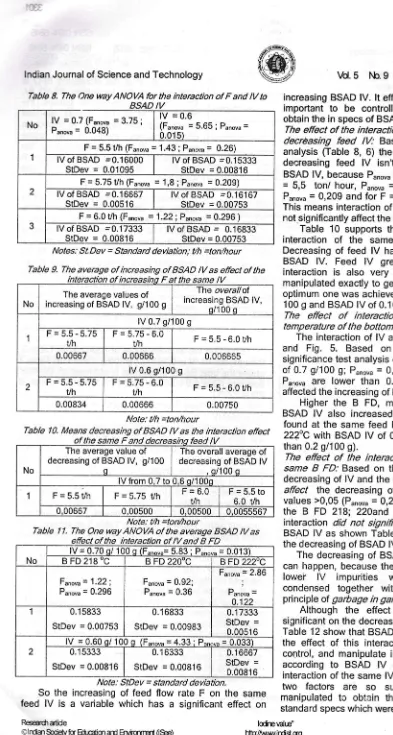
Table 8. The One way ANOVA for the interaction of F and lV to
Notes: St.Dev = Standard devbtion; yh =ton/hour
Table 9. The average of increasing of BSAD lV as effect of the
Note: t/h =ton/hour
Table 10. Means decreasing of BSAD lV as the interaction effect
Note: t/h =ton/hour
Table I /. The One way ANOVA of the average BSAD lV as
Note: StDev = standard deviation.
So the increasing of feed flow rate
F
on the same feedlV is a
variable which hasa
significant effect onResearch atide
: lrdian Socjdy fs ftfi.rcdlm
ai
&rvirtrrrert (See)330C
Vd.5
Nb.g (Sep.2012)
ISSNI G746846increasing BSAD lV. lt effect can't be neglected and very important
to be
controlledor
manipulated exactly to obtain the in specs of BSAD lV.The effect of the interaction of same feed flow rate F and decrbasing
feed
lW
Basedon the
one
way ANOVA analysis (Table8,
6) the interaction of the same F anddecreasing
feed
lV
isn't
significanton
decreasing ofBSAD lV, because Punouu is greater than Pcr = 0,05 (for F
=
5,5
ton/ hour, Punouu=
0,26 ; for F=
5,75 ton/hour ;Punoru = 0,209 and for F = 6,0 ton/ hour ; Purou, = 0.296). This means interaction of F and lV within the Table 4 did
[image:5.612.73.466.14.749.2]not.significantly affect the decreasing of BSAD lV.
Table 10 supports
the
explanationof
effectof
the interactionof
the
same
F
and
decreasingfeed
lV.Decreasing of feed
lV
has impact on the decreasing of BSADlV.
Feed
lV
greatly
affected BSADlV.
This interactionis
also very
importantto
be
controlled or manipulated exactly to get the in specs of BSAD IV. The optimum one was achieved at F = 6 ton/hour, lV = 0.6 g/100 g and BSAD lV of 0.16833 g/ 100 g (Table 8).
The effect
of
interactionof
feed iodine
value andtemperature of the bottom flash distiller on BSAD
lV
The interaction of lV and B FD is shown in the Fig. 3
and
Fig.
5.
Based
on the
data
in
the
Table
11,significance test analysis of variance Punou" = 0,013 for lV of 0.7 9/100 Qi Panova = 0,033 for lV of 0.6 9/100 g. Both
Prnor"
are
lowerthan 0.05 (Po).
lncreasingof B
FDaffected the increasing of BSAD lV or vice versa.
Higher
the
B
FD,
morethe
lodine value and the BSADlV
also increased. The optimum BSADlV
was found at the same feed lV 0.6g/
100 g and ata
B FD222oc with BSAD tV of 0.16667
g/
100 g (This is tower than 0.2 g/100 g).The effect
of
the interactionof
lV
decreasing and the sameB
FD.' Based on the Table 1''1, the interaction of decreasing of lV and the same B FD did not significantlyaffect
the decreasingof
BSADlV,
becauseall
P"no*values >0,05 (Punovu = 0,296 ; 0.36 and
0]22for
each ofthe B
FD
218; 220and 220oC). This meanslV.B
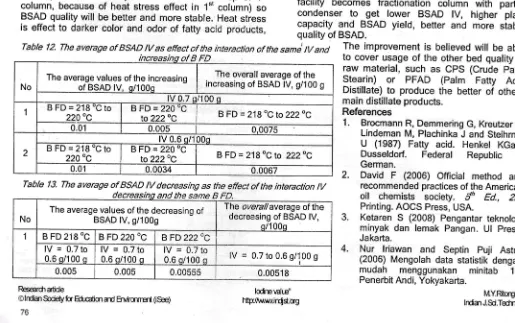
FDinteraction did not significantly affect the decreasing cf BSAD lV as shown Table 2. See the overallaverage of the decreasing of BSAD lV in Table 13.
The decreasing of BSAD lV in this case (see Fig. 5)
can happen, because the decreasing of lV impacted on
lowel
lV
impurities
which
was
evaporated and condensed togetherwith
BSAD.This
matches the principle of garbage in garbage out.Although
the
effect
of
this
interactionwas
not significant on the decreasing of BSAD lV, the data in the Table 12 show that BSAD lV was decreased. That's whythe
effectof
this
interactionare
alsoso
important to control, and manipulate in orderto
obtain the in specs accordingto
BSADlV
quaiity standard, besides the interaction of the same lV and lncreasing of BFD. Thesetwo
factors
are
so
sufficientto
be
controlled or manipulaiedtc
obtainthe
qualityof
BSADlV as
itsstandard specs which were settled.
.@
of lodiremlrc" Ittp/Aarr,wlindsLog tvlY.RitorEa lrdanJ.Si.Tednd. BSAD IVNo lV = 0.7 (Funouu = 3.75 ; Punoyu = 0.048)
lV = 0.6
(Funouu = 5.65 ; Punouu = 0.015)
1
F = 5.5 t/h (Fanoua = 1.43 ; Puno*
=
0.26) lV of BSAD =0.16000StDev = 0.01095 lV of BSAD --0.15333StDer, = 0.00816 2
F = 5.75 Uh (Fanov" = 1,8 ; Panora = 0.209)
lV of BSAD =0.16667
StDev = 0.00516
lV of BSAD --0.16'167
StDev = 0.00753
J
F = 6.0 Uh (Funou" = 1.22 i P"nov" = 0.296 ) IV of BSAD --0.17333
StDev = 0.00816
lVof BSAD
=
0.16833 StDev = 0.00753interaction of increasino F IV
No
The average values of increasing of BSAD lV, gi 100 g
fhe overallo'f increasing BSAD lV,
o/1 00 q
1
lV 0.7 g/100 g F=5.5-5.75
Uh
F=5.75-6.0
Uh F = 5.5 - 6.0 t/h
0.00667 0.00666 0.006665
2
lV 0.6 s/l00 g
F=5.5-5.75
Vh
F=5,75-6.0
i/h F=5.5"6.0Yh
0.00834 0.00666 0.00750
the same F and decreasino feed lV
No
The average value of decreasing of BSAD lV, g/100
o
The overall average of decreasing of BSAD lV
, q/'100 q
lV from 0,7 to 0,6 o/100o
1 F=5.5Uh F = 5.75 Uh F=6.0 UN
F=5.5to 6.0 uh
0,00667 0,00500 0,00500 0,0055567
effect of the interaction of lV and B FD
No
lV = 0.70 g/ 10C g (Funnuu= 5.83 ; Puno,u = 0.013)
B FD 218 OC B FD 220,C BFD222,C
Funon"= 1,22;
Punoru = 0.296
Fanovu = 0.92;
Punoru = 0.36
Funoru = 2.86
;
t)-I anova
-4.122
I 0.1 5833
StDev = 0.00753
0.1 6833
StDev = 0.00983
0.'17333
StDev = 0.00516
2
lV = 0.60 o/ 100 q (Funo*=4.33 ; P"nou, = 0.033) 0.1 5333
StDev = 0.00816
0.1 6333
StDev = 0.00816
0.1 6667
lndian Journal of Science and Technology
0.1:0
0.r55
--7
ml
Vd.5
1.b.9 (Sep.2012)
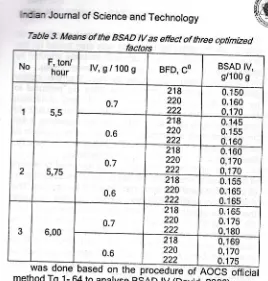
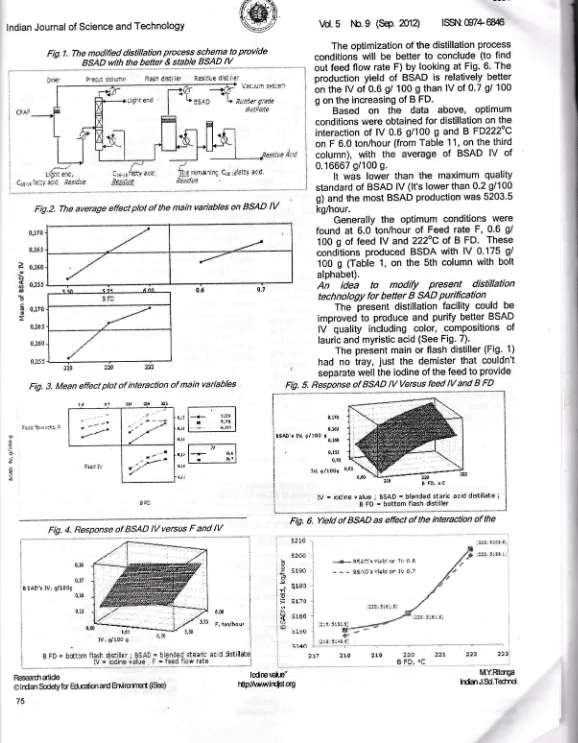
lsst\t G7+6&16 The optimization of the distillation process conditionswill
be betterto
conclude (to find out feed flow rate F) by looking at Fig. 6. The production yieldof
BSADis
relatively better on the lV of 0.6 g/ 100 g than lV of 0.7 gl 100g on the increasing of B FD.
Based
on
the
data
above,
optimumconditions were obtained for distillation on the interaction
of
lV 0.6 g/100 g and B FD222oCon F 6.0 ton/hour (from Table 11, on the third column),
with
the
averageof
BSADlV
of 0.16667 g/100 g.It
was
lower thanthe
maximum quality standard of BSAD lV (lt's lower than 0.2 9/100 g) and the most BSAD production was 5203'5 kg/hour.Generally
the
optimum conditions were foundat
6.0 ton/hourof
Feed rate F, 0.6 g/ 100 g of feedlV
and 222oC of BFD.
These conditions produced BSDA withlV
0'175 g/ 100g
(Table 1, on the Sth column with bolt alphabet).An
idea
to
modify
present
distillation technology for befter B SAD purificationThe
present distillation facility could be improvedto
produce and purify better BSADlV
quality includingcolor,
compositions of lauric and myristic acid (See Fig. 7).The present main or flash distiller (Fig. 1)
had
no
tray,just the
demister that couldn't'
separate well the iodine of the feed to provideFig. 5. Response of BSAD lV Versus feed lV and B FD
Ii*'
?:
{
ar)
d
o a
0,
7.
0,1{J
0*1ss
0.15J
0.1?0
0,165
0,I60
li
il;i :l)r' til:, t
r----
-::l
r-i: l+I r 5.731
qr( L_:__jIlJ
(lt0
0.t6J
&sAO', ,V. Urtoo , o.r,
,.,,1=-n;
| - o''l O,IJJ 0,?0 [image:6.612.22.600.24.767.2],!!:
o|::orns1
of BSAD tv*ny:_, ,r!_lv
gaAF'5 Iv, q/1008
i
e fn = bottom flash distiller; ESAD = bpnded dearic;
IV = iodine valuB. F = fecd IloH rEtet*-^^-*@*^.,*,
Researdatide
olndan Societyfor Elucatim ard ErvircrrErtft$e) 75
IV o/loog B'6j
2I8 g FD, oc
lV - iodine value j BSAD - blended staric acld distillate;
B FO = bottom flash distiller
Fig. 6. Yeld of BSAD as effect of the interaction of the
,.'....** iE:i'r ?;ili tr tt, C a
0,11 0,16
' r?10
f croo
- 51e'l
rE
: F ;1?6
< 5rov
I 515il
I
i {14i1 : i
1
I
t
t,
ll
l
"l_i
*-21*
acid iistillatel
lodrrwlrc'
fflp/
^!wjnq$.sg
21,3 3?i 22r
IvtY.Rito'ga
lrdanJ.$i.Tedrd
'@
Fig.1. The modified distillation process schema t9 Provide BSAD- with tle be!91e sbble B_SAD tV
.
Jaei ft{":i ({i!r;l Fn!'" clSi! it. i'tilite
c;s:rilt'-Fig.2. The average effect ptot of the main variables on BSAD
lV
?, *,r
a
aie
:io
217Fig. 3. Mean effect plot of interaction of main variables !j;g r*ra:riq C:;.::f;iS ffi{
3@ lrdianJournalof
scienceandrechnotogy @
vd.5
tb.9 (sep.2012)
rsst\t@746846Fig. 7. An idea to modify the present distillation facility to provide BSAD
with
inciudes BSAD.the befter and stable iodine
value
Conclusionspurification through distillation
are:
0.60 g/100 g of feed lV, 6.0 ton/hour of feed flowr'ntd!'t*
r
botton flush distilleits
FEjl on the BSADr
this optimization met the BSAD's lV quality:
standard
by
separating
rubber
gradei i,iili erd, c1113 r'att1 *t d i;1e. rerjr, ri 10 .::.:. :-4t:y .,:,d. distillate aS bypfOdUCt. All thg main faCtOfS
C... . '-F::r ;c f ;i_:,du, {*5,6,-6 .ii_. }-. f
lV
0.175 9/100g.
Blended Stearic Acid Distillate of RBDPS which was obtained inTable /2. The average of BSAD lV as effect of the interaction of the sam/ lV
and
The improvementis
believed will be able lmprovement of this equipmentwiil
be,""t
o"rriii"
i"
:1:"1?d the BSAD lV' These factors.can be manipulated achieve lowerlv
and more expansive"upuii1!?i5ni5
and,controlled to achieve better and more stable BSADpurification through fractionat
distiilation,'"i;;";;"-;H
lv^'.lncreasingof
the
feedflow
rateF,
temperature ofi",ist'e. capabilit|
to
compete
in
the
,.,.,r"t"'"i
H!t!,-,"'ir.J,S:",:,:T#t"?SSXJ]irrJ,r"l1i"':r#t,,:il1
"'.TH[:TfllitY
o""n
cristiler corumn
to
main
The interactions betwe-en the main fact.rs had impact onfractionationitor 1z'; cotumn in Fig. 7) with
rp""Lr
irrr" :f-
PSADlV,
although someof
the interaction effects (that have highei efficiency) and "doubte"#d*;;;;;;
yu-f-.not
significant'These
interactionshad
to
bepartial conderisers will increise efficiency
t"
1"*";
o"irirJ
manipulatedand
controlled carefullyand
exactly to point (includes iodine value or double Uo|Olu-"alr;;;
i:.TI"
BSAD lV which is according to the specs.boilinj
pointin sefiration
of
DoA
unai,i.,irn:ti;;:;;;
sussestionsi""#:tffi,?d';:l,lni,"u;*ixLas,*i*ilijs"*m
ed;yT0'.1y":::?x1','I
3l'i"":i;'iJ""Hffii:
S1,X1,HI*iaJ::liru:fif:f
;:t"1[i*:1"##;ti
;:"?"""J::'31Xi11il1X':'['i,,"
va,ue or 0 6 s/100 g or and odor better also. The partialconoensel'iliti;;;
lower
by
improving
the
present
hydrogenation easier separation of tight end, cotor ano oooi,;[; ilj;;
.
iperating
conditionsvalue (that can't be
ieparated
moreon
th;
;;;
';i'j;
2'
To -improve2nd
columnof
the
present distillation cotumn, becauseot
freaiitiess
;t;;,
i;i"";i;r;) ;"
facility
becomes fractionation columnwith
partiat BSAD quatity wiu be better and more stabte.;;;i';ii";;
condenserto
get
lower
BSAD
lv,
higher
plant is effeci to darker coror and odor of ruttv u"io'pltirii.,
;iffi,?t[
33Xo1too
vield,
betterand
more stableFD
No
The average values of the increasing of BSAD lV, q/100o
The overall average of the increasing of BSAD lV, g/100 g
lV 0.7 q/100 q
1 BFD=218"Cto
22Q"C
B FD = 22AoC
to222oC B FD = 219 "C to 2220C
0.01 0.005 0,0075
2
lV 0.6 q/l00q
BFD=218oCto
2200C B FD = 220oCto222oC
BFD=21goC:
222',C0.01 0.0034 0.0067
Table /3. The average of BSAD lv decreasing as the effect of the interaction
ll/
and the same B FD.No The average values of the decreasing ofBSAD lV, g/1009
The overallaverage of the decreasing of BSAD lV,
o/1 00o
I B FD 218 0C B FD 220 "C BFD 222OC
lV = 0.7 to 0.6 o/100 o
lV
=
0.7 to0.6 s/100 q lV = 0.6 s/100 0.7 toq lV
=
0.7 to 0.6 9/100 g0.005 0.005 0.00555 0.00518
to cover usage of the other bed quality of raw material, such as CPS (Crude palm
Stearin)
or
PFAD (Patm Fatty
AcidDistillate)
to
produce the betterof
others main distillate products.References
1.
Brocmann R, Demmering G, Kreutzer U,Lindeman M, Plachinka J and Steihrner
U
(1987) Fattyacid.
Henket KGaA,Dusseldorf.
Federal
Republlc
ofGerman.
David
F
(2006) Officiat method andrecommended practices of the American
oil
chemists society.d'
Ed.,
2ndPrinting. AOCS Press, USA.
Ketaren
S
(2008) Pengantar teknologi minyak dan lemak Pangan.Ui
press. Jakafta.Nur
lriawanand
SeptinPuji
Astuti (2006) Mengolah data statistik denganmudah
menggunakanminitab
14.Penerbit Andi, Yokyakarta.
IilYRitorga lrdanJ.fti.Tednd. a
Z.
3.
4.
Researd artide
olrdian Soci@ fa ElLratim ard EnvirCI nBt (See)
-5
[image:7.612.57.559.27.695.2] [image:7.612.73.588.438.761.2]-3303
Vd.s
tS.g
(Sep.2012)
lsst\t @746846d;G;1\
rdian Journalof science and
rechnologv
'it[ryj|fl
5.
Muhammad YR (2009) Refined bleached deodorized palm oil (RBDPO) Sebagai Bahan B-aku PembuatanV tegs
S" J. Sisiim Teinik lndust., Fakultas Teknik,usu.
10(1),769-780.5.
Muhammad YR (2010) Optimization of palmitic acidcomposition
in
crude
oleic
acid
to
providespecifications of titer and cloud point of distillate oleic
acid
using a flash distiller.J.
Technol' & Sci' IPTEK, LPPM, lTS. 21(4), 203-213.7.
MuhammadVn
1ZOt1) Providing vegetable stearic acid superV
1895S
from hydrogenatedof
crude vegetabie stearicacid
HCV 1895S
with
A
single fraltionation column. |JET/IJENS. 1 1(4)' 5-15'8.
Peter W, Faesler, Karl Kolmezt and Wang SengKek (2004) Revamp strategiesfor
fatty .acid distillation sectionin
oteo-chemical plants. Sulzer Chemtech' Singapore.9.
pf
iiX
(2005) Production planning control' Medan, lndonesia.10. Roberl
C
Hastert (1990) Hydrogenating fatty acids: Hardening, furlher, faster a-nd cheaper' Proc' World Conf. Oleohemical into2'l't
Century, AOCS, Kuala Lumpur. PP: 16S171'11. Yusuf Riionga M (2010) Optimization of the providing of stearic
aiid
based on refined bleached deodorized palm stearin (RBDPS) whichis
stable according to ihe quality standard. FM\PA, USU. Medan, lndonesia' pp: 2-6.Researd artide
o lrdian Society fcr tlucdim ard Envirmrcri (See) 77
lodirE\alrc'
htipl^ rw.inq*W
lVtYRitorga