IDENTIFICATION AND EXPRESSION OF SKINNING
INJURY-RESPONSIVE GENES AND CURING PROPERTIES
IN SWEETPOTATO
JOLLANDA EFFENDY
SCHOOL OF GRADUATE STUDIES BOGOR AGRICULTURAL UNIVERSITY
This is to declare that the dissertation titled “Identification and Expression of
Skinning Injury-Responsive Genes and Curing Properties in Sweetpotato” is the
result of my original research under the direction of the supervisory committee and that no part of this dissertation has not been submitted for a higher degree to any other University or Institution. Any other sources of information that have been mentioned in this dissertation from published or unpublished works of other authors are fully acknowledged in accordance with the standard reference practices.
Based on this assertion, I hereby transfer the copyright of this dissertation to Bogor Agricultural University
Bogor, August 2015
Jollanda Effendy
Terhadap Skinning dan Manfaat Curing pada Ubijalar. Dibimbing oleh DARDA EFENDI, NURUL KHUMAIDA, GUSTAAF ADOLF WATTIMENA, dan DON R. LA BONTE.
Kehilangan kulit dari permukaan umbi bertanggungjawab terhadap kehilangan hasil pascapanen yang signifikan akibat penyakit pada tempat penyimpanan dan kehilangan bobot karena respirasi dan transpirasi yang berlebihan. Sayangnya, tidak ada laporan tentang gen-gen yang terlibat dalam penyembuhan luka pada ubijalar dan pengetahuan yang lebih mendalam tentang penyembuhan luka akan memfasilitasi perbaikan strategi pemuliaan. Sistim Annealing control primer
(ACP) digunakan untuk mengidentifikasi gen-gen yang terekspresi setelah kerusakan akibat skinning (kulit yang terkelupas) dari ubijalar kultivar LA 07-146. Total didapati 70 gen yang terekspresi berbeda (DEG) yang dapat direproduksi. Dari 70 DEG ini, 58 terinduksi dan 12 tereduksi. Empat puluh dua diklon dan dari 250 klon yang disolasi, 119 klon dikirim untuk disekuensing. Dari 119 klon ini, 101 klon sama dengan DEG dari tanaman. Kelompok DEG tersebut mewakili 63 unigen: 19 kontig (sekuens dengan tumpang tindih sekurang-kurangnya 50 nukleotida) dan 44 singleton (tidak dapat dibentuk menjadi kontig). Fungsi anotasi dari DEG menunjukkan gen-gen yang terlibat dalam protein yang berhubungan dengan stress dan pertahanan, penyandian redoks, metabolism, sintesis protein dan lokasi akhir protein, regulasi dan signal transduksi.
Perubahan ekspresi gen akibat adanya kerusakan akibat skinning yang berhubungan dengan 18 DEG dipelajari lebih lanjut. Ke-18 DEG ini menyandikan gen-gen yang terlibat dalam respons terhadap stress abiotik, biosintesis lignin dan suberin, regulasi transkripsi dan penyandian. Penelitian tentang ekspresi dari 18 DEG meliputi studi tentang kuantitatif dan semi kuantitatif transkripsi balik rantai reaksi polimerase (q/sq RTPCR) dibagi menjadi tiga kategori: gen dengan respon cepat, gen dengan respon lambat dan gen yang respon tidak beraturan. Hasil penelitian menunjukkan bahwa gen-gen dengan respon cepat umumnya berhubungan dengan stress secara umum, gen-gen dari lintasan biosintesis lignin dan suberin ekspresinya meningkat setelah 8 – 12 jam setelah pelukaan (gen-gen dengan respon lambat). Gen-gen yang lain menunjukkan regulasi ekspresi yang meningkat atau menurun tergantung dari waktu pengambilan sampel setelah terjadi pelukaan yang disengaja.
terhadap mekanisme kerusakan karena skinning yang berasosiasi dengan biosintesis lignin dan pembentukan suberin pada kedua kultivar. Penelitian ini menunjukkan bahwa gen-gen ini diregulasi secara berbeda pada kultivar resistan dan rentan dari ubijalar dengan perbedaan pada waktu induksi pada kondisi curing. Hasil penelitian ini juga menunjukkan bukti dari aliran signal tranduksi yang terkordinasi dari gen pada lintasan biosintesis pra- lignin dan lignin. Keseluruhan, penelitian ini mendemonstrasikan perbedaan besar pada toleransi terhadap skinning antara kedua kultivar tersebut disebabkan karena kemampuan kultivar resisten untuk mengatur aktivitas transkripsi yang lengkap antara gen-gen pada lintasan biosintesis pra-lignin dan lignin selama perlakuan skinning, suatu karakteristik yang hanya ditemukan pada kultivar yang resisten. Penelitian curing ini juga menunjukkan regulasi dan ekspresi yang berbeda pada gen-gen pada lintasan biosintesis lignin dan suberin pada kultivar ubijalar yang menunjukkan perbedaan kemampuan untuk menyembuhkan luka yang terjadi pada level paska panen untuk membantu memperbaiki kualitas umbi ubijalar.
Responsive Genes and Curing Properties in Sweetpotato. Supervised by DARDA EFENDI, NURUL KHUMAIDA, GUSTAAF ADOLF WATTIMENA, and DON R. LA BONTE.
Loss of the skin from the surface of the roots, is responsible for significant postharvest loss resulting from storage diseases and weight loss. Unfortunately, there is no report on the genes involved in wound healing of sweetpotato and a better understanding will facilitate improved breeding strategies. An annealing control primer (ACP) system was used to identify genes that expressed after skinning injury of sweetpotato cultivar LA 07-146 storage roots. In total, 70 unambiguous and reproducible differentially expressed genes (DEGs) were identified. Of these, 58 were up-regulated and 12 down-regulated. Forty two were cloned and from 250 total clones isolated, 119 independent clones were sent for sequencing. Of these, 101 clones were related to plants DEGs. These DEGs represented 63 unigenes: 19 contigs (assembled sequences that were overlapping by 50 nt) and 44 singletons (that did not have any assemble into a contig). Functional annotation of the DEGs represented genes involved defense- and stress-related proteins, redox signaling, metabolism, DNA, RNA stress-related, and gene expression, intercellular transport, transport facilitation and transport routes, cellular communication and signal transduction pathway.
The skinning injury changes in gene expression in genes corresponding to eighteen DEGs were studied further These DEGs encoded genes involved in abiotic stress responses, lignin and suberin biosynthesis, and transcriptional regulation/signaling. The expression study of 18 DEGs through quantitative- and semiquantitative reverse transcription-polymerase chain reaction in response to skinning injury in sweetpotato roots were divided into three categories: genes with early response, genes with late response, and genes with transient expression. The study showed that lignin and suberin pathways were up-regulated after 8 and 12 hours of skinning. Other genes showed up- or down-regulation in their transcript abundance depending on the time the storage roots were sampled after intentional skinning.
cascade in pre- and lignin biosynthesis pathway. Taken together, this study demonstrated that major differences in skinning tolerance between these two cultivars were due to the ability of the skinning resistant cultivars to maintain a complete transcription activity of pre-lignin and lignin biosynthesis pathways genes during curing treatment following skinning, characteristics were only observed in skinning resistant cultivars. This curing research also showed differential regulation and expression of the genes in lignin and suberin biosynthesis pathways in sweetpotato cultivars that lead to determination the ability to heal skinning wound occurred during postharvest level to help improve the quality of storage roots of sweetpotato.
© Copyright IPB, 2015
Copyright protected under the law
Dissertation
Submitted in partial fulfillment of the requirements for the degree Doctor of Philosophy
in
Plant Breeding and Biotechnology Study Program
IDENTIFICATION AND EXPRESSION OF SKINNING
INJURY-RESPONSIVE GENES AND CURING PROPERTIES
IN SWEETPOTATO
SCHOOL OF GRADUATE STUDIES BOGOR AGRICULTURAL UNIVERSITY
BOGOR 2015
Examiner during Closed Defense: Prof Dr Ir Sudarsono, MSc Dr Ir Trikoesoemaningtyas, MSc
ACKNOWLEDGEMENTS
Glory be to God Almighty for His everlasting blessings, I was able to finish writing this dissertation.
I would like to thank my senior supervisor, Dr Darda Efendi for his supervision, patience, advice and encouragement throughout my study and during the preparation of this dissertation. A similar appreciation is also addressed to Dr Ir Nurul Khumaida MSi, Prof Dr G.A. Wattimena, MSc, and Prof. Dr. Don R. La Bonte for being my supervisory committee, and help shaping my PhD research. Especially to Dr. La Bonte, thank you for discussing my project and reviewing my dissertation.
I was grateful to Prof Dr Ir Sudarsono, MSc and Prof Dr Ir Bambang S. Purwoko as Oral Pre-Qualitifaction Examiners. Prof Sudarson MSc and Dr Ir Trikoesoemaningtyas MSc to serve as closed examiners. Same appreciation was given to Prof Dr Ir Sudarsono, MSc and Dr Satya Nugroho to serve as doctoral promotion examiners. Dr. Yudiwanti Wahyu EK, MS as a chair of Plant Breeding and Biotechnology major thank you for your kindness. Similar appreciation also went to Dr. Trikoesoemaningtyas.
I would thank Dr. Baisakh for sharing his knowledge and expertise in molecular biology, in addition to his guidance, advice, discussion, and help in editing and revising the manuscripts for publication. I was indebted to Dr. La Bonte for providing the sweetpotato cultivars LA 07-146 and LA 10-70 to be used in this research. The project would never have been running without permission from SPESS LSU AgCenter. Other very important contributions were made by the member of the Molecular Biology Lab at School of Plant, Environmental, and Soil Sciences at LSU AgCenter. They were Arnold, Nizar, Bode, Renesh, Lina, Julio, Ramana, and Andres.
To all the lecturers who taught me during my study in IPB, thank you very much for sharing the knowledge. To all the employee in the Department of AGH thank you for your help and good laugh.
I wish to express my sincere gratitude to BPPS for funding my PhD study. My gratitude to Borlaug Fellowship from USDA-FAS and USDA-NIFA for providing grants to do research in the United States of America. Also to DIKTI Funding for a Sandwich Program Fellowship to do research in the USA.
For PBT 2010 thank you for the support and friendship through the 5 years. Ms. Hesti, thanks for sharing your motorbike with me and to Mr. Ismail thanks for sharing your skill in powerpoint presentation and good discussions.
I also place on record, my sense of gratitude to one and all who directly or indirectly have lent their hand in this journey.
Finally, I would like to thank my family who always support me through their prayers, love, encouragement and understanding.
Bogor, August 2015
LIST OF FIGURES APPENDICES
1 INTRODUCTION 1
Research Background 1
Problem Statement 2
Research Objectives 2
Hypotheses 3
Novelty of the Research 3
Scope and Framework of the Research 4
2 LITERATURE REVIEW 6
Skin Formation in Storage Roots and Wound Healing Processes 6
Skinning Injury and Postharvest Loss 8
Physical Factors 8
Physiological Factors 8
Biological Factors 8
Strategies Adopted by Plants to Avoid Skinning Injury 9 Skinning Injury Induced Changes in Gene Expression 9
Genes Involved in Lignin and Suberin Biosynthesis Pathways 10
Genes Involved in Protein Fate 10
Genes Involved in Cell Wall Modification 11
Genes Involved in Transcription and Protein Synthesis 11
Genes Involved in Stress Response and Defense 12
Molecular Biology as Tools to Study Gene Expression in Response to Sknning Injury in Storage Roots of Sweetpotato 13
ACP Technology as a Tool to Isolate Differential Expressed Genes
in Storage Roots of Sweetpotato 13
Real-time Quantitative Polymerase Chain Reaction as a Tool to Study the Temporal and Developmental Regulation of the
Expression of Skinning Responsive Genes in Sweetpotato 13
3 FUNCTIONAL CLASSIFICATION OF SKINNING INJURY
RESPONSIVE GENES IN STORAGE ROOTS OF SWEETPOTATO 15
INTRODUCTION 16
MATERIALS AND METHODS 17
Time and Place of Research 17
Plant Materials and Skinning Treatment 17
RNA Isolation 17
cDNA Preparation and ACP-Based Gene-Fishing PCR 18
Cloning and Sequencing of DEGs 19
Nucleotide and Deduced Amino Acid Sequencing Analyses 20
RESULTS AND DISCUSSION 20
Effect of Skinning treatment on Storage Root RNA Populations 20 Cloning and sequencing of skinning injury responsive DEGs 21
CONCLUSIONS 37 4 IDENTIFICATION AND EXPRESSION OF SKINNING
INJURY-RESPONSIVE GENES IN SWEETPOTATO1 39
INTRODUCTION 40
MATERIALS AND METHODS 41
Time and Place of Research 41
Plant Materials and Skinning Treatment 41
RNA Isolation, cDNA Preparation, and ACP-Based Gene-Fishing PCR 42
Cloning and Sequencing of DEGs 42
Semiquantitative Reverse Transcription Polymerase Chain Reaction
(sqRT-PCR) Analyzes of Selected DEGs 43
Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-
PCR) DEGs 43
RESULTS AND DISCUSSION 43
Isolation of DEGs under Skinning Injury 43
Transcript Abundance Analysis of DEGs 46
Transcript Abundance Analysis of Stress-Responsive Genes Involved
in Abiotic Stresses 46
Transcript Abundance Analysis of Genes Involved in Lignin and
Suberin Biosynthesis 48
Transcript Abundance Analysis of Genes Involved in Transcriptional
Regulation Signaling 49
CONCLUSIONS 51
5 CURING ALTERS THE EXPRESSIN OF SKINNING INJURY-
INDUCED GENES IN TWO CULTIVARS OF SWEETPOTATO 52
INTRODUCTION 53
MATERIALS AND METHODS 54
Time and Place of Research 54
Plant Materials, Skinning and Curing Treatment 54
RNA Isolation and cDNA Preparations 54
Quantitative Reverse Transcription Polymerase Chain Reaction
(qRT-PCR) Analyzes of Selected DEGs 55
RESULTS AND DISCUSSION 55
Expression of an Abiotic Stress-Responsive Gene (IbELIP3) 57
Expression of a Wound-Response Gene (IbTAL) 58
Expression of Lignification-Associated Genes 60
IbPAL 60
IbCCOMT 62
IbCAD 63
Expression of IbExt, a Suberin-Related Gene 64
CONCLUSIONS 66
6 GENERAL DISCUSSIONS 67
7 CONCLUSIONS AND RECOMMENDATIONS 70
Conclusions 70
Recommendations 70
REFERENCES 71
Figure 1.1 Research flowchart 5 Figure 2.1 Potato native and wound periderm 7 Figure 2.2 The monolignol biosynthesis pathway and typical lignin
distribution in monocot Switchgrass and dicot 11 Figure 3.1 Representative gel pictures from PCR with ACP2 with
different primers 21
Figure 3.2 Alignment of the deduced amino acid of fifteen DEGs 25 Figure 3.3 Distribution of DEGs length and percentage of transcripts
with BLASTX hits 29
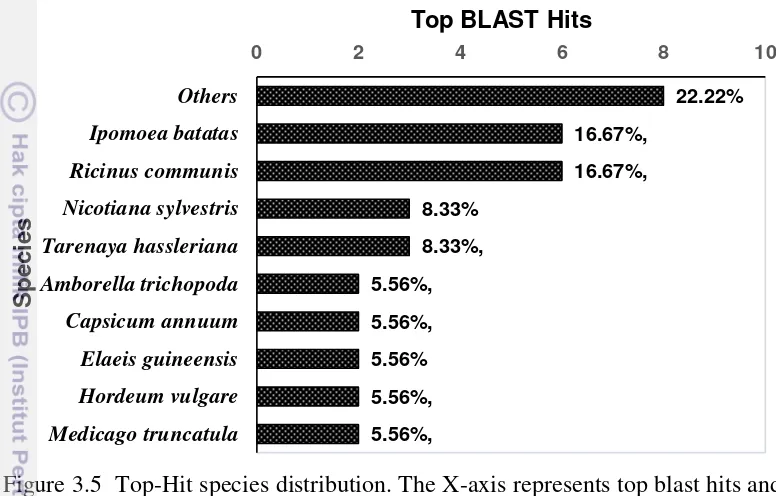
Figure 3.4 Sequence identity distribution 30 Figure 3.5 Top-Hit species distribution 31
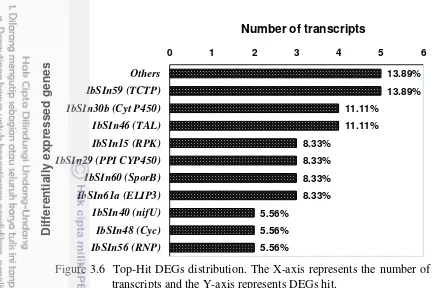
Figure 3.6 Top-Hit DEGs distribution 32
Figure 4.1 Representative gels from PCR with annealing control
primers (ACP) 45
Figure 4.2 Semiquantitative reverse transcription PCR analysis of differentially expressed genes in storage roots of
sweetpotato 46
Figure 4.3 Expression of differentially expressed genes in storage root of sweetpotato at 2, 4, 8 and 12 h relative to 0 h
after skinning 50
Figure 5.1 Expression of ELIP3 (Early light-inducible protein) IbSIn61a) ) in storage roots of LA 07-146 and
LA 10-70 cultivars of sweetpotato, skinned and cured (C) (at 28-29.5 oC with RH 85-90%) and non-cured (NC) (24 ± 1 oC with RH 50%) at 0, 2,
4, 8, 12, and 24 h. 58
Figure 5.2 Expression of TAL (Transaldolase) (IbSIn46) ) in
storage roots of LA 07-146 and LA 10-70 cultivars of sweetpotato, skinned and cured (C) (at 28-29.5 oC with RH 85-90%) and non-cured (NC) (24 ± 1 oC
with RH 50%) at 0, 2, 4, 8, 12, and 24 h. 59 Figure 5.3 Expression of PAL (Phenylalanine ammonia lyase)
(IbPAL) ) in storage roots of LA 07-146 and LA 10-70 cultivars of sweetpotato, skinned and cured (C) (at 28-29.5 oC with RH 85-90%) and non-cured (NC)
methyltransferase) (IbCCOMT) ) in storage roots of
LA 07-146 and LA 10-70 cultivars of sweetpotato, skinned and cured (C) (at 28-29.5 oC with RH 85-90%) and non-cured (NC) (24 ± 1 oC with RH 50%) at 0, 2,
4, 8, 12, and 24 h. 62
Figure 5.5 Expression of CAD (Cinnamyl alcohol dehydrogenase)
(IbCAD) in storage roots of LA 07-146 and LA 10-70
cultivars of sweetpotato, skinned and cured (C) (at 28-29.5 oC with RH 85-90%) and non-cured (NC)
(24 ± 1 oC with RH 50%) at 0, 2, 4, 8, 12, and 24 h. 63 Figure 5.6 Expression of Ext (Extensin) (IbExt) ) in storage roots
Of LA 07-146 and LA 10-70 cultivars of sweetpotato, skinned and cured (C) (at 28-29.5 oC with RH 85-90%) and non-cured (NC) (24 ± 1 oC with RH 50%) at 0, 2,
to RNA from storage root of sweetpotato subjected to
skinning injury 22
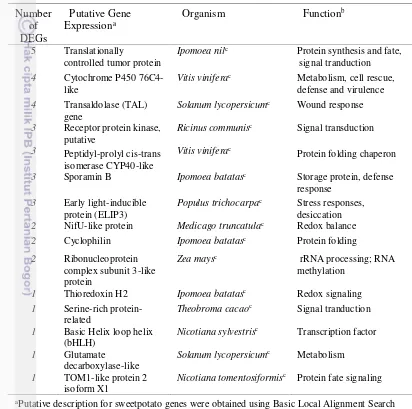
Table 3.2 Functional annotation of fifteen DEGs in response to
skinning injury 33
Tabel 4.1 Differentially expressed genes (DEGs) induced in
response to skinning 44
Table 5.1 Genes used for expression profiling under curing and non-curing conditions of sweetpotato storage root
1 INTRODUCTION
Research Background
Sweetpotato, the world’s seventh important food crop, is the main staple in 82 developing countries. The world sweetpotato production is estimated to be 8.18
Mt (FAO β01γ). Asia and the Pacific Islands account for 87.γ% of world’s sweetpotato production. Indonesia is the world’s fourth largest sweetpotato
production in the world with total production 2.386 Mt and total harvested area 0.161MHa with the average yield 14.7 ton/ha.
In Indonesia, sweetpotato is the second most important tuber crops after cassava. Besides high in starch and sugar, sweetpotato is also high in vitamin C, provitamin A, vitamin B, (thiamine) and iron. Some variety of sweetpotato are rich in ß-carotene and anthocyanine. Despite its important as a food crops, research in sweetpotato in Indonesia have mainly focused on breeding for sweetpotato cultivars with traits such as: adapted to different climate conditions, improve taste or nutritional value, better cope with diseases or pests in the field, or to use water or nutrients more efficiently and high yield production. There is need to improved postharvest handling and processing of storage roots (Saleh and Hartojo 2003). Due to its thin and delicate skin, sweetpotato roots are often subjected to skinning during harvest, transport from field to the market or to storage facilities. Skinning, the loss of skin (periderm) from the surface of the roots is the main culprit of reduced root weight by water loss associated with increase rate of moisture and weight loss, shriveling of the root surface, increase susceptibility to pathogen attack and unattractive appearance. Skinning injury is unavoidable in developed and developing countries. In developed countries, skinning injury is due to mechanical injury, while in developing countries is due to hand-harvested. Skinning tolerance is thus equally important in developed and developing countries.
Many studies have shown altered gene expression in response to skinning injury. The rapid induction of mRNA transcripts related to defense and wound healing may protect plants from attack by fungi and pathogens during storage (Bowles 1990; Chen et al. 2005). These defense genes were activated through
transcriptional, post-transcriptional and post-translational regulation. Furthermore, lignin deposition are accompanied by changes in genes involved in lignin biosynthesis pathway. In addition to the role of lignin as a resistance factor, induced lignification has been proposed as an active resistance mechanism of plants against fungi; at sites of wounding or pathogen attack. Lignin formation has been observed, to strengthen the cell wall at the location of damage (Vance et al. 1980;
Borg-Olivier and Monties 1993; Hawkins and Boudet 1994). Lignin biosynthesis has been proposed to be controlled by two signal transduction pathways, one involved in the development of lignified tissues and the other in plant defense response (Walter 1992).
Problem Statement
Sweetpotato is the third most economically important root crops after potatoes and cassava. It has been estimated that losses in sweetpotato due to physical wounding, such as skinning, cuts and bruises can be as high as 40%. Skinning is inevitable due to the rigors of bulk harvesting in sweetpotato, and thus skinning resistance is a prerequisite to developing sweetpotato that can withstand subsequent postharvest loss caused by storage disease and insect predicament.
Wound responses in plants have been of interest to researches for many years, especially in those plants that are of economic and nutritional importance. One of the main reasons for studying wound response is to gain an understanding of the processes that plants will undergo to minimize infection and fluid loss after injury. When plant tissue is damaged, a variety of physiological and anatomical changes occurs in the cells surrounding the wound. Plant organs are generally protected from desiccation and infection by pathogens by substances such as cutin, suberin, and lignin. When injury to surfaces of plants occurs, cells exposed to the environment may become desiccated and (or) infected unless impermeability is rapidly reestablished. Wound responses may differ among different plant species and organs. The ability of plant tissue to heal wounds is vital to prevent excessive water loss and pathogen invasion. This is exploited to improve storability of root crops after harvest by a process termed curing, in which they are placed in an environment to promote healing of wounds incurred during harvesting and handling.
Understanding the genetics of wound healing through detail analysis of specific genes in different regulatory/signaling pathway of sweetpotato will help breeding for storage roots with tough skin acquiescent to mechanized harvesting in the developed countries while in developing countries, tough skin may help extended marketing shelf life and also reduce postharvest losses.
Research Objectives
The major objective of my research was to isolate, identify, and characterize novel skinning injury responsive genes in sweetpotato storage roots and to examine their expression when subjected to skinning injury. A further objective was to elucidate the role of curing on the expression of skinning injury responsive genes in two cultivars of sweetpotato in order to determine if curing treatment mediates changes in gene expression of skinning injury responsive genes.
1. The objective of Experiment I was to isolate, identify and characterize the function of the genes/transcripts that are responsive to skinning injury due to intentional skinning in storage roots of sweetpotato.
2. The objective of Experiment II was to understand the temporal and developmental regulation of the expression of skinning-responsive genes. 3. The objective of Experiment III was to differentiate wound healing efficiency
in response to curing due to skinning in skinning-injury resistant and –
Hypotheses
This research were based on several assumptions:
1. There were up-regulation and down-regulation of differentially gene expressions in response to skinning injury in storage roots of sweetpotato. 2. There were differences in the level and time of gene expressions in response
to skinning injury in storage roots of sweetpotato.
3. There were differences in the expression of genes induced during curing due to skinning in skinning injury resistant and -susceptible cultivars of sweetpotato
Novelty of the Research
Research in sweetpotato is not as advance as research in potato. However, until now, no molecular data exists for skinning in potato as well. Due to its delicate and thin skin (periderm), sweetpotato is prone to several forms of postharvest losses during transportation from the field and in storage. Furthermore, skinning cause an increase in moisture and weight losses. Curing is pre-requisite to alleviate postharvest loss due to the loss of skinning from the surface of the roots. Gene expression studies in sweetpotato still focus on the aspect of understanding storage root formation and development, transcription profiling in storage root formation and lignin and starch biosynthesis, initiation of storage root development, sucrose metabolism, (a)biotic stress responses. No study outside the present work have addressed directly the effect of curing on the induction of skinning injury responsive genes. This dissertation presents experimental research that is unique:
1. Identified, characterized and classified the functions of skinning injury responsive genes are identified, characterized and classified.
2. Studied the spatial and temporal expression of skinning injury responsive genes in a time course manner.
3. Investigated the effect of curing on the expression of genes involved in general stress response, wound inducible response, lignin and suberin biosynthesis pathways in skinning injury resistant and –susceptible cultivars of sweetpotato.
4. Elucidated the role of TAL (pre-lignin) gene in lignin biosynthesis pathway. 5. Assessed the role of CAD as the final key step in lignin biosynthesis pathways
important for lignin formation.
6. Made a recommendation of using cinnamyl alcohol dehydrogenase (CAD) gene as a molecular marker specific for lignin biosynthesis.
7. Differentiated the role of early (short term rapidly induced) and late gene response with respect to wound healing in response to skinning injury. 8. Determined that the genes identified in this research are not limited to study
Scope and Framework of the Research
This research is consisted of three experiments. The first stages is to isolate, identify, and characterize the functions of the genes/transcripts that are responsive expression of eighteen selected genes that are induced during skinning injury. The last stage is to study the expression of six selected genes under cured and non-cured condition in susceptible- and resistant cultivars of sweetpotato (Figure 1.1).
In Experiment I, skinning injury induced changes in gene expression were analyzed at mRNA level using ACP-based DD-RTPCR). This experiment began with isolation of total RNA from sweetpotato cultivar LA 07-146 from three independent roots subjected to skinning at 0 (control), 2, 4, 8 and 12 hr. The next step was to performe cDNA preparation and ACP-based gene fishing PCR. Following agarose gel electrophoresis, the selected bands of interest were excised from the gel, extracted using Qiaquick Gel extraction kit and then cloned into pGEM®-T Easy Vector (Promega). After removing the vector backbone and poly (A), DEGs were sent for sequencing. Functional classification of DEGs was performed by compared against all sequences in the non redundant database at NCBI using BlastN and BlastX (Altschul et al. 1997). These distinct identifications
were grouped into their functional categories.
In Experiment II, Quantitative and semi quantitative Reverse Transcription-Polymerase Chain Reaction q(sq) RT-PCR were used to study the expression of skinning induced genes. Fifteen selected DEGs from Experiment I and three additional genes (lignin and suberin related genes) were selected and primers were designed using Primer3.
In potato, curing has been shown to induce changes in a(biotic)-stress-related genes. In present study, the role of curing in regulating the selected skinning responsive expression of gene(s) corresponding to skinning responsive cDNA was determined:
1. By subjecting roots to curing and not-curing treatments after skinning treatments at 0, 2, 4, 8, 12 and 24 h.
Experiment I:
Experiment II:
Experiment III:
Cloning and Sequencing of DEGs
· Isolate and Elute the DEGs fragments
· Gel Extraction of DEGs to elute DNAs
· Ligation of DEGs
· Transformation of DEGS
· Growing the Bacterial Overnight
· PCR Bacterial Suspension Cultures
· Run the Agarose Gel to Check the Inserts
· Sequencing the DEGs
Differentially Expressed Genes (DEGs)
Study the Expression of Genes induced during Skinning Injury
Using Susceptible and Resistant Cultivars of Sweetpotato to Study the Effect of Curing on the Expression of Skinning-induced Genes
in LA 07-146 and LA 10-70 Cultivars of Sweetpotato Identification of Genes/Transcripts that are Responsive
to Wound Injury due to Skinning in Storage Roots of Sweetpotato
Functional Annotation of DEGs
Isolation of Total RNA from LA 07-146 and LA 10-70
cDNA synthesis
QRT-PCR to Identify Genes/Transcripts That Responsive to Curing
Quantitative Reverse Transcription PCR Semiquantitative Reverse
Transcription PCR
Fragments of DEGs
cDNA Preparation and ACP-Based Gene Fishing PCR Isolation of Total RNA from Storage Roots of Sweetpotato
2 LITERATURE REVIEW
Sweetpotato is the seventh largest food crop, just after cassava, with an annual production around 110 Mt and a cultivated area of 8.18 Mha (FAO 2013). It produces stable crop yields under a wide range of environmental conditions and one of the staple diets in many countries. Over 95% of the global sweetpotato crop is produced in the developing countries, where it is the fifth most important crop on fresh weight basis after rice, wheat, maize, and cassava (Plucknett 1991). Indonesia
is the forth world’s largest in sweetpotato production after China, Uganda and Nigeria with the total production 2.39 Mt (FAO 2013). In Indonesia, sweetpotato is utilized mainly as human food, 88% of total production in 1996 (World Bank 1998–
99 report summarized by CIP, 1999) although it could be a lower figure if it is true as claimed by Jusuf (2002) that the crop’s use as food in recent years in Indonesia has decreased but reliable statistics are lacking. Thus, varieties widely grown are those preferred on the basis of traits related to eating quality such as taste and flavor and root appearance rather than root yield (Jusuf 2002; Zuraida 2003). The most of the popular local varieties of sweetpotato in Indonesia have colored flesh ranging from cream to orange and at least in one case (Samarinda), purple (Zuraida 2003).
Skin Formation in Storage Roots and Wound Healing Processes
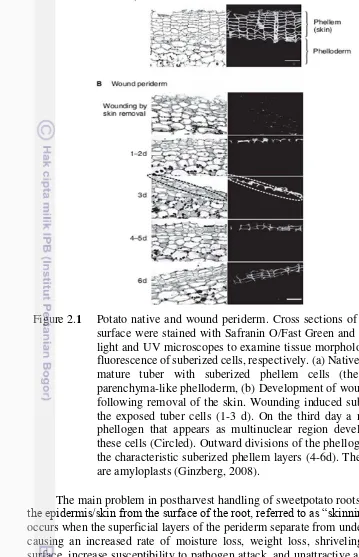
The storage roots of sweetpotato consist of the inner flesh, which is mainly composed of parenchyma cells with starch granules (storage parenchyma), covered by the skin. The skin is the secondary protective layer or periderm that consists of three layers: phellem, phelloderm, and phellogen (Järvinen et al. 2011). The
phellogen originates the phelloderm toward the inside of the root and the phellem toward the outside. The phellem is composed of several layers of cells devoid of starch granules and positioned in a radial manner toward the phellogen. The phelloderm is more difficult to identify as it resembles cortical cells and it usually is distinguished by radial position of the cells in reference to the phellogen (Kono and Mizoguchi 1982; Villavicencio et al. 2007; Firon et al. 2009).
The outer peridermal cells of sweetpotato become partly lignified during growth and are progressively sloughed off. The phellogen layer, however, remains active until harvest, and its activity caused the thickness of the periderm layer to remain constant (Artschwager and Starrett 1931; Villavicencio et al. 2007; Firon et al. 2009). The capacity to produce new periderm upon wounding is retained by
The main problem in postharvest handling of sweetpotato roots is the loss of
the epidermis/skin from the surface of the root, referred to as “skinning”. Skinning
occurs when the superficial layers of the periderm separate from underlying tissue; causing an increased rate of moisture loss, weight loss, shriveling of the root surface, increase susceptibility to pathogen attack, and unattractive appearance. In
the United States, one of the most popular cultivars is ‘Beauregard’ because of its smooth skin, color, and high yields. This cultivar, however is very prone to skin loss during harvest and handling (Firon et al. 2009).
In sweetpotato storage roots, the process of wound-healing involves desiccation of the surface cell layers, followed by lignifications of underlying cell layers and finally the formation of a wound periderm by cell division (Artschwager Figure 2.1 Potato native and wound periderm. Cross sections of potato tuber
and Starrett 1931). At low humidity, poor wound healing is associated with a thick desiccated layer and slow incomplete lignifications (van Oirschot et al. 2006).
Continuity of the lignified layer is vital for effective wound-healing, presumably to act as an effective barrier to control fluxes of water, mineral nutrients and essential gasses, and also defend against incorporation of toxic compounds, water loss, insect predicament and pathogen invasion (van Oirschot et al. 2006; Ranathunge et al.
2011). A method for assessing efficiency of wound-healing based on van Oirschot
et al. (2002) assessing the continuity of lignified layers by phloroglucinol staining
(lignifications score: LS) was developed (van Oirschot et al. 2002; 2006), and has
been used as a tool for screening sweetpotato germplasms.
Villavicencio et al. (2007) suggested that because skin loss occurs due to
breakage of the cell walls of peridermal cells, lignin content and polygalacturonase (PG) and pectin methylesterase (PME) activities could be part of the processes or factor that lead to increased or decreased skin adhesion in sweetpotato roots. If this is the case skin adhesion might correlate with these variables, making it possible to use them as indicators of susceptibility to skinning.
Skinning Injury and Postharvest Loss
Physical Factors
Mechanical damage is the most important harvest factor, much of which is sustained during harvest itself, and during transport and marketing. Harvesting in the tropics is usually manual, employing a variety of tools such as digging sticks, spades, hoes and knives. Sweetpotato roots are often cut, skinned and bruised by the harvesting tools.
Physiological Factors
In sweetpotato, respiration and transpiration contribute to loss in weight and alteration of internal and external appearance. Transpiration losses are due to` evaporative loss of the cellular water caused by vapor pressure difference between the root interior and the outside environment. In addition, wounding of sweetpotato roots resulted in an increase in both the respiration rate and subsequent weight loss. Furthermore, loss of moisture leads to a condition known as
‘pithiness‘ characterized by the formation of cavities within the tissues. Prolonged
moisture losses, as occur in tropical conditions may cause collapse of tissues starts at the distal end of the roots. This is commonly found in small sized root which could lead to total desiccation (Picha 1986).
Biological Factors
Strategies Adopted by Plants to Avoid Skinning Injury
Plants have evolved different mechanisms for protection against injury from insect and micro-organism due to skinning. Structurally, plants have a polyester coating composed of cutin and suberin (Kolattukudy 1980). This coating normally isolates the plant tissues from competing organisms and plants are therefore relatively immune from the presence of these competitors even on their surface. However, if a break or wound occurs in this surface coating, then competing
organisms gain entrance into the plant’s tissues where they can cause injurious
damage to those tissues. Consequently, plants have developed a complex response to wounding that dramatically alters the cellular physiology of plant tissues and results in the production of defenses. These defenses are particularly protection against microorganisms and are effective against small herbivores (Constabel et al.
2000).
A major phase of the wound-response is a generalized activation of plant defenses. Because the majority of microbial infections occur in plants following a wound, plants have developed a range of biochemical defenses to inhibit invading pathogens and small herbivores (Zhou and Thornburg 1999). The accumulation of phytoalexins after wounding has been a worldwide area for study. Phytoalexin, a small molecular weight defensive compounds, has been shown to have a biological activity against microorganisms or herbivores. For example, phenolic, terpenoid, and alkaloid compounds are a major component of plant secondary metabolism (Zhou and Thornburg 1999).
Plants protect themselves by physical barriers (such as wax coatings and harden tissues), and chemical metabolites against damage from outside (Chen et al.
2005). Mechanical wounding and pathogen infection stimulate plants to produce local and systemic signals, which in turn activate signal pathways within cells (Bergey et al. 1999). Several compounds, including hydrogen peroxide, nitric
oxide, calcium, protein kinase, jasmonate, salicylic acid, and ethylene, were involved in the signal pathways of defense systems (Delaney et al. 1994; Song et al. 1995; O'Donnell et al. 1996; McConn et al. 1997; Zimmermann et al. 1997;
Foissner et al. 2000; Orozco-Cárdenas et al. 2001; Gould et al. 2002; Jih et al. 2003;
Deng et al. 2013).
Skinning Injury Induced Changes in Gene Expression
with respect to skinning injury and other related biotic and abiotic stresses are: (1) what genes are induced or repressed? (2) what is the function of the encoded gene products? And (3) how are these genes regulated? (Bray 1993).
A common technique that has been used to identify and isolate skinning/wound injury responsive has been the screening of cDNA library constructed from poly(A)+ RNA from skinning injury plants. The encoded products of the genes isolated by this technique are believed to play a role in a number of processes including lignin and suberin biosynthesis pathways, protein fate, cell wall modification, transcription and protein synthesis, and stress response and defense. Genes Involved in Lignin and Suberin Biosynthesis Pathways
Suberin, a complex polymer, consists of aliphatic and aromatic domains (Bernards 2002). The aliphatic suberin is a glycerol-based polyester of long and
very long chain of ω-hydroxyacids and fatty α, ω-diacids with small amounts of esterified hydroxycinnamic acids, mainly ferulic acid. The aromatic suberin is a lignin-like polymer mostly made of hydroxycinnamic acids. Regarding candidate genes for aliphatic suberin pathway, two genes induced in potato skin are long-chain acyl-CoA synthethase (LACS) apparently related to the activation of fatty acids before elongation (Pollard et al. 2008) and a cytochrome P450 of the CYP94A
subfamily, whose putative orthologue in Nicotiana tabacum catalyses the oxidation
of fatty acids to α, ω-diacids (Le Bouquin et al. 2001). For aromatic suberin
pathway, it is worth to stress the presence of phenylalanine ammonia lyase (PAL) and caffeic acid 3-O-methyl transferase (COMT) (Fig. 2.2). PAL encodes the
enzyme that catalyses the first step in the phenylpropanoid pathway (Kolattukudy 1981) whose enzymatic activity in periderm is concomitant with suberin deposition (Bernards et al. 2000; Lulai 2008). COMT catalyzes the multi-step methylation
reactions of hydroxylated monomeric lignin precursors. It is believed to occupy a pivotal role in the lignin biosynthesis pathway. A cDNA (TaCM) was identified from wheat and expressed constitutively in stem, leaf, and root tissues (Ma and Xu 2008). The deduced amino acid sequence of TaCM showed a high degree of identity with COMT from other plants, particularly in SAM binding motif and the residues responsible for catalytic and substrate specificity (Ma and Xu 2008).
Genes Involved in Protein Fate
A group of protein encoded by the cDNA clones are involved in protein fate, some of which are previously reported to be induced under various stress treatments. Parvulin-type peptidyl-prolyl cis-trans isomerase (PPIase) is believed to play a role in the folding of certain proteins by catalyzing the cis-trans isomerization of X-Pro peptide bonds. Accumulation of PPIase mRNA was found in plant responses to various environmental stress (Godoy et al. 2000; Reilly et al.
2007; Effendy et al. 2013). Gene of Soy max translationally controlled tumor
protein (SmTCTP) involved in protein fate (Gnanasekar et al. 2009). SmTCTP was
Genes Involved in Cell Wall Modification
Changes in cell wall modifications are crucial during tuber periderm differentiation as a result of cell extension and suberin deposition. At the subcellular level, suberin is deposited inside the primary cell wall. Within subdomains of cell walls, suberin occurs in three forms, depending on the developmental stage of the suberized tissue (Ranathunge et al. 2011). Genes involved in cell wall growth and
remodeling such as pectin-glucuronyltransferase (Iwai et al. 2002; Soler et al.
2011), extensin (Cannon et al. 2008; Soler et al. 2011; Effendy et al. 2013;
Neubauer et al. 2013), xyloglucan endotransglucosylase/hydrolase (Fry 2004; Soler et al. 2011) and -D-glucan exohydrolase (Hrmova and Fincher 2001) have been
found in the potato skin SSH library (Soler et al. 2011).
Genes Involved in Transcription and Protein Synthesis
Recent studies have revealed differentially expressed genes that involved in transcription and protein synthesis (Zhou and Wu 2009; Gray et al. 2012).
Transcriptional and posttranscriptional regulations are important for gene expression in eukaryotes. Transcription factors fulfill the work through trans-regulation to control plant responses to stress (Singh et al. 2002). The NAC protein
family is one of the largest families of plant specific transcription factors (Olsen et al. 2005; Zheng et al. 2009). Genes from this family involved in various biological
processes including biotic and abiotic stress responses including drought and salt stress (Zheng et al. 2009). Zinc finger proteins belong to the largest family of
regulatory transcription factors. They have very different structures and functions Figure 2.2 The monolignol biosynthesis pathway and typical lignin
for example RNA or DNA recognition, RNA packaging, transcriptional activation, protein folding and assembly, and lipid binding (Laity et al. 2001; Zhou et al.
2009a; Zhou and Wu 2009).
Genes Involved in Stress Response and Defense
Periderm is a tissue that replaces the epidermis early in potato-tuber development (Reeve et al. 1969). It was found that periderm is a tissue with high
exogenous and endogenous oxidative stress (Pla et al. 2000). Genes involved in
abiotic and biotic stress tolerance such as patatin-like phospholipase, catalase and ascorbat peroxidase have been identified in both native and wound-healing periderm (Chávez et al. 2005; Krits et al. 2007; Barel and Ginzberg 2008; Ginzberg et al. 2009; Soler et al. 2011). Studies by Bernards (2002) showed that the
occurrence of stress proteins in periderm has been related with detoxification of the reactive oxygen species (ROS). Furthermore, genes that regulate the protein redox state, such as thioredoxin, are also up-regulated in periderm (Bernards 2002). Taken together, the presence of genes involved in antioxidant activity and in redox homeostasis suggests the importance of redox signaling (Buchanan and Balmer 2005; Foyer and Noctor 2005), which could have a main role in periderm.
Lectins have also been identified as defense-related proteins. Lectins are carbohydrate-binding proteins, and commonly found in viruses, bacteria, plants, and animals (Barondes 1988). Most lectin bound glycoproteins, glycolipids, and polysaccharides (Goldstein and Hayes 1977), and were believed to help defend plants against pathogen or insect infection (Zhu-Salzman et al. 1998;
Bandyopadhyay et al. 2001). Lectins are classified into seven families according to
their structures and evolutionary origins, namely legume lectins, type 2 ribosome-inactivating proteins, chitin-binding lectins, monocot mannose-binding lectins, cucurbitaceae phloem lectins, jacalin-related lectins (Chang et al. 2012), and others
(Damme et al. 1998).
Numerous defense-related genes have been studies in Arabidopsis such as pathogenesis-related genes, general defense and stress-related genes, genes involved in fatty acid signaling and metabolism, genes for aromatic amino acid metabolism, genes with unknown functions. Furthermore, studies had found evidences that octadecanoid signaling pathway mediated responses to mechanical wounding, insect attack, and ultraviolet irradiations. (Conconi et al. 1996; Howe et al. 1996). Proteins encoded by wound-inducible genes have various functions.
Several help repair damaged tissue, whereas others may produce specific compounds to inhibit insect growth. Wound-inducible proteins also played a role in activating the signaling pathway and regulating plant metabolism (León et al.
2001). For example the ipomoelin (IPO) gene was isolated from sweetpotato, and
its expression was induced by the application of methyl jasmonate and mechanical wounding (Imanishi et al. 1997; Chen et al. 2003). Additionally, experimental
results of feeding IPO protein from Escherichia coli to silkworm confirmed that the
Molecular Biology as Tools to Study Gene Expression in Response to Sknning Injury in Storage Roots of Sweetpotato
ACP Technology as a Tool to Isolate Differential Expressed Genes in Storage Roots of Sweetpotato
Several RNA-profiling techniques, such as cDNA-AFLP, have been used earlier as an initial step to identify differentially expressing genes (DEGs) under various physiological stages or experimental conditions. The difficulty of
identifying (or “fishing out”) a gene responsible for a specialized function during a
certain biological stage is often encountered because the gene is expressed at low levels, while mRNA transcripts within a cell are highly abundant (Hwang et al.
2004). However, to discover DEGs, it is important to choose a simple PCR-based method that is sensitive and reproducible. A recently developed annealing control primer (ACP) system provides a suitable primer with annealing specificity that specifically targets sequence hybridization to the template through a polydeoxyinosine poly(dI) linker (Kim et al. 2004). This system used ACP- based
on a unique tripartite structure consisting of γ′-end and 5′-end distinct portion separated by a regulator (Hwang et al. 2004; Kim et al. 2004). There are two sets
of ACPs that play important roles in amplification of a specific target gene. The first primer, dT-ACP1, reverse transcribed the RNA templates to produce first
cDNA strand. The procedure is achieved by hybridizing the γ′-end core portion of dT-ACP1 with a sequence complementary to the polyA region of mRNA transcripts. The first-strand cDNA produced at the first stage possessed the universal sequence of dT-ACP1 at its 5′-end. The second primers consist of combination of arbitrary ACPs (forward primers) and dT-ACP2 (reverse primers), possessed a hybridising sequence complementary to a region on the first strand cDNA, and are participating in synthesizing the second strand cDNA by PCR. This ACP-based system allows amplification of specific target gene by stringent hybridization of the ACPs with the intended template. Therefore, gene expression profiles can be characterized with elimination of false positive result. Due to its high-annealing specificity during PCR, the application of the ACP to DEG identification generates reproducible, accurate, and long (100 bp to 2 kb) PCR products that can be visualized on agarose gels.
Real-time Quantitative Polymerase Chain Reaction as a Tool to Study the Temporal and Developmental Regulation of the Expression of Skinning Responsive Genes in Sweetpotato
generate variable amount of PCR product. Only during the exponential phase of the PCR reaction is it possible to extrapolate back in order to determine the starting quantity of template sequence. The measurement of PCR products as they accumulate (i.e., real-time quantitative PCR) allows quantitation in the exponential phase of the reaction and therefore removes the variability associated with conventional PCR.
Since the first documentation of real-time PCR, it has been used for an increasing and diverse number of application including mRNA expression studies, DNA copy number measurements in genetic or viral DNA, allelic discrimination assays, expression analysis of specific splice variants of genes and gene expression in paraffin-embedded tissues and laser captured micro-dissected cells.
Initially, intercalator dyes are used to measure real-time PCR products. The primary disadvantage to these dyes is that they can detect accumulation of both specific and nonspecific PCR products.
Small molecules that bind to double-stranded DNA can be divided into two classes: intercalators and minor groove binders. Regardless the binding method, there are two requirements for a DNA binding dye for real-time detection of PCR such as increased fluorescence when bound to double-stranded DNA and no inhibition of PCR. The SYBR Green I dye chemistry uses the SYBR Green I dye to detect polymerase chain reaction (PCR) products by binding to double-stranded DNA formed during PCR.
When SYBR Green I dye is added to a sample, it immediately binds to all double-stranded DNA present in the sample. During the PCR, AmpliTag Gold® DNA Polymerase amplifies the target sequence, which creates the PCR products or
‘amplicons’. The SYBR Green I dye then binds to each new copy of double-stranded DNA. As the PCR progresses, more amplicons are created. Since the SYBR Green I dye binds to all double-stranded DNA, the result is an increase in fluorescence intensity proportionate to the amount of PCR product produced.
3 FUNCTIONAL CLASSIFICATION OF SKINNING INJURY
RESPONSIVE GENES IN STORAGE ROOTS OF
SWEETPOTATO
ABSTRACT
Skinning injury in sweetpotato due to loss of skin or periderm which occurred during harvest is inevitable and account for financial loss due to dehydration, pests, and pathogens. Hence, studies on gene expression changed due to skinning injury can provide important information about this protective tissue and for improving the life of storage roots. New candidate genes involved in skinning injury were isolated with an Annealing Control Primer (ACP). Using 20 ACP primers, a total of 103 differentially expressed genes (DEGs) were retrieved and clustered into 19 contigs and 44 singletons. In this study, the functional annotation of these selected 15 up-regulated DEGs (10 contigs and 5 singletons) were further characterized. The
results showed that these 15 “DEG-unigenes” are mainly associated with defense
and stress responses, regulation and signaling, protein synthesis and fate, and metabolism may play an important role in the primary responses to skinning injury in storage roots of sweetpotato. This study showed the importance of defense and stress responses genes to the formation of wound periderm. Furthermore, this results can be used for better understanding of the molecular mechanism of skinning/mechanical injury-related genes in the storage roots of sweetpotato as well as to all stems, fruits, and roots of all plants.
INTRODUCTION
Sweetpotato storage roots are underground storage organs covered by skin or periderm, a suberized layer that protects inner flesh from dehydration and pathogens. Skinning, the loss of the skin from the surface of the storage roots of sweetpotato, is responsible for significant postharvest losses due to mechanical injury, weight loss, sprouting, pests and diseases. Thus, because of its short shelf-life, in developing countries the sweetpotato storage roots has to be consumed or marketed within 1 to 2 weeks after harvesting (Rees et al. 2008).
Periderm formation which characterized by secondary growth are usually found in the plants following wounding under conditions of high temperature and humidity (curing). Studies on the molecular processes associated with periderm formation is of great importance for a better understanding of this protective tissue and for improving the storage life of storage roots. Moreover, to understand the molecular mechanisms underlying skinning injury, attempts have turned toward the isolation of genes regulated by skinning injury. This permits insight into their functions and the pathways that lead to their expression. Although several responses of plants to skinning injury, including short term metabolic and physiological changes may not require changes in gene expression, the majority are predicted to rely on alterations in gene expression. For example, wounding alters changes in gene expression in response to mechanical wounding and insect attack has been studied in Arabidopsis leaves using cDNA microarrays (Reymond et al. 2000), and
potato skin using a suppression subtractive hybridization library (Soler et al. 2011).
They showed that in response to wounding, the highest mRNA transcripts were dominated by genes involved in stress and defense followed by genes involved in signal transduction and regulations (Reymond et al. 2000; Soler et al. 2011).
Studies by Soler et al. (2011) also showed the activation of genes involved in
suberin and wax and cell wall in response to wounding in potato skin. Furthermore, study by Lulai and Neubauer (2014) in potato skin showed the spatial and temporary wounding induced suberization genes that involved in the closing layer and wound periderm formation.
In order to understand the molecular basis of skinning injury responses, the differentially expressed genes (DEGs) in skinning-treated storage roots must be characterized. Low levels of DEGs and their transcripts are sometimes hard to detect in abundant mRNA within tissues. A highly reliable, accurate, and reproducible specific polymerase chain reaction (PCR) amplification is required. A novel annealing control primer (ACP)-based DD-RTPCR technology is based on a unique tripartite structure of a specific oligonucleotide primer with its 3' and 5' ends separated by a regulator, and the interaction of each end of this primer during a two-stage PCR. Because of the high-annealing specificity during PCR, the application of the ACP to DEG identification generates reproducible, accurate, and long (100 bp to 2 kb) PCR products that are detectable on agarose gels (Hwang et al. 2003).
This method specifically targets the template sequence for hybridization through a polydeoxyinosine linker (Hwang et al. 2003). Here, ACP-based DD-RTPCR is
cDNA from skinned storage roots to determine the common genes. Freshly harvested sweetpotatoes were used for this approach. Briefly, this work presents 15 DEGs that could be used to further investigate their role in skinning injury and wound healing responses at the molecular level. The objective of this work was to describe functional classification of DEGs in LA 07-146 in response to skinning injury in storage roots of sweetpotato at 2, 4, 8, and 12 h.
MATERIALS AND METHODS
Time and Place of Research
Research was carried out in the Molecular Biology Laboratory, School of Plant, Environmental, and Soil Sciences, Louisiana State University Agricultural Center, Baton Rouge, Louisiana, United States of America from July to October, 2011.
Plant Materials and Skinning Treatment
Freshly harvested storage roots of sweetpotato cultivar LA 07-146 were washed, blot-dried, and carefully scraped with a razor scraper (Titan 11030; Star Asia-USA, Renton, WA) to remove the thin outer pigmented skin and stored at room temperature (24 ± 1 oC), 50% relative humidity, and 400 lx light. ‘LA 07-146’ was chosen for this study because this cultivar is uniquely suited to sweetpotato french fry processing and skins when bulk-harvested. The entire roots were skinned and the skinned roots were peeled to the same thickness (1.2 mm) at 0 (control), 2, 4, 8, and 12 h after skinning and the peels were immediately frozen in liquid nitrogen and stored at –80 oC for RNA extraction. Three independent roots were used for each time point as replicates.
RNA Isolation
including any precipitate that may have been formed, then were transferred to an RN easy spin column (pink) placed in a 2 mL collection tube (supplied) and then were centrifuged for 15 s at ≥8000 x g (≥10,000 rpm). The flow-through was then discarded. If the sample volume exceeds 700 µL, the successive aliquots were centrifuged in the same RNeasy spin column. The flow-through was discarded after each centrifugation. After that, 700 µL Buffer RW1 were added to the RNeasy spin
column and the centrifuged for 15 s at ≥8000 x g (≥10,000 rpm) to wash the spin
column membrane. After centrifugation, the RNase spin column were carefully removed from the collection tube so that the column will not be contacted the flow-through and also be sure to empty the collection tube completely. Following centrifugation, 500 µL Buffer RPE were added to RNeasy spin column and then
were centrifuged 15 s at ≥8000 x g (≥10,000 rpm) to wash the spin column
membrane. After discarding the flow through, 500 µL of Buffer RPE were added
to the RNeasy spin column and then were centrifuged for β min at ≥8000 x g (≥10,000 rpm) to wash the spin column membrane. After centrifugation, the
RNeasy spin column was removed carefully from the collection tube so that the column will not contact the flow-through. Following centrifugation, the RNeasy spin column were placed in a new 1.5 mL collection tube and the old collection tube with the flow-through were discarded. Fifty to thirty µL RNase-free water were added directly to the spin column membrane and were then centrifuged for 1 min
at ≥8000 x g (≥10,000 rpm) to elute the RNA. If RNA yield >γ0 µg were expected,
the previous step was repeated using another 30 – 50 µL RNase-free water or using the eluate from previous step if high RNA concentration was required by reusing the previous collection tube.
cDNA Preparation and ACP-Based Gene-Fishing PCR
First strand cDNA synthesis was performed using a GeneFishingTM DEG premix kit (Seegene, Rockville, MD) as per the manufacturer’s instruction. An
aliquot of 3 µg RNA extracted from storage roots at different replicates were pooled at each time course experiment except for 8 h and 12 h were pooled together. In brief, 3 µg of pooled RNA were mixed with (dT) 18-ACP1 primer in a final volume of 9.5 µL and were incubated at 80 oC for 3 min followed by ice-chilling for 2 min. Subsequently, the reverse transcription reaction were performed at 42 oC for 90 min in a final reaction volume of 20 µL containing 4 µ L of 5 x reaction buffer, 5 µL of dNTP mix (2 mM), 20 U RNase inhibitor (Promega, Madison, WI), 2 µL of 10 µM dT-ACP1 (Seegene, Rockville, MD), and 1 µL Moloney murine leukemia virus transcriptase (200 U/µL; Promega, Madison, WI). First-strand cDNAs were diluted 5 x with nuclease-free water for further use.
one cycle at 94 oC for 1 min, followed by 50 oC for 3 min, and 72 oC for 1 min. After second-strand cDNA synthesis were completed, the second stage PCR amplification profile were as follows: 40 cycles of 94 oC for 40 s, followed by 65 oC for 40 s, 72 oC for 40 s, and a 5 min final extension at 72 oC. The amplified PCR products using 20 ACP primers were resolved in a 2% agarose gel, stained with ethidium bromide, and visualized under UV and documented in a Kodak Gel Logic200 system (Carestream Health Inc, Rochester, NY).
Cloning and Sequencing of DEGs
Based on intensity or presence/absence) between control and skinning were
performed following the manufacturer’s instruction (QIAquick® Gel Extraction
Kit Qiagen, Valencia, CA). Essentially, the DEGs were cut from the gel with clean, sharp scapel. The sliced gel then were weighted in a colorless tube and then 3 volumes Buffer QG were added to 1 volume of gel (100 mg – 100 µL). For >2% agarose gel, 6 volumes Buffer QG were added. The tube was then incubated at 50 oC for 10 min (or until the gel slice has completely dissolved). The tube were then vortexed every 2 – 3 min to help dissolve gel. After the gel slice had been dissolved completely, the color of the mixture turned yellow (similar to Buffer QG without dissolved agarose). If the color of the mixture were orange or violet, 10 µL 3 M sodium acetate, pH 5.0 were added, and mixed. After that, the color of the mixture turned yellow. One gel volume of isopropanol were added to the sample and mixed. A QIAquick spin column was placed in a provided 2 mL collection tube. The sample were then applied to the QIAquick column to bind DNA and centrifuged for 1 min until all the samples had passed through the column. The flow-through were discarded and placed in a QIAquick column back into the same tube. For sample volume of >800 µL, the sample were loaded and spinned again. To wash, 0.75 mL Buffer PE were added to QIAquick column and centrifuged for 1 min. The flow-through was discarded and QIAquick column were placed back to the same tube. The QIAquick column were centrifuged once more in the provided 2 mL collection tube for 1 min at 17,900 x g (13,000 rpm) to remove residual wash buffer. QIAquick column were then placed into a clean 1.5 mL microcentrifuge tube. To elute DNA, 50 µL Buffer EB was added (10 mM Tris-Cl, pH 8.5) or water to the center of QIAquick membrane and then centrifuged for 1 min. After adding Buffer EB to the QIAquick membrane, additional increasing incubation time to up to 4 min increased the yield of purified DNA.
DEGs were cloned into pGEM-T Easy Vector Systems following the manufacturer’s instructions. The plasmid DNA was then transformed into JM109
High Efficiency Competent Cells by the heat-shock method whereby the reaction was incubated on ice for 20 min and subsequently heat-shocked in a water bath for 20 s at 4 oC without shaking. The transformed cells were immediately transferred to ice, followed by addition of 450 µL SOC medium. The cultures were then incubated at 37 oC for 2 h with shaking (250 rpm). Aliquots of bacterial culture (50
– 100 µL) were spread on pre-warmed agar plates containing 50 µg/mL kanamycin, and incubated at 37 oC overnight. Plasmid DNA was isolated using the Qiagen
plasmid purification kit following the manufacturer’s instructions (Qiagen,
same primers used to amplify the original cDNAs. PCR products were separated in a 2% agarose gel in 0.5 x TBE. The nucleotide sequence of positive cDNA clones
were determined using the M1γ reverse (5’-CAGGAAACAGCTATGACC-γ’) and
M1γ FORWARD (5’-GTAAAACGACGGCCAGT-γ’) universal primers.
Sequencing was carried out by an ABI 311 automatic sequencing machine (Perkin Elmer, Foster City, CA, USA).
Nucleotide and Deduced Amino Acid Sequencing Analyses
The DEGs were cut from the gel and cloned into pGEM®-T Easy vector (Promega, Madison, WI). The positive colonies from DEGs were confirmed by colony PCR using M13F and M13R primers. Plasmids isolated from these clones were single-pass sequenced with T7 primer in an ABI 3730x1 genetic analyzer.
DNA sequences were processed manually to clean the vector backbone and the poly (A) tail and searched against the nonredundant nucleotide and protein database of National Center for Biotechnology Information using using BLASTN and BLASTX interference (http://www.ncbi.nlm.nih.gov/BLAST) (Altschul et al.
1997).
RESULTS AND DISCUSSION
Effect of Skinning treatment on Storage Root RNA Populations
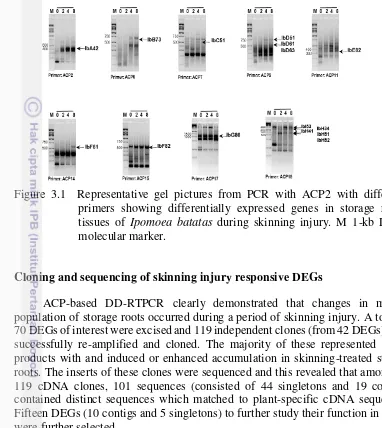
To examine the effect of skinning treatment on storage root RNA populations, freshly harvested storage roots were exposed to skinning treatment. In order to determine how rapidly changes in the mRNA population occurred following the onset of a skinned treatment, time course experiments were carried out. RNA was isolated from sweetpotato storage roots for 0 (control), 2, 4, 8 + 12 h following the onset of a skinning treatment and used for ACP-based DD-RTPCR. The RNAs results obtained were then used for ACP-based DD-RTPCR with 20 ACP primers. Differentially expressed genes were used to examine and compare the patterns of expressed mRNAs present in skinning injury treated storage roots. Subsequently, a number of skinned –induced partial cDNAs were isolated and examined in roots subjected to skinning. The results showed that skinning induced changes in the accumulation of a number of different DEGs (Fig. 3.1); indicative of a skinned-induced activation and repression of gene expression. A number of changes in mRNA accumulation that occurred in skinned-treated roots occurred rapidly within 2 h after subjected to skinning injury.
ACP-based DD-RTPCR products were classified into skinned-induced, pregulated and –downregulated DEGs (Table 3.1).
Cloning and sequencing of skinning injury responsive DEGs
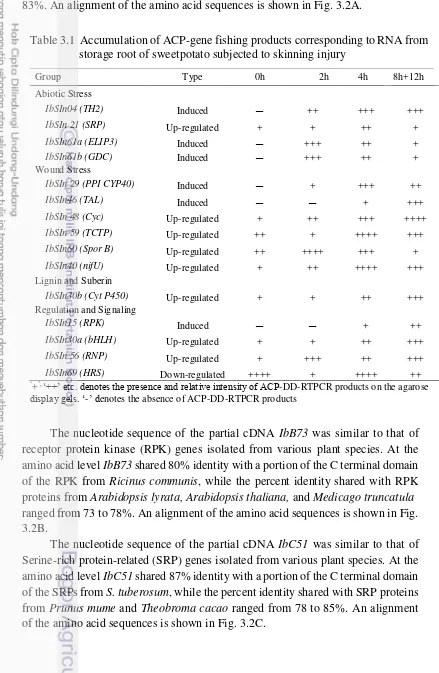
ACP-based DD-RTPCR clearly demonstrated that changes in mRNA population of storage roots occurred during a period of skinning injury. A total of 70 DEGs of interest were excised and 119 independent clones (from 42 DEGs) were successfully re-amplified and cloned. The majority of these represented DEGs products with and induced or enhanced accumulation in skinning-treated storage roots. The inserts of these clones were sequenced and this revealed that among the 119 cDNA clones, 101 sequences (consisted of 44 singletons and 19 contigs) contained distinct sequences which matched to plant-specific cDNA sequences. Fifteen DEGs (10 contigs and 5 singletons) to further study their function in plants were further selected.
Each of the 15 DEGs sequences (Fig. 3.2) was submitted to the NCBI server for comparison to non redundant database sequencings residing in the GenBank nucleotide and dbEST database using BLAST programs (Altschul et al. 1997). All
DEGs products were similar to known sequences deposited in these database. In this study for multiple alignments, DEGs that represented a(biotic) stress(s) (IbA42, TH2; IbC51, SRP; IbH51, ELIP3; IbH52, GDC ), wound stresses (IbD51, PPI CYP450; IbF61, TAL; IbF82, Cyc; IbH34, TCTP; IbH41; IbE82, NifU), lignin and suberin (IbD63, Cyt P450), and regulation and signaling (IbSInB73, RPK; IbD61, bHLH; IbG86, RNP; IbI53, TOM1) (Fig. 3.2) were further selected.
The nucleotide sequence of the partial cDNA IbA42 was similar to that of
thioredoxin H2 (TH2) genes isolated from various plant species. At the amino acid level, IbA42 shared 100% identity with a portion of the C terminal domain of the
TH2 proteins from Ipomoea batatas, while the percent identity shared with TH2
Figure 3.1 Representative gel pictures from PCR with ACP2 with different primers showing differentially expressed genes in storage roots tissues of Ipomoea batatas during skinning injury. M 1-kb DNA