i
EFFECT OF BEE VENOM TO CELL DEATH AND
SPATIAL MEMORY IN MICE (
Mus musculus
)
RIAN OKTIANSYAH
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
iii
STATEMENT ABOUT THESIS
I hereby declare that thesis entitled Effect of Bee Venom to Cell Death and Spatial Memory in Mice (Mus musculus) is original result of my own research supervised under advisory committee and has never been submitted in any form at any institution before. All information from other authors cited here are mentioned in the text and listed in the reference at the end part of the thesis.
Bogor, June 2016
iv
SUMMARY
RIAN OKTIANSYAH. Effect of Bee Venom to Cell Death and Spatial Memory in Mice (Mus musculus). Supervised by BERRY JULIANDI, KANTHI ARUM WIDAYATI, and VETNIZAH JUNIANTITO.
Bee venom has been used as an alternative medicine as well as prevention of various diseases. It contains complex compounds and some of its content act as allergen and inflammation agents. It is expected to cause cell death in brain and to affect spatial memory. The objective of this study was to determine dose of bee venom that causes neuronal cell death in dentate gyrus, amygdala, and cerebral cortex as well as analyze the alteration of mice behaviour, particularly spatial memory. Fifteen male mice of Deutche Denken Yoken (DDY) were divided into control and treatment groups. Bee venom was injected six times for two weeks intraperitoneally with doses 1.88 mg/kg (P1), 3.76 mg/kg (P2), 5.6 mg/kg (P3), and 7.48 mg/kg (P4), respectively. Brain slices were made by paraffin method in 5 µm coronal section and stained by haematoxylin eosin. The Y maze method was used for behaviour assay. Parameters observed were the number of neuronal cell death in hippocampus, amygdala, and cerebral cortex, and percentage of alteration mice behaviour in Y maze. This study used complete randomized design method which consists of five treatments and three repetitions. Data was analyzed using one-way (ANOVA) in SPSS.
The results showed differences in the mean of neuronal cells death in dentate gyrus, amygdala, and cerebral cortex. Mean of neuronal cells death varied in each treatment compared to control. The highest mean of dead neuron in dentate gyrus, amygdala, cortex upper layer (2-4), and cortex deep layer (5-6) was 1510 cell/mm2,
1754 cell/mm2, 1415 cell/mm2, and 1168 cell/mm2 while the lowest mean was 220 cell/mm2, 326 cell/mm2, 420 cell/mm2, and 365 cell/mm2, respectively. The highest mean of alteration after 24 h bee venom injection was 74.57 % and the lowest was 61.07 %. The highest mean of alteration after 72 h bee venom injection was 76.03 % and the lowest was 57.50 %. Analysis of variance (ANOVA) showed that effect of bee venom was significantly different in the number of neuronal cell death in dentate gyrus and amygdala, but no significant effect in cerebral cortex as well as mice behaviour. The Duncan’s Multiple Range Test (DMRT) results showed that P4 significantly different to neuronal cells death in dentate gyrus and amygdala compared to other treatments. Based on the study, bee venom induced neurotoxic effect because it caused neuronal cell death in dentate gyrus, amygdala, and cerebral cortex and tend to affect spatial memory.
v
RINGKASAN
RIAN OKTIANSYAH. Pengaruh Racun Lebah terhadap Kematian Sel dan Memori Spasial Mencit (Mus musculus). Dibimbing oleh BERRY JULIANDI, KANTHI ARUM WIDAYATI, dan VETNIZAH JUNIANTITO.
Racun lebah digunakan sebagai obat alternatif untuk berbagai penyakit. Racun lebah mengandung senyawa kompleks dan beberapa senyawa tersebut berperan sebagai alergen dan agen inflamasi. Senyawa tersebut diduga menyebabkan kematian sel di otak dan mempengaruhi memori spasial. Tujuan penelitian ini adalah untuk menentukan dosis racun lebah yang menyebabkan kematian sel saraf di hipokampus, amygdala, dan korteks dan menganalisis perubahan perilaku mencit, terutama memori spasial. Mencit jantan galur Deutche Denken Yoken (DDY) sebanyak lima belas ekor dibagi kedalam kelompok kontrol dan kelompok perlakuan. Racun lebah diinjeksi enam kali selama dua minggu secara intraperitoneal dengan dosis 1.88 mg/kg, 3.76 mg/kg, 5.6 mg/kg, dan 7.48 mg/kg. Sayatan histologi otak digunakan metode parafin dengan ketebalan 5 µ m secara koronal dan diwarnai dengan hematoksilin eosin. Metode Y maze digunakan untuk pengujian perilaku. Parameter yang diamati adalah jumlah neuron yang mati dan persentase perubahan perilaku mencit. Penelitian ini menggunakan metode rancangan acak lengkap yang terdiri atas lima perlakuan dan tiga ulangan. Data dianalisis menggunakan analisis one-way ANOVA di software SPSS statistics 20.
Hasil penelitian menunjukkan jumlah neuron yang mati di hipokampus (dentate gyrus), amygdala, dan korteks dan perubahan perilaku mencit. Rata-rata tertinggi jumlah neuron yang mati di dentate gyrus, amygdala, korteks lapisan atas (2-4), korteks lapisan bawah (5-6) adalah 1510 sel/mm2, 1754 cell/mm2, 1415 cell/mm2, dan 1168 cell/mm2 sedangkan terendah adalah 220 sel/mm2, 326
cell/mm2, 420 cell/mm2, and 365 cell/mm2. Rata-rata tertinggi perubahan perilaku mencit setelah 24 jam injeksi racun lebah adalah 74.57 % dan terendah 61.07 %. Rata-rata tertinggi perubahan perilaku mencit setelah 72 jam injeksi racun lebah adalah 76.03 % dan terendah 57.50 %. Analisis varians (ANOVA) menunjukkan bahwa pengaruh racun lebah berbeda signifikan pada jumlah neuron yang mati di hipokampus dan amygdala, namun tidak signifikan berpengaruh pada bagian korteks dan perilaku mencit. Hasil uji Duncan menunjukkan bahwa P4 berbeda signifikan terhadap jumlah neuron yang mati di hipokampus dan amygdala dibandingkan perlakuan lain. Berdasarkan penelitian, racun lebah memiliki efek neurotoksik karena menyebabkan kematian sel saraf di dentate gyrus, amygdala, dan korteks, serta cenderung mempengaruhi memori spasial.
vi
© Copy Right by IPB, 2016
All rights reserved
It is prohibited to cite all or a part of this thesis without referring to and mentioning the source. Citation is permitted for the purpose of education, research, scientific paper, report, or critism writing only; and it does not defame the name and honour of Bogor Agricultural University.
i
A Graduate Thesis
in partial fulfillment of Master Science degree in Animal Biosciences Faculty of Mathematics and Natural Science
EFFECT OF BEE VENOM TO CELL DEATH AND SPATIAL
MEMORY IN MICE (
Mus musculus
)
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2016
ii
iv
PREFACE
I thank to Allah SWT for all His blessings so that this thesis successfully completed. The title of this thesis is Effect of Bee Venom in Cell Death and Spatial Memory in Mice (Mus musculus).
I want to send my gratitude to my supervisors Dr Berry Juliandi, MSi, Dr Kanthi Arum Widayati, MSi, and drh Vetnizah Juniantito, SKH PhD for all guidance and encouragement as well as invaluable academic advices for the whole period of my study and research, and to my examiner, Prof drh Arief Boediono, PhD PAVet for the generous support and great discussion. Thank to Lembaga Pengelola Dana Pendidikan (LPDP) for the financial support. Special thank to my parents and my family for your love and support, BSH 2014 for togetherness, and to all people in Zoo Corner, for their supports for my study. Hopefully this paper can be useful.
v
CONTENTS
LIST OF FIGURES vi
INTRODUCTION 1
MATERIALS AND METHOD 2
Time and Place 2
Research Animal 2
Experimental Unit 2
The Treatments of Animals 2
Mice Brain Histology 2
Spatial Learning Test 2
Experimental Design and Data Analysis 3
RESULTS 3 Effect of Bee Venom to Neuronal Cells Death 3
Spatial Learning 6
DISCUSSION 7 Effect of Bee Venom to Neuronal Cell Death 7
The Ability of Spatial Learning in Mice 9
CONCLUSION 9
REFERENCES 10
vi
LIST OF FIGURES
1 Mean of neuronal cells death in hippocampus (dentate gyrus), amygdala,
and cortex 3
2 Representative image of hippocampus (dentate gyrus) in each treatment
stained by haematoxylin eosin 4
3 Representative image of amygdala in each treatment stained by
haematoxylin eosin 5
4 Representative image of cerebral cortex stained by haematoxylin eosin 6
1
INTRODUCTION
Bee venom is used as an alternative medicine as well as prevention of various diseases, such as arthritis, rheumatism, pain, cancer, and neurodegenerative diseases (Bellik 2015; Worlitzer et al. 2012; Yang et al. 2010; Kwon et al. 2001). Bee venom is produced by two glands in bee worker sting. Bee venom increases during the first week of adult bee worker life and reaches the maximum amount when bee worker involves in nest defense and forage. However, Bee venom decreases along with age (Krell 1996). Bee venom contains complex compounds, including polypeptides, enzymes, lipids, and amino acids. Some of the content of bee venom act as allergen, anti-inflammation, and inflammation agents (Lee and Bae 2016). It is expected to cause cell death.
Cell death in multicellular organisms play an important role during development. Cell death controls the number of cells and protects the organism by removing all of damaged cells that caused by disease, aging, infections, genetic mutations, and exposure of toxic substances. Cell death in nature is categorized into two types, namely necrosis and apoptosis (Saikumar and Venkatachalam 2009).
Necrosis and apoptosis due to the external factors, such as toxic substances and allergens is prominently found (Cudrici et al. 2006). Morphologically, necrosis indicated by shrinkage, fragmentation, or fusing the nucleus of the cells. In contrast to necrosis, apoptosis is characterized by the presence of the bleb on the plasma membrane and separation of cytoplasm and organelles of cells (apoptotic body) (Majno and Joris 1995). Necrosis and apoptosis can occur in tissue or organ, including in the brain regions, such as amygdala, cerebral cortex, and hippocampus that affect emotion, motoric response, and spatial memory, respectively.
Spatial memory is the capability to code, store, and retrieve information relate to spatial space in environment. Spatial memory has important function for human to learn a route, path, direction, or remember the location of objects. (Kessels et al. 2000; Langley 2012; Banikowski 1999). There is a product alleged to affect spatial memory but it is not reported yet scientifically, that is bee venom. There are several studies showed that bee venom can also cause cell death.
2
MATERIALS AND METHOD
Time and Place
The study was conducted in Division of Animal Function and Behaviour, Departement of Biology, Faculty of Matemathics and Natural Sciences and Laboratory of Pathology, Faculty of Veterinary Medicine, Bogor Agricultural University. The study was carried out on October 2015 until March 2016.
Research Animal
The study used three months old male mice Deutche Denken Yoken (DDY), with weight ranging from 20-30 g. Mice were obtained from Non Ruminant Laboratory and Prospective Animals, Faculty of Animal Sciences, Bogor Agricultural University.
Experimental Unit
Fifteen mice used for this study were divided into control and treatments groups. Each treatment group consisted of three mice. Mice were reared to different cages, according to the group.
The Treatments of Animals
Mice were acclimatized for a week before treatments. Mice were maintained in the animal maintenance unit at room temperature and with wood chips. Mice were given drink and feed continuously throughout the maintenance (Clark et al. 2008). Bee venom was injected intraperitoneally after acclimatization. Bee venom was injected for two weeks at days 1, 4, 7, 10, 13, and 16 with doses 1.88 mg/kg (P1), 3.76 mg/kg (P2), 5.6 mg/kg (P3), and 7.48 mg/kg (P4) (Bogdanov 2015). Bee venom was obtained from apitheraphy clinic.
Mice Brain Histology
Brain tissues isolation was done three days after the last injection of bee venom using perfusion method (Matsuda 2007; Erwin et al. 2013). Morphological examination on brain tissue was performed with sectioned tissue stained by Haematoxylin-eosin (HE). Each section was observed by using light microscope.
Spatial Learning Test
3
Experimental Design and Data Analysis
This study used complete randomized design method which consists of five treatments and three repetitions. Parameters observed were the percentage of mice to explore correct Y maze arms and the number of neuronal cell death in dentate gyrus, amygdala, and cerebral cortex. Data was analyzed using one-way analyses of variance (ANOVA) in SPSS statistics software.
RESULTS
Effect of Bee Venom to Neuronal Cells Death
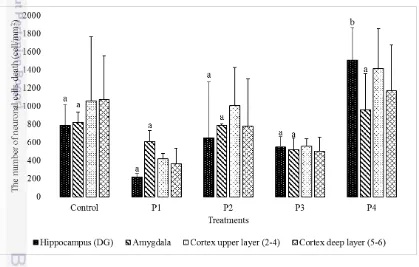
Based on observation, there were variation of mean neuronal cells death in several brain regions (Figure 1). The variation showed that bee venom has influence to neuronal cells death in hipocampus (dentate gyrus), amygdala, and cerebral cortex (Figure 2, Figure 3, and Figure 4). The results showed differences in the mean of neuronal cells death in dentate gyrus, amygdala, and cortex. The mean of neuronal cells death varied in each treatment compared to control. The highest mean of dead neuron in dentate gyrus, amygdala, cortex upper layer (2-4), and cortex deep layer (5-6) was 1510 cell/mm2, 1754 cell/mm2, 1415 cell/mm2, and 1168 cell/mm2 while the lowest mean was 220cell/mm2, 326 cell/mm2, 420 cell/mm2,
and 365 cell/mm2, respectively.
4
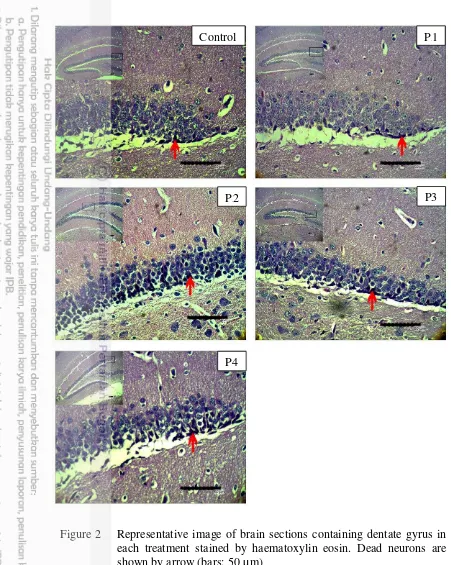
Figure 2 Representative image of brain sections containing dentate gyrus in each treatment stained by haematoxylin eosin. Dead neurons are shown by arrow (bars: 50 µm)
Brain histology in dentate gyrus stained by haematoxylin eosin showed normal and dead neuron. Dead neuron were showed by dark colour cell and nucleus was unseen (Figure 2). Analysis of variance (ANOVA) showed that effect of bee venom was significantly different in the number of neuronal cell death in dentate gyrus (F = 7.040, p < 0.01). It means that bee venom has a role in neuronal cell death regulation in dentate gyrus. Thus, the Duncan’s Multiple Range Test (DMRT)
Control PP1
PP2 P3
5
is required to examine the effect of treatments in neuronal cells death regulation in dentate gyrus.
The DMRT results showed that P4 significantly different to other treatments. Treatment with the highest dose showed the highest number of neuronal cells death in dentate gyrus. Based on the study, doses of bee venom gave significant effect on neuronal cells death. Furthermore, the effect of bee venom against neuronal cells death significantly was showed by P4 (7.48 mg/kg).
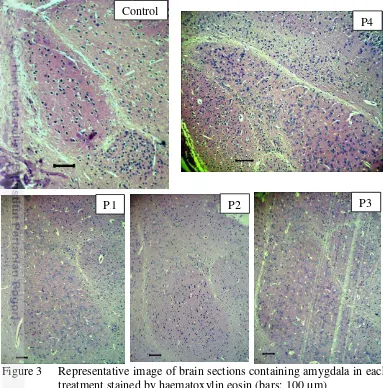
Figure 3 Representative image of brain sections containing amygdala in each treatment stained by haematoxylin eosin (bars: 100 µm)
Amygdala was also examined to show effect off bee venom to neuronal cells death (Figure 3). Results of ANOVA showed that effect of bee venom was significantly different in the number of neuronal cell death in amygdala (F = 3.940, p < 0.05). It means that bee venom has a role in neuronal cell death regulation in amygdala. The DMRT results showed that P4 was significantly different to other treatments. Treatment with the highest dose showed the highest number of neuronal cells death in amygdala. Based on the study, doses of bee venom gave the significant effect on neuronal cells death. Significant effect of bee venom to neuronal cells death was showed by P4 (7.48 mg/kg).
Control
P4
6
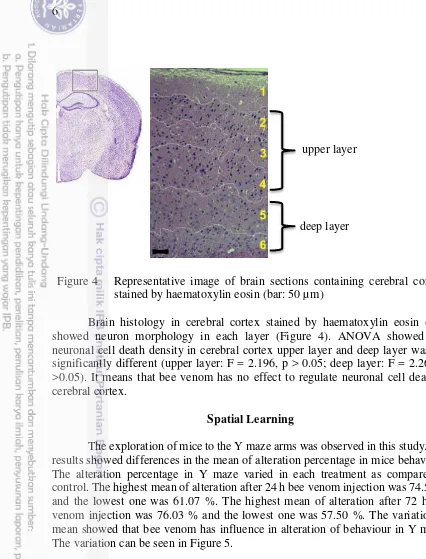
Figure 4 Representative image of brain sections containing cerebral cortex stained by haematoxylin eosin (bar: 50 µm)
Brain histology in cerebral cortex stained by haematoxylin eosin (HE) showed neuron morphology in each layer (Figure 4). ANOVA showed that neuronal cell death density in cerebral cortex upper layer and deep layer was not significantly different (upper layer: F = 2.196, p > 0.05; deep layer: F = 2.267, p >0.05). It means that bee venom has no effect to regulate neuronal cell death in cerebral cortex.
Spatial Learning
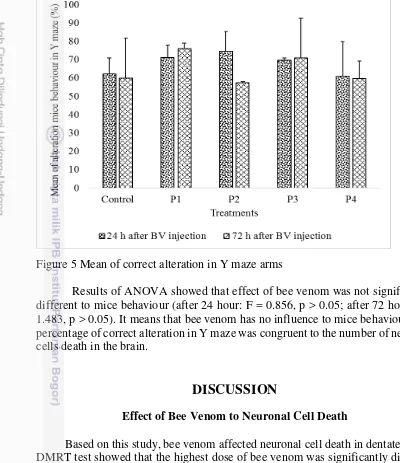
The exploration of mice to the Y maze arms was observed in this study. The results showed differences in the mean of alteration percentage in mice behaviour. The alteration percentage in Y maze varied in each treatment as compared to control. The highest mean of alteration after 24 h bee venom injection was 74.57 % and the lowest one was 61.07 %. The highest mean of alteration after 72 h bee venom injection was 76.03 % and the lowest one was 57.50 %. The variation of mean showed that bee venom has influence in alteration of behaviour in Y maze. The variation can be seen in Figure 5.
upper layer
7
Figure 5 Mean of correct alteration in Y maze arms
Results of ANOVA showed that effect of bee venom was not significantly different to mice behaviour (after 24 hour: F = 0.856, p > 0.05; after 72 hour: F = 1.483, p > 0.05). It means that bee venom has no influence to mice behaviour. The percentage of correct alteration in Y maze was congruent to the number of neuronal cells death in the brain.
DISCUSSION
Effect of Bee Venom to Neuronal Cell Death
Based on this study, bee venom affected neuronal cell death in dentate gyrus. DMRT test showed that the highest dose of bee venom was significantly different to other treatments on neuronal cell death in dentate gyrus. Dose of 4 mg/kg body weight of rats is the maximum dose of bee venom to anti-inflammation effect while the higher dose shows neurotoxic effects (Bogdanov 2015). Figure 2 showed that neuronal cells death found in subgranular zone (SGZ). According to study, we proposed that dead neuron was neural progenitor cells (NPC) because it was found in the innermost layer of SGZ. However, it needs special markers to confirm the cell types.
8
layers (Figure 4). Each layer has kind of neuronal type and different functions (Feldmeyer 2012). Layers III and V of the cerebral cortex contains a large number of pyramidal neurons with high levels of acetylcholinesterase activity (Mesulam and Geula 1991; Viswanathan et al. 2006) while layer II and IV receive information from hippocampus (Gomez-Isla 1996). Cell death in tissues or organs can upset the function. The redundant of neuronal cells death in brain can cause variety of neurodegenerative diseases, such as parkinson (Ruberg et al. 1997; Anglade et al. 1997), alzeimer (Shimohama 2000; Bamberger and Landreth 2002), huntington (Dean 2008). Taken together, these result suggested that bee venom treatment contribute to the poor performance in learning and memory test.
Increasing in the number of neuronal cells in dentate gyrus, amygdala, and cerebral cortex in P4 suggested as an effect of inflammatory compounds of the bee venom. Previous studies have demonstrated the role of melittin, phospholipase A2, apamin, and adolapin in high doses contained in bee venom can cause allergies, lysis of erythrocytes, myonecrosis, and neurotoxic effects (Ovcharov et al. 1976; Ownby et al. 1997; Raghuraman & Chattopadhyay 2006; Ali 2012; Abdu & Alahmari 2013; Elhakim et al. 2014; Bogdanov 2015; Eze et al. 2016; Lee & Bae 2016). Therefore, bee venom can affect the peripheral tissues.
The influence of bee venom in peripheral tissues is expected to produce cytokines which sends signals to the brain, so that it can cause neuronal cells death in hippocampus (dentate gyrus), amygdala, and cerebral cortex. Dantzer et al. (2008), Dilger & Johnson (2008), and Maier et al. (2012) demonstrated that cytokines from peripheral tissues transported into the brain through the endocrine system and induce neuronal cell death. Palombella & Vilcek (1989) reported that melittin and phospholipase A2 can activate cytokines, which is TNF. Although it still needs further confirmation, administration of bee venom is also supposed to have indirectly effect on neuronal cells death in the brain through cytokines.
Another suggestion about the mechanism of neuronal cell death because of bee venom, is the ability of bee venom compounds in passing through the blood brain barrier (BBB). BBB plays an important role in regulating the molecule that can be in and out of the brain (Ransohoff & Engelhardt 2012; Takeshita & Ransohoff 2012). Oller-Salvia et al. (2013) and Mourre et al. (1997) reported that apamin, compound of bee venom, can pass through the blood brain barrier and cause neuronal cell death. However, other compounds of bee venom have not been reported to pass through the BBB. Therefore, apamin suggested neuronal cells death in brain.
9
The Ability of Spatial Learning in Mice
Spatial learning ability of mice is shown by the correct-arm alternation in Y-maze. The higher percentage of correct-arm alternation showed that the better spatial memory in mice.Y-maze was used to examine spatial learning underlying mice activity to explore new environment. Wolfe (1969) reported that mice prefers to investigate new object and environment. ANOVA test showed that number of neuronal cell death was not significantly different to alteration mice behaviour. Conrad and Roy (1993) reported that as much as 80% of neuronal cell death in dentate gyrus do not affect spatial memory. Nonetheless, the results showed that bee venom administration tend to affect the spatial learning ability of mice (Figure 5).
The tendency of these effects can be caused by neuronal cells death in the dentate gyrus. Dentate gyrus plays a role in memory formation, distinctively related to cognitive function and spatial memory (Silva et al. 1998; Eichenbaum et al. 1999). The results showed that treatment with the most of dead neuron had the lowest correct-arm alternation in Y-maze (Figure 5). We suggested that NPC was dead neuron in SGZ. Dead or damage NPC causes the mature neuron will be reduced while the mature neuron that still remain will undergo programmed cell death. It will affect the process of learning and spatial memory in mice. Lawyer et al. (2006), Plessen et al. (2006), Thomann et al. (2012), and Juliandi et al. (2015) reported that the abnormal morphology in the hippocampus associated with the reduce neurogenesis which correlated with the cognitive deficits.
Cognitive deficits associated with bee venom-treated might not due to the abnormal morphology in hippocampus (dentate gyrus), there is possibility responses in emotion and motoric function that caused by abnormal in amygdala and cerebral cortex. We have shown neuronal cell death in amygdala and cerebral cortex (upper and deep layer) (Figure 1). Preston and Eichenbaum (2013) demonstrated that hippocampus and cerebral cortex engages to form meaningful contexts in which relate to memory retrieval, as well as the amygdala. Amygdala and hippocampus act synergistically when emotion and memory came together (Richter-Levin and Akirav 2000; Phelps 2004). Hippocampus, amygdala, and cerebral cortex have a role in limbic systems, link to another brain region. Abnormalities in those region will affect the tissues functionally. Deficiency in amygdala and cerebral cortex contributes to cognitive deficit. However, other brain regions contribute to the deficits. It still needs further investigation to complete possibility.
CONCLUSION
10
REFERENCES
Abdu F, Alahmari A. 2013. Anti-inflammatory effect of melittin on mice jejunum. GARJEST. 2 (3): 68-76.
Ali MAAM. 2012. Studies on bee venom and its medical uses. IJOART. 1: 1-15. Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J,
Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. 1997. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol Histopatol. 12 (1): 25-32.
Bamberger ME, Landreth GE. 2000. Inflammation, apoptosis, and alzheimer's disease. Neurosci. 8 (3): 276-283.
Banikowski AK. 1999. Strategies to enhance memory based on brain-research. Educ Psychol. 32 (2): 1-22.
Bellik Y. 2015. Bee venom: its potential use in alternative medicine. Anti-Infective Agents 13 (1): 1-14.
Bogdanov S. 2015. Bee Venom: Composition, Health, Medicine: A Review. Bee Prod Sci. 1-20.
Clark PJ, Brzezinska WJ, Thomas MW, Rhyzenko NA, Toshkov SA, Rhodes JS. 2008. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in c57bl/6j mice. Neurosci. 155: 1048-1058.
Conrad CD, Roy EJ. 1993. Selective loss of hippocampal granule cell following adrenalectomy: implications for spatial memory. J Neurosci. 13 (6): 2582-2590.
Cudrici C, Niculescu T, Niculescu F, Shin ML, Rus H. 2006. Oligodendrocyte cell death in pathogenesis of multiple sclerosis: protection of oligodendrocytes from apoptosis by complement. J Rehabil Res Dev. 43(1): 123-132.
Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 9(1): 46-56.
Dean E. 2008. Apoptosis in neurodegeneration: programmed cell death and its role
in alzheimer’s and huntington’s diseases. Eukaryon. 4 (1): 42-47.
Dilger RN, Johnson RW. 2008. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 84: 932-939.
Eichenbaum H, Paul D, Emma W, Matthew S, Heikki T. 1999. The hippocampus, memory, and place cells: is it spatial memory or a memory space?. Neuron. 23: 209-226.
Erwin, Tri WP, Sitarina W. 2013. Kepadatan sel hipokampus insulin imunoreaktif pada formasi hipokampus mencit yang diinduksi berulang dengan streptozotosin. J Vet. 14 (2): 126-131.
Eze OBL, Nwodo OFC, Ogugua VN. 2016. Therapeutic effect of honey bee venom. J Pharm Chem Biol Sci. 4 (1): 48-53.
11
Gajski G, Garaj-Vrhovac V. 2011. Bee venom induced cytogenetic damage and decreased cell viability in human white blood cells after treatment in vitro: A multi-biomarker approach. Environ Toxicol Pharmacol. 32: 201-211.
Gomez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. 1996. Profound loss of layer II entorhinal cortex neurons occurs in very mild
alzheimer’s disease. J Neurosci. 16 (14): 4491-4500.
Hedrych-Ozimina A, Behrendt K, Hao Z, Pofahl R, Ussath D, Knaup R, Krieg T, Haase I. 2011. Enhanced contact allergen- and UVB-induced keratinocyte apoptosis in the absence of CD95/Fas/Apo-1. Cell Death Differ. 18: 155-163. Jeong JK, Moon MH, Bae BC, Lee YJ, Seol JW, Park SY. 2011. Bee venom phospholipase A2 prevents prion peptide induced-cell death in neuronal cells. Int J Mol Med. 28: 867-873.
Juliandi B, Kentaro T, Katsuhide I, Takashi T, Yusuke F, Maky O, Noriko M, Daigo I, Masahiko A, Tsukasa S et al. 2015. Reduced adult hippocampal neurogenesis and cognitive impairments following prenatal treatment of the antiepileptic drug valproic acid. Stem Cell Rep. 5: 1-14.
Kessels RPC, Postma A, Kappelle LJ, de Haan EHF. 2000. Spatial memory impairment in patients after tumour resection: evidence for a double dissociation. J Neurol Neurosurg Psychiatry. 69: 389-391.
Krell R. 1996. Value-added products from beekeeping. FAO Agric Serv Bull. 124: 1-13.
Kwon HK, Kim GC, Hwang JS, Kim Y, Chae CS, Nam JH, Jun CD, Rudra D, Surh CD, Im SH. 2016. Transcription factor NFAT1 controls allergic contact hypersensitivity through regulation of activation induced cell death program. Sci Rep. 1-15.
Kwon YB, Lee JD, Lee HJ, Han HJ, Mar WC, Kang SK, Beitz AJ, Lee JH. 2001. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 90: 271-280.
Langley P. 2012. Intelligent behavior in humans and machines. Advances in Cognitive Systems. 2: 3-12.
Lawyer G, Nyman H, Agartz I, Arnborg S, Jonsson EG, Sedvall GC, Hall H. 2006. Morphological correlates to cognitive dysfunction in schizophrenia as studied with bayesian regression. BMC Psychiatry. 6 (31): 1-16.
Lee G, Bae H. 2016. Bee venom phospholipase A2: yesterday’s enemy becomes
today’s friend. Toxins. 8 (48): 1-12. Anti-inflammatory effect of bee venom on type II collagen-induced arthritis. Am J Chin Med. 32 (3): 361-367. Lee SM, Yang EJ, Choi SM, Kim SH, Baek MG, Jiang JH. Effects of bee venom
on glutamate-induced toxicity in neuronal and glial cells. J Evid Based Complementary Altern Med. 1 (1): 1-9.
Maier SF, Linda R. 2012. Consequences of the Inflamed Brain. Dana Alliance. 1-4.
Majno G, Joris I. 1995. Apoptosis, oncosis, and necrosis an overview of cell death. Am J Pathol. 146 (1): 3-15.
12
Mesulam MM, Geula C. 1991. Acetylcholinesterase-rich neurons of the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Compar Neurol. 306 (2): 193-220.
Mourre C, Fournier C and Soumireu-Mourant B. (1997). Apamin, a blocker of the calcium-activated potassium channel, induces neurodegeneration of purkinje cells exclusively. Brain Res. 778 (1): 405-408.
Oller-Salvia B, Teixido M and Giralt E. (2013). From venom to BBB shuttles: Synthesis and blood-brain barrier transport assessment of apamin and a nontoxic analog. Biopolymers. 100 (6): 675-686.
Onaolapo OJ, Adejoke YO, Tolulope JM, Oningbinde OA, Oyedele RA. 2012. Elevated plus maze and y-maze behavioral effects of subchronic, oral low dose monosodium glutamate in swiss albino mice. Int J Pharm Biol Sci. 3 (4): 21-27.
Ovcharov R, Shkenderov S, Mihailova S. 1976. Antiinflammatory effects of apamine. Toxicon. 14: 441-447.
Ownby CL, Powell JR, Jiang MS, Fletcher JE. 1997. Melittin and phospholipase A2 from bee (Apis mellifera) venom cause necrosis of murine skeletal in vivo. Toxicon. 35 (1): 67-80.
Palombella VJ, Vilcek J. 1989. Mitogenic and cytotoxic actions of tumor necrosis factor in balb/c cells. J Biol Chem. 264 (30): 18128-18136.
Phelps EA. 2004. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr Opin Nerobiol. 14 (1): 198-202.
Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J et al. 2006. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 63 (7): 795-807.
Preston AR, Eichenbaum H. 2013. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 23 (17): 764-773.
Ransohoff R M and Engelhardt B. 2012. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 12 (9): 623-635.
Raghuraman H, Chattopadhyay A. 2006. Melittin: a membrane-active peptide with diverse functions. Biosci Rep. 27: 189-223.
Richter-Levin G, Akirav I. 2000. Amygdala-hippocampus dynamic interaction in relation to memory. Mol Neurobiol. 22 (1-3): 11-20.
Ruberg M, France-Lanord V, Brugg B, Lambeng N, Michel PP, Anglade P, Hunot S, Damier P, Faucheus B, Hirsch E et al. 1997. Neuronal death caused by apoptosis in Parkinson disease. Rev Neurol. 153 (8-9): 499-508.
Saikumar P, Venkatachalam MA. 2009. Apoptosis and cell death. Mol Pathol Lib. 14: 29-41.
Shimohama S. 2000. Apoptosis in Alzheimer's disease-an update. Apoptosis. 5 (1): 9-16.
Silva AJ, Karl PG, Nikolai BF, Paul WF, Jeffrey HK, 1998. Molecular, cellular, and neuroanatomical substrates of place learning. Neurobiol Learn Mem. 70: 44-61.
13
Thomann PA, Seidl U, Brinkmann J, Hirjak D, Traeger T, Wolf RC, Essiq M, Schroder C. 2012. Hippocampal morphology and autobiographic memory in mild cognitive impairment and Alzheimer's disease. Curr Alzheimer Res. 9 (4): 907-915.
Truskinovsky AM, Dick JD, Hutchins GM. 2001. Fatal infection after a bee sting. Clin Infect Dis. 32: 36-38.
Viswanathan A, Gray F, Bousser MG, Baudrimont M, Chabriat H. 2006. Cortical neuronal apoptosis in CADASIL. Stroke. 37 (1): 2690-2695.
Wagner AH, Witjen I, Stojanovic T, Middel P, Meingassner JG, Hecker M. 2008. Signal transducer and activator of transcription 1 decoy oligodeoxynucleotide suppression of contact hypersensitivity. J Allergy Clin Immunol. 121 (1): 158-165.
Wilson C J, Finch C E and Cohen H J. 2002. Cytokines and cognition—The Case for A Head-to-Toe inflammatory paradigm. J Am Geriat Soc. 50 (12): 2041-2055.
Wolfe J L. (1969). Exploratory activity and new object response of wild and laboratory house mice. Commun Behav Biol. 4(1): 13-16.
Worlitzer MMA, Eva CB, Kathrin H, Jens CS. 2012. Anti-inflammatory treatment induced regenerative oligodendrogenesis in parkinsonian mice. Curr Stem Cell Res Ther. 3: 33.
14
BIOGRAPHY