DIVERSITY OF CELLULOLYTIC, OLEAGINOUS AND
CELLULO-OLEAGINOUS YEASTS FROM INDONESIAN
RESOURCES
ATIT KANTI
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT OF ORIGINALITY
1I certify that Ph.D dissertation entitle Diversity of Cellulolytic, Oleaginous and Cellulo-oleaginous Yeasts from Indonesian Resources is my original work directed by supervisor committe has not been submitted to other university in any form. Source of information used in this study are cited at the end of this dissertation. By this I transfer the copyright to Graduate School of Bogor Agricultural University.
Bogor, August 2014 Atit Kanti NIM:G36110001
1 Copy right transfer of joint research collaboration with external agency of
RINGKASAN
ATIT KANTI. Diversity of Cellulolytic, Oleaginous and Cellulo-oleginous Yeasts from Indonesian Resources. Supervised by NAMPIAH SUKARNO, ENDANG SUKARA, LATIFAH K DARUSMAN and KYRIA BOUNDY-MILLS.
Penelitian diversitas khamir yang diisolasi dari alam kebanyakan dilakukan di daerah sub tropika, hanya sedikit yang dilakukan di daerah tropika. Untuk mengungkapkan diversitas khamir di alam, perlu dilakukan isolasi khamir yang berasal dari Indonesia. Pengetahuan tentang diversitas khamir di Indonesia lebih banyak didapatkan dari makanan fermentasi tradisional.
Krisis energi yang menimpa dunia pada abad ke 20, memacu penelitian pencarian sumber energi alternatif. Akhir-akhir ini biofuel diproduksi untuk menanggulangi krisis energi minyak bumi. Akan tetapi masih ada silang pendapat yang mempertanyakan biofuel sebagai energi alternatif karena sumber biofuel yang juga merupakan sumber pangan. Biofuel yang menggunakan mikroba diekplotasi dengan menggunakan single cell oils yang diproduksi oleh mikroba pengakumulasi minyak seperti khamir dapat dijadikan sebagai alternatif unutuk sumber energi baru yang mempunyai keunggulan diantaranya tidak memerlukan tempat yang besar untuk proses produksi, dan dapat diproduksi dalam waktu yang lebih singkat.
Penelitian ini bertujuan untuk mengungkapkan keragaman khamir yang berasal dari berbagai macam sumber sampel, penemuan jenis baru, mempelajari karakter fisiologi khamir untuk mendegradasi selulosa, dan kemampuan mengakumulasi minyak. Penelitian ini juga mempelajari proses akumulasi minyak yang dipengaruhi oleh berbagai macam sumber karbon.
Khamir diisolasi dari sumber sampel yang dikoleksi di daerah Sulawesi Tenggara, Raja Ampat, Nusa Tenggara Timur, Bali dan Jawa Barat. Sampel yang dikoleksi dari daerah Sulawesi Tenggara meliputi daun, seresah, tanah, insek, insek larva, kayu lapuk dan jamur. Tanah merupakan sampel yang diambil dari Raja Ampat. Nusa Tenggara Timur diwakili oleh sampel yang berasal dari tanah dan seresah. Sampel yang berasal dari Jawa Barat meliputi daun dan bunga. Material tanaman Piper betle danPiper nigrum merupakan sumber sampel yang dikoleksi dari daerah Bali dan Gunung Salak. Identifikasi khamir dilakukan dengan pengamatan karakter morfologi, fisiologi dan pendekatan molekular dengan pemetaan daerah D1/D2 (26S) rDNA. Khamir yang berpotensi sebagai jenis baru dikonfirmasi dengan identifikasi molekular pada daerah ITS1-5.8S rDNA ITS5.
Empat ratus lima belas khamir diisolasi dari berbagai macam sumber sampel. Dari Sulawesi Tenggara berhasil diisolasi 38 isolat dari Mekongga, dan 260 dari Papalia. Dua puluh tiga isolat diisolasi dari Raja Ampat, 15 isolat dari Nusa Tenggara Timur, 47 isolat dari Bali, 24 isolat dari Gunung Salak dan 8 isolat dari Cibodas.
22 marga termasuk dalam kelompok Basidiomycota. Keragaman marga yang paling tinggi sebanyak 32 marga ditemukan di Sulawesi Tenggara yang terdiri dari 28 marga yang termasuk kelompok Ascomycota dan 4 marga termasuk ke dalam kelompok Basidiomycota. Sembilan marga ditemukan dari sampel yang berasal dari Raja Ampat yang terdiri atas 4 marga yang termasuk ke dalam kelompok Ascomycota 5 marga yang termasuk ke dalam kelompok Basidiomycota. Sampel dari material tanaman yang dikoleksi dari Bali menunjukkan bahwa hanya kelompok khamir yang termasuk ke dalam kelompok Basidiomycota yang berhasil diisolasi yang terdiri atas 4 marga. Tiga marga kelompok Ascomycota merupakan khamir yang berasal dari sampel yang dikoleksi dari Cibodas. Khamir paling banyak ditemukan pada 3 jenis sampel yaitu : daun, seresah, dan bagian usus insek. Hasil ini menunjukkan bahwa Indonesia memiliki keragaman jenis khamir yang tinggi.
Analisis homologi daerah D1/D2 menunjukkan 204 isolat memiliki nilai substitusi > 1 % sehingga merupakan kandidat jenis baru. Sebagian besar kandidat jenis baru berasal dari insek. Keragaman jenis khamir yang cukup tinggi pada insek menunjukkan ada keterikatan antara khamir dan insek. Jenis-jenis baru yang diisolasi termasuk kedalam Ascomycota (clade Yamadazyma, Wickerhamomyces, Ogateae, Metschnikowia, Candida, Kodamaea) dan Basidiomycota (clade Bulleromyces). Dua jenis baru diverifikasi secara lengkap sebagai Yamadazyma sp. nov. yang termasuk dalam phylum Ascomycota , order Saccharomycetales, family Debaryomycetaceae, diisolasi dari insek, sedangkanCiteromyces sp. nov., termasuk dalam ordeSaccharomycetales, family Wickerhamomycetaceaediisolasi dari seresah.
Sebanyak 157 isolat selulolitik yang termasuk anggota Ascomycota dan Basidiomycota diisolasi dari daun, seresah, insek usus, tanah, kayu lapuk, dan bagian dari tanaman (batang, daun, bunga dan buah dari Piper betle danPiper nigrum) mempunyai distribusi ekologi yang luas. Didapatkan lebih dari 10 isolat merupakan kandidat jenis baru yang mempunyai kemampuan selulolitik. Sporobolomyces poonsookiae Y08RA07, Rhodosporidium paludigenum Y08RA29 and Cryptococcus flavescens Y08RA33 adalah jenis khamir yang paling potential yang diisolasi dai Raja Ampat, Papua.
Dua ratus dua strain khamir mempunyai kemampuan mengakumulasi lipid (oleaginous) yang diisolasi dari seresah, usus insek, kayu lapuk, dan sirih ( Piper betle L dan Piper nigrum L), yang dijumpai di Sulawesi Tenggara, Raja Ampat, Nusa Tenggara Timur, Bali dan Jawa Barat. Khamir pengakumulasi lipid dicirikan oleh sel yang menggambang pada medium gliserol 10 %, dan memiliki gumpalan lipid di dalam sel. Candida adalah kelompok marga yang paling dominan. Anggota dari Candida mengakumulasi lipid sekitar 20-40 %. Marga lain yang mampu mengakumulasi lipid adalah Rhodosporidium, Sporidiobolus, Metschinkowia dan Cryptococcus. Penelitian ini menunjukkan khamir pengakumulasi lipid adalah polyphelitic.
cellulo-oleaginous adalah Sporodiobolus, Aureobasidium, Rhodosporidium, Pseudozyma, Debaryomyces, Pichia, dan Cryptococcus.
Beberapa sumber karbon seperti glukosa, gliserol, xilosa, dan CMC diuji untuk media tumbuh khamir Candida intermedia PLE6DP6, Candida orthosilopsis InaCC Y-302/Y09GS34, Candida oleophila InaCC Y-306/Y09GS48), Crytococcus flavescent PL3DP6), (Cryptococcus humicola PLE3DP9), (Cryptococcus luteolus InaCC Y-265/ Y10BS72), Yamadazyma aff. mexicana PL1W2) menggunakan khamir Lipomyces starckeyii NBRC 10831 sebagai strain pembanding. Komposisi FAME yang dihasilkan dipengaruhi oleh jenis sumber karbon. Berdasarkan komposisi FAME diketahui Candida orthosilopsis InaCC Y-302/Y09GS34, Candida oleophila InaCC Y-306/Y09GS48), Crytococcus flavescent PL3DP6) merupakan khamir cellulo -oleaginous yang potential untuk dikembangkan dalam penelitian biofuel dengan menggunakan bahan limbah pertanian.
SUMMARY
ATIT KANTI. Diversity of Cellulolytic, Oleaginous and Cellulo-oleginous Yeasts from Indonesian Resources. Supervised by NAMPIAH SUKARNO, ENDANG SUKARA, LATIFAH K DARUSMAN and KYRIA BOUNDY-MILLS.
The studies of yeasts from natural habitats have been conducted extensively in temperate regions, but very few studies conducted in tropical region. At the microbial diversity level, further study is needed to verify the species richness in Indonesian resources. The information about yeasts diversity in Indonesia are mostly obtained from traditional fermented food.
The energy crisis that hit world since the 20th centuries triggers intensive exploration of alternative energy resources. Recently, biofuel is produced to address under supply of transportation fuel, but it is still suffering from social controversy due to resource competition between food sufficiency particularly in developing countries and huge energy demand due to rapid population growth. Microbial based biofuel through exploiting Single Cell Oils (SCOs), popular as microbial oil, produced by oleaginous yeasts, could offer an alternative for renewable energy sources since consuming less space, and rapid biomass production. This can be achieved by development of strains able to convert low cost substrates, grow quickly to high density, and produce larger quantities of neutral lipid, and development of improved harvesting. Microbial oils do have some inherent advantages over plant oils: microbial biodiesel could be produced year-round (given available feedstock).
The present study focus on isolation, identification of yeasts from Indonesian resources, and proposal of new taxa as well as yeasts diversity study and assessment of physiological character include analysis of hydrolyses carboxymethyl-cellulose, lipid accumulation, and verification of lipogenesis of selected strain using varying carbon sources. Source of microorganism were from Indonesian natural resources obtained from Eastern and Western Part of Indonesia includes South East Sulawesi, Papua, East Nusa Tenggara, Bali and West Java. The natural resources were collected from varying sources include leaf surface, leaf litter, soil, insect, insect larva, insect larva gut, insect tunnel, decay wood and mushroom from South East Sulawesi; soil from Papua; soil and litter from East Nusa Tenggara;litter and flower from West Java; and plant material of Piper betle and Piper nigrum from Bali and Piper betle from West Java. The yeasts diversity discussion is started in sequential from two hot spot biodiversity areas: Papalia and Mekongga in South East Sulawesi, followed by the most pristine area Raja Ampat, Papua and dry savana area in Kupang, East Nusa Tenggara, and ended with plant associated yeasts in Jembrana, Bali represent low land and Gunung Salak and Cibodas Botanical Garden, West Java for high land. Yeasts strains were identified by morphology and by amplification of their D1/D2 domain of the end of the large subunit (26S) rDNA. For the selected strains for proposal of new taxa, the identifications were confirmed by sequencing of their ITS1-5.8S rDNA ITS5 regions.
by 260 strains, Mekonga area by 38 strains, Raja Ampat by 23 strains, Kupang by 15 strains, Bali 47 strains and West Java area by 24 strains from Gunung Salak ecosystem, and 8 strains from Cibodas Botanical Garden.
Molecular identification revealed that the yeasts strains are taxonomically diverse, 40 genera reside under the Ascomycota and 22 genera under Basidiomycota. The most diverse genera (32 genera), include 28 genera under Ascomycota and 4 genera under Basidiomycota, were occurred in Sulawesi. While 9 genera (5 under Basidiomycota and 4 under Ascomycota ) were recorded from Raja Ampat, and only 4 genera under Basidiomycota obtained in Bali, and 3 genera under Ascomycota obtained from Cibodas. Leaf, leaf litter and insect gut were 3 most-richest yeasts sources. These results were implying enormous diversity of yeasts in Indonesian resources. Insect gut is the new source of microorganism used in this study. Soils were nutrient rich substrate, however less divers of yeasts were isolated. This would be due to other microorganisms out compete yeasts in soil ecosystem. More advance isolation and culturing technique should be developed to obtain more divers soil yeasts.
Homology and phylogenetic analyses reveal that 204 yeasts strains showing greater than 1% nucleotide substitution in the D1/D2 region of LSU rDNA, and they are likely belong to different species. Most of the candidate for new taxa were isolated from insect include larvae gut, insect larvae, and insect’s body surface. High diversity of insect yeasts including novel taxa would imply that insect live cycle is closely associated with yeasts. Novel taxa isolated from varying Indonesian resources were phylogenetically diverse and distributed within the Ascomycota (clade Yamadazyma, Wickerhamomyces, Ogateae, Metschnikowia, Candida, Kodamaea) and Basidiomycota (clade Bulleromyces). Two novel species: Yamadazyma sp. nov. reside within phylum Ascomycota , order Saccharomycetales, family Debaryomycetaceae, representing yeasts inhabiting insect, whileCiteromyces sp. nov., belonging to order Saccharomycetales, family Wickerhamomycetaceae representing yeasts inhabiting leaf litter are further described for complete proposal of new species.
Based on ability to hydrolyze carboxymethyl cellulose, 157 isolated strains of Ascomycota and Basidiomycota inhabiting leaf, leaf litter, insect gut, soil, decay wood, and part of plant (stem, leaf, flower and fruit of Piper betle and Piper nigrum) were obtained implying that cellulolytic yeasts were polyphyletic. More than 10 strains were candidate for novel species. Sporobolomyces poonsookiae Y08RA07, Rhodosporidium paludigenum Y08RA29 and Cryptococcus flavescens Y08RA33 are most potential cellulolytic yeasts isolated from Raja Ampat.
Our interest was also obtaining yeasts that hydrolyze cellulose and accumulate large amount of lipid. This group of yeasts are defined as cellulo-oleaginous. Based on this definition about 78 strains were included in this group. Cellulo-oleaginous yeasts were mostly isolated from leaf, leaf litter, Piper betle, insect larvae and insect tunel. We found several Candida were cellulo-oleaginous yeasts, they were isolated from wide resources include leaf, leaf litter, Piper betle, insect larvae and insect tunel implying that cellulo-oleaginous yeasts were ecologically distributed in organic rich substrates. Sporodiobolus, Aureobasidium, Rhodosporidium, Pseudozyma, Debaryomyces, Pichia, Cryptococcus are other important yeasts that would be potentially explored for hydrolyzing lignocellulose hydrolysate for biofuel production.
Several carbon sources were evaluated for lipogenesis character of selected strains include Candida (Candida intermedia PLE6DP6, Candida orthosilopsis InaCC Y-302/Y09GS34, Candida oleophila InaCC Y-306/Y09GS48), and highest lipid accumulator (Crytococcus flavescent PL3DP6), their relative (Cryptococcus humicola PLE3DP9),(Cryptococcus luteolus InaCC Y-265/ Y10BS72) and strains candidate for novel species (Yamadazyma aff. mexicana PL1W2) using Lipomyces starckeyii NBRC 10831 as a reference strain for oleaginous yeasts. FAME species compositions were affected by C-source feeding for lipid accumulation. Finally based on FAME species composition, we confirm that selected oleaginous yeasts with CMC-ase activity are good candidate for biofuel production using cheap materials such as agricultural wastes.
© Copy Right IPB, 2014
This work is under copy right of IPB 2014. No part of this work can be copied without citing the source. Citation is allowable solely for education, research, scientific paper writing, reporting, assay or review; and the citation will not cause liability to IPB
A dissertation submitted to the Department of Biology and the committee on graduate school of Bogor Agricultural
University in partial fulfillment of the requirements for the degree of doctor of philosophy in microbiology
DIVERSITY OF CELLULOLYTIC, OLEAGINOUS AND
CELLULO-OLEAGINOUS YEASTS FROM INDONESIAN
RESOURCES
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Examiner for internal examination : Prof. Dr. Indrawati Gandjar Dr. Gayuh Rahayu
Dissertation Title : Diversity of Cellulolytic,Oleaginous and Cellulo-oleginous Yeasts from Indonesia Resources
Name : Atit Kanti
NIM : G361100012
Approved by
Dr.Nampiah Sukarno Principal Advisor
Prof. Dr. Latifah K Darusman Advisor committe
Prof. Dr.Endang SukaraAdvisor committe
Kyria Boundy-Mills, PhD. Advisor committe
Signed by
Head of Microbiology Study Pogram
Dean of Bogor Agricultural Graduate School
ACKNOWLEDGEMENTS
First and foremost I want to thank God for blessing me to finish writing Ph.D dissertation entitle: Diversity of Cellulolytic, Oleaginous and Cellulo-Oleaginous Yeasts from Indonesian Resources. The research works were started in September 2011.
I want to express my most sincerely gratitude to the advisor committee Dr. Nampiah Sukarno, Prof. Dr. Latifah K Darusman of Bogor Agricultural University, Prof. Dr. Endang Sukara of Indonesian Institute of Sciences and Kyria Boundy- Mills, Ph.D of University California Davis, USA for all their contributions of time, and ideas to make my Ph.D experience productive and stimulating.
I would like to extent my gratitude to all members of InaCC for laboratory assistance, and my colleagues that have contributed immensely to my personal and professional time at Bogor Agricultural University during Ph.D course.
I would like to expess my sincere gratefull to all the lecturers, administrative staffs of post graduate program of Bogor Agricultural University for their advisory, assistance and help during my study.
I gratefully acknowledge the funding sources that made my Ph.D work possible. I was funded by the Indonesian Ministry of Science and Technology through post graduate study grant. Material used in this study is a part of research collaboration between University of California Davis and the Government of the Republic of Indonesia, funded by Grant Number U01TW008160 from the US National Institutes of Health Fogarty International Center, the NIH Office of Dietary Supplements, the National Science Foundation and the Department of Energy. This project was supported by the USDA Agricultural Food Research Initiative of the National Food and Agriculture, USDA, Grant #35621-04750.
I wish this study contribute to science and technology development particularly on exploiting potential use of tropical microorganism.
CONTENT
LIST OF TABLES vi
LIST OF FIGURES vii
LIST OF APPENDICES viii
LIST OF TABLES
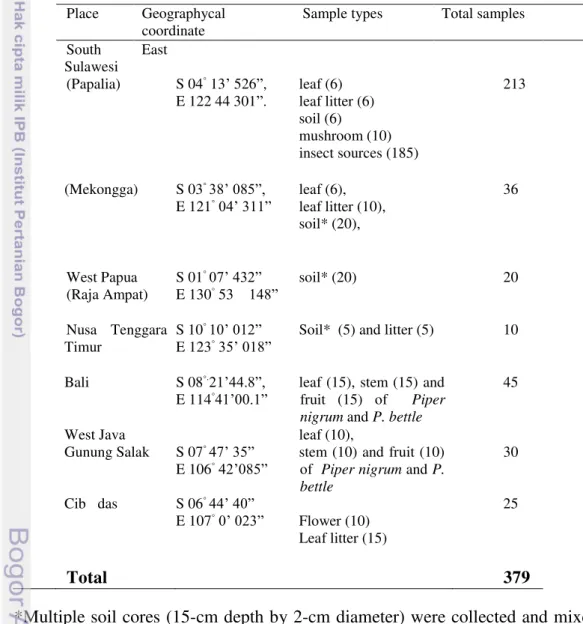
1 List of samples collected as biotope for oleaginous and cellulolytic yeasts studies
17 2 Parameter of the instrument GCMS for lipid species identification 21 3 Number of strains of yeasts isolated from varying soures: leaf surface, and soil, in South East Sulawesi
27 5 Taxonomic placement of yeasts species isolated from insect larva gut in
South East Sulawesi
31 6 Taxonomic placement of yeasts species isolated from insect and insect
tunel, in South East Sulawesi, Indonesia
33 7 Taxonomic placement of yeasts species isolated from mushroom in
South East Sulawesi, Indonesia
34 8 Taxonomic placement of yeasts species isolated from decay wood,
decomposed wood, infected wood in South East Sulawesi, Indonesia
35 9 Taxonomic placement of yeasts isolated from soil from Raja Ampat,
Papua
36 10 Diversity of yeasts species isolated from leaf litter and soil in East Nusa
Tenggara, Indonesia
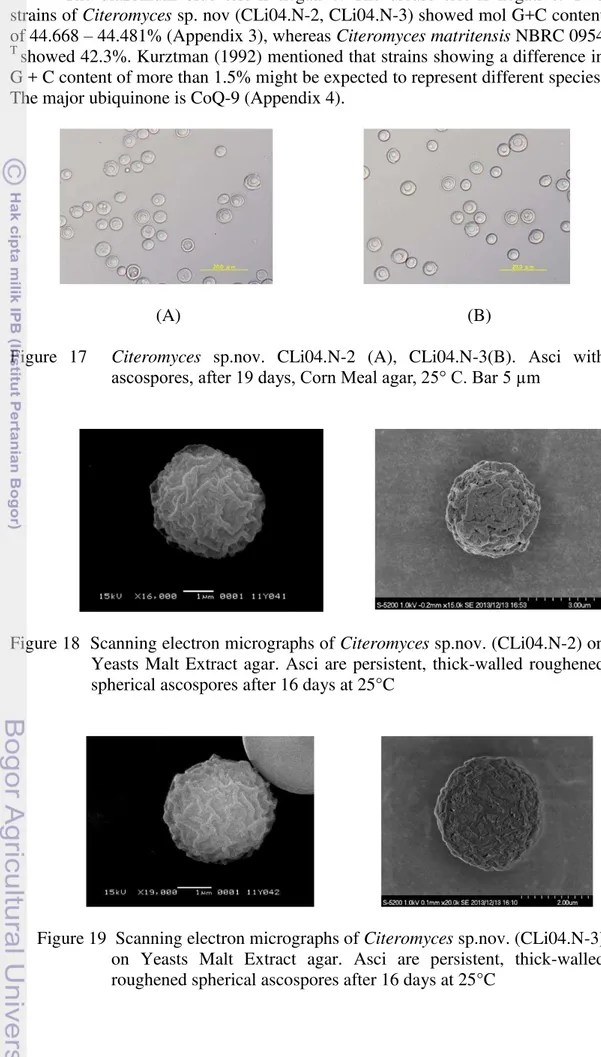
compared with those of Y. mexicana and Y. philogaea
49 15 The growth characteristics of the Citeromyces sp.nov strains are
compared with those of C.matritensis NBRC 0954T
52 16 Diversity of cellulolytic yeasts isolated from various biotopes in South
East Sulawesi, Raja Ampat, NTT, Bali and Gunung Salak
54 17 Cellulolytic yeasts isolated from South East Sulawesi. Sample sources
were leaf-litter, litter, soil, decay wood, insect gut and soil
55 18 Cellulolytic yeasts isolated from South East Sulawesi. Sample sources
were insect, insect larva, insect tunel, insect frass, and mushroom
56 19 Cellulolytic yeasts isolated from NTT (East Nusa Tenggara), sample
types were litter and soil
57 20 Cellulolytic yeasts isolated from Piper betle and Piper nigrum from Bali
and Gunung Salak. Sample code started with character B and GS indicate materials were obtained from Bali and Gunung Salak
respectively. L, F, Fr and S represent leaf, flower, fruit and stem respectively. Whereas PB and PN represent Piper betle and Piper nigrum respectively
21 Cellulolytic yeasts isolated from soil in Raja Ampat Papua 59 22 Diversity of oleaginous yeasts isolated from secondary forest in South
East Sulawesi. Sample sources were leaf, leaf-litter, soil, decay wood and gut insects
66
23 Diversity of oleaginous yeasts isolated from secondary forest in South East Sulawesi. Sample sources insect, insect larva, mushroom, and gut insects
68
24 Diversity of oleaginous yeasts from soil of Raja Ampat, Papua. Soil from tropical rain forest was used as yeasts sources
70 25 Diversity of oleaginous yeasts from East Nusa Tenggara (NTT). Litter
from savanah was used as yeasts sources
72 26 Diversity of oleaginous yeasts from litter and flower collected in
Cibodas Botanical Garden
72 27 Diversity of oleaginous yeasts isolated from Piper bettle and Piper
nigrum. Sample sources were stem, leaf, flower and fruit leaf-litter, soil, decay wood and gut insects
73
28 Number of genera, species and strains of oleaginous yeasts isolated from Piper nigrum L and Piper betle L collected from Bali
73 29 Number of species and strains of oleaginous yeasts isolated from Piper
betle L collected from West Java
74 30 Number of species and strains of oleaginous yeasts isolated from Piper
betle L collected from West Java
74 31 Lipid composition of oleaginous yeasts grown on N-limited 75 32 Cellulo-oleaginous yeasts reside within Ascomycetous yeasts 79 33 Cellulo-oleaginous yeasts reside within Basidiomycetous yeasts 80 34 Fatty Acid Methyl Esther (FAME) compositition of total lipid procedure
from varying C-source
86
LIST OF FIGURES
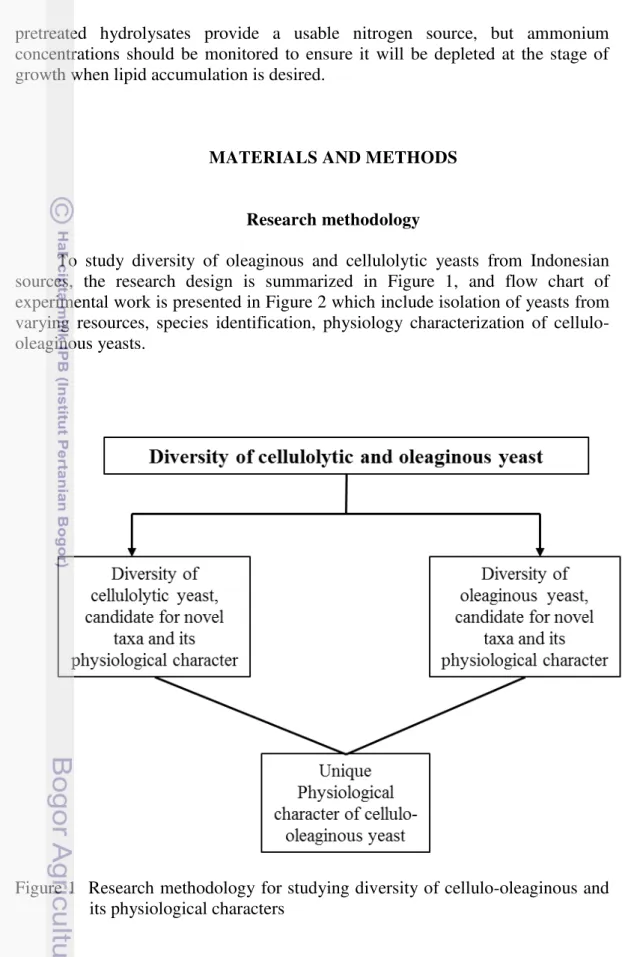
1 Research methodology for studying diversity of cellulo-oleaginous and its physiological character
14
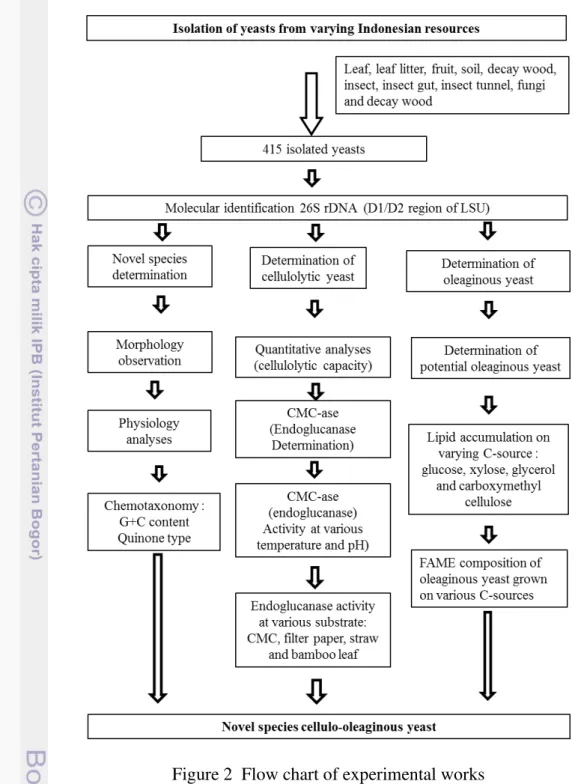
2 Flow chart of experimental works 15
3 Sampling sites for isolation of yeasts from Indonesian sources, sampling was conducted in 2010, 2011, and 2012. Samples were collected from soil, litter, plant materials, larvae of wood insect, leaf, stem, and fruit of Piper nigrum and Piper betle
16
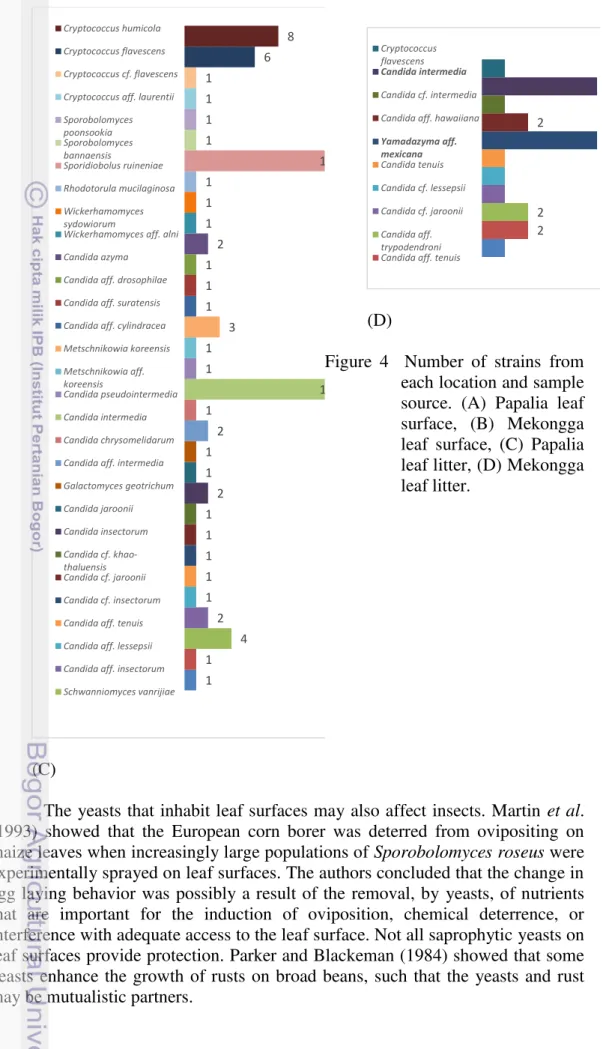
4 Number of strains from each location and sample source. (a) Papalia leaf surface, (b) Mekongga leaf surface, (c) Papalia leaf litter, (d) Mekongga leaf litter
30
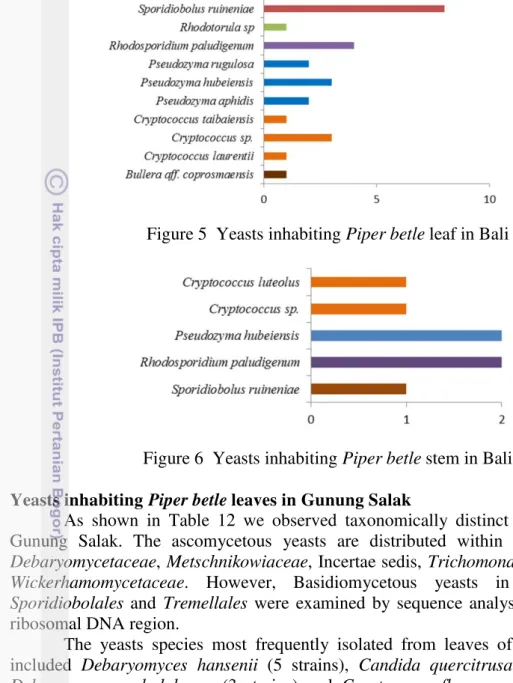
5 Yeasts inhabiting Piper betle leaf in Bali 40
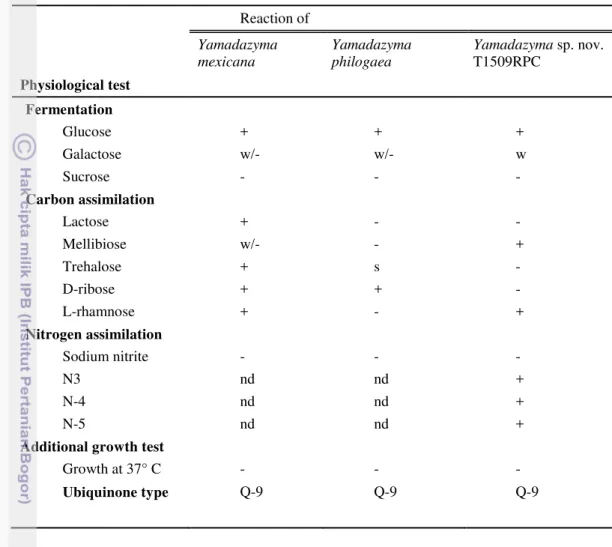
6 Yeasts inhabiting Piper betle stem in Bali 40
7 Yeasts inhabiting Piper betle leaf in Gunung Salak 41 8 Number of strains obtained from varying sources proposed as novel
taxa. Strain proposed as new taxa when strains showing greater than 1% nucleotide substitution in the D1/D2 region of LSU rDNA
44
9 Phylogenetic placement of Wickerhamomyces clade and related species determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
44
10 Phylogenetic placement of Ogataea clade and related species determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
45
11 Phylogenetic placement of Metschnikowia and related species determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
45
12 Phylogenetic placement of Kodamaea clade and related species determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
46
13 Phylogenetic placement of Bulleromyces clade and related species determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
46
14 Phylogenetic placement of Yamadazyma sp.nov among neighbouring yeasts determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
48
15 Yamadazyma sp.nov. T1509RPC. Cells, after 3 days on Yeasts Malt Extract Agar, at 25° C
49 16 Phylogenetic placement of Citeromyces sp.nov among neighbouring
yeasts determined from neighbor-joining analysis of D1/D2 LSU rRNA gene sequences. Bootstrap values are from 1000 replicates
51
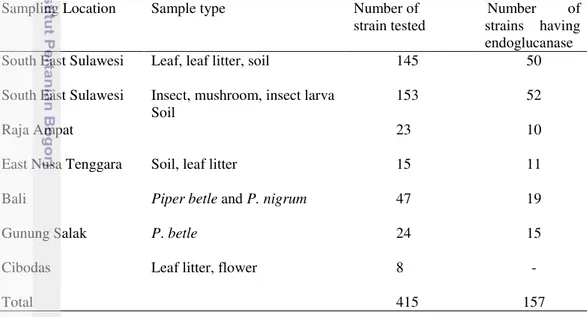
17 Citeromyces sp.nov. CLi04.N-2 (A), CLi04.N-3(B). Asci with ascospores, after 19 days, Corn Meal agar, 25° C. Bar 5 µm 53 18 Scanning electron microscrops of Citeromyces sp.nov. (CLi04.N-2). On
spherical ascospores after 16 days at 25°C
19 Scaning electron microscrops of Citeromyces sp.nov. (CLi04.N-3). On Yeasts Malt Extract agar. Asci are persistent, thick-walled roughened spherical ascospores after 16 days at 25°C
53
20 Morphological characters of growing colonies in PDA media after 5 days incubation
57 21 Clear zone formation of cellulolytic Yeastscellulolytic
yeastsSporobolomyces poonsookiae Y08RA07 (A), Rhodosporidium paludigenum Y08RA29 (B) and Cryptococcus flavescens Y08RA33 (C)
60
22 Profile of CMC-ase activity of tested isolated grown for 7 days in media with CMC was the sole carbon sources
60 23 Profile of CMC-ase activity of Sporobolomyces poonsookiae
Y08RA07as affected by temperature and pH
61 24 Profile of CMC-ase activities of Rhodosporidium paludigenum
Y08RA29 during cultivation at various pH and temperature
61 25 CMC-ase activity profile of Cryptococcus flavescens Y08RA33 grown
in CMC used as the carbon sources at various pH and temperature
62 26 Cellulase activity of Sporobolomyces poonsookiae Y08RA07grown in
bamboo leaf, paper waste, and paddy straw
62 27 Cellulase activity of Rhodosporidium paludigenum Y08RA29 grown in
bamboo leaf, paper waste, and paddy straw
63 28 CMC-ase activity profile of Cryptococcus flavescens Y08RA33 grown
in bamboo leaf, paper waste, and paddy straw
63 29 Morphology of oleaginous yeasts: (A) floating cell on glycerol medium,
(B) intracellular lipid bodies (C and D)
66 30 Diversity of oleaginous yeasts isolated from Indonesian resources.
Sample sources: leaf litter; litter; decay wood; Piperbetle and Pipernigrum,obtained from South East Sulawesi, Raja Ampat, NTT, Bali and West Java, Indonesia
66
31 Number of strains accumulate lipid >30 % (total lipid/cell dry weight, w/w)
67 32 Number of strain accumulate lipid greater than 20 % but less than 30 %
(total lipid/cell dry weight, w/w)
67 33 Strains accumulate Lipid > 40 % (total lipid/cell dry weight, w/w) 67 34 Morphology of oleaginous yeasts Candida intermedia PLE6DP6,
Candida orthosilopsis InaCC Y-302/Y09GS34, Candida oleophila InaCC Y-306/Y09GS48) grown on N-limited with CMC as the sole C-source
81
35 CMC-ase activity and lipid accumulation of Candida intermedia PLE6DP6, Candida orthosilopsis Y09GS34, Candida oleophila Y09GS48, Crytococcus flavescent PL3DP6, Cryptococcus humicola PLE3DP9, Yamadazyma aff. mexicana PL1W2, and yeasts Lipomyces starckeyii NBRC 10831 used as reference strains for oleaginous yeasts
83
36 Lipid accumulation by oleaginous yeasts at varying C-sources: (A) glucose, (B) glycerol, (C) xylose and (D) CMC.
84 37 Metabolic pathways of lipid accumulation using glucose and acetate as
C-sources
LIST OF APPENDICES
1 Physiological characteristic of Yamadazyma sp. nov. 111 2 Physiological characteristic of Citeromyces sp. nov. 112 3 Guanine + Cytosine data of Citeromyces sp.nov. 113
4 Ubiquinone type of Citeromyces sp.nov. 114
5 Lipid compositition of Y09GS34 /InaCC Y302 analyzed by GCMS
115 6 Published Paper 1: Cellulolytic yeasts isolated from Raja Ampat,
Indonesia
7 Published Paper 2: Indonesian oleaginous yeasts isolated from Piper betle and Piper nigrum
8 Published Paper 3: Oleaginous yeasts with CMC-Ase activities for biofuel production
INTRODUCTION
Yeasts are unicellular fungi and include in two big groups of fungal taxa, ascomycetous and basidiomycetous, which contain 149 genera and nearly 1500 species currently described. Taxonomist make clear distinction between yeasts and those dimorphic filamentous fungi that often produce abundant yeasts-like growth. Yeasts can be defined as fungi whose asexual growth predominantly results from budding or fission, and which do not form their sexual states within or upon a fruiting body (Kurtzman et al. 2011). For ascomycetous yeasts, this distinction has been substantiated by molecular comparisons, which demonstrate that budding and fission yeasts are phylogenetically distinct from one another. One exception is the genus Eremascus, which has unenclosed asci, but budding cells are not formed. A similar distinction can be made for basidiomycetous yeasts, which are often phylogenetically separate from the mushrooms and other taxa that form complex fruiting bodies. In summary, yeasts, are generally characterized by budding or fission in ascomycetes and budding in basidiomycetes as the primary means of asexual reproduction, and have sexual states that are not enclosed in fruiting bodies (Kurtzman, 2001).
Reproductive system of yeasts, like fungi, is quite complex. They may reproduce through asexual and sexual reproductive cycles. The most ubiquitous mode of vegetative growth is through asexual reproduction by budding. The asexual life cycle start with formation of a small bud (also known as a bleb), or daughter cell, is formed on the parent cell. The nucleus of the parent cell splits into a daughter nucleus and migrates into the daughter cell. The bud continues to grow until it separates from the parent cell, forming a new cell. The daughter cell produced during the budding process is generally smaller than the mother cell. Some yeasts, including Schizosaccharomyces pombe, reproduce by fission instead of budding, thereby creating two identically sized daughter cells. In general, under high-stress conditions such as nutrient starvation, haploid cells will die; under the same conditions, however, diploid cells can undergo sporulation, entering sexual reproduction (meiosis) and producing a variety of haploid spores, which can go on mating (conjugate), reforming the diploid cell.
2
maintenance of sex in S. cerevisiae. Some pucciniomycete yeasts, in particular species of Sporidiobolus and Sporobolomyces, produce aerially dispersed, asexual ballistoconidia.
Yeasts are chemoorganotrophic, as they use organic compounds as a source of energy and do not require sunlight to grow. Carbon is obtained mostly from hexose sugars, such as glucose and fructose, or disaccharides such as sucrose and maltose. Some species can metabolize pentose sugars such as ribose (Barnett et al. 2000) alcohols, and organic acids. Yarrowlia lipolytica assimilate glycerol, and accumulate lipid when nitrogen is depleted (Sorger, 2002). Some yeasts such as Trichosporon fermentans produce endoglucanase, exoglucanase and β-glucosidase (Zhou et al. 2004). Yeasts having cellulolytic characters and accumulating large amount of lipids are intensively studies for biofuel production (Liang & Jiang, 2013).
Yeasts species either require oxygen for aerobic cellular respiration (obligate aerobes) or are anaerobic, but also have aerobic methods of energy production (facultative anaerobes). Unlike bacteria, no known yeasts species grow only anaerobically (obligate anaerobes). Yeasts grow best in a neutral or slightly acidic pH environment. With these physiological characters yeasts have been a focus for many industrial product development (Zhang, 2011).
Yeasts may grown in many various temperature range for their best growth. For example, Leucosporidium frigidum grows at - 2 to 20°C (28 to 68°F), Saccharomyces telluris at 5 to 35 °C (41 to 95°F), and Candida slooffi at 28 to 45 °C (82 to 113 °F). The cells can survive freezing under certain conditions, however their viability are decreasing over time. Their ability to grow at wide temperature ranges is other physiological advantages to use yeasts for many research interest (Wilson & Talbot, 2009).
In general, yeasts are grown in the laboratory on solid growth media or in liquid broths. Common media used for the cultivation of yeasts include potato dextrose agar or potato dextrose broth, nutrient agar, yeasts peptone dextrose agar, and yeasts mould agar or broth. Home brewers who cultivate yeasts frequently use dried malt extract and agar as a solid growth medium. The antibiotic cycloheximide is sometimes added to yeasts growth media to inhibit the growth of Saccharomyces yeasts and select for wild/indigenous yeasts species.
The appearance of a white, thready yeasts, commonly known as kahm yeasts, is often a by product of the lacto fermentation (or pickling) of certain vegetables, usually the result of exposure to air. Although harmless, it can give pickled vegetables a bad flavor and must be removed regularly during fermentation Schoneck, 2002. Yeasts were isolated from Indonesian fermented foods such as tape, brem, and dadih (Limyati & Juniar, 1998). The consistent occurrence of yeasts in traditional fermented food would suggest yeasts having untapped economic potentials.
3 soil yeasts ecology mostly comprised surveys conducted to determine yeasts biodiversity in this habitat (Botha, 2006). It must be noted however, that the functional diversity among soil microorganisms may outweigh the diversity among taxonomic entities. Thus, unless the physiology of specific taxa playing a key role in an ecosystem is known, the mere listing of microbial taxa occurring within a particular habitat may be more of value to the taxonomist than to the ecologist. Similarly, the potential of soil yeasts communities to affect ecosystem function may be unrelated to yeasts species delineated using either selected morphological and physiological tests, or analyses of taxonomic informative gene sequences. It is well known that many yeasts species show either intraspecific diversity or no difference when certain physiological traits such as assimilation of specific carbon or nitrogen sources are compared (Kurtzman and Fell, 2006).
Characteristics that commonly occur among yeasts species frequently encountered in soil are the ability to utilize L-arabinose, D-xylose and cellobiose aerobically (Lian et al. 2013). These carbohydrates are the products of hydrolytic enzymes, which originate from bacteria or moulds and act on lignocellulosic plant material. Some soil yeasts are also found to assimilate intermediates of lignin degradation i.e. ferulic acid, gallic acid, 4-hydroxybenzoic acid, protocatechuic acid and vanillic acid (Sampaio, 2001).
The black yeasts or pigmented fungi mostly forms hyphal states (Sterflinger, 2006). Typical representatives of this group are Aureobasidium, Coniosporium and Exophiala, are known for the ability to grow oligotrophically on mineral surfaces or to degrade recalcitrant materials such as complex aromatic hydrocarbons as well as polymeric components of wood (Sterflinger, 2006). In order to survive as saprotrophs in soils, containing either low nutrient concentrations or recalcitrant woody materials as potential carbon sources the non-filamentous budding yeasts, are unable to degrade these materials, therefore to obtain nutrients they should perform other metabolism. A strategy that microorganisms, including those in soil, frequently employ to overcome nutrient limitation is to interact with other organisms and form symbioses (Van der Heijden and Sanders, 2002).
Phylloplane yeasts may represent a life form that changes from one type of ecological interaction to another as its habitat changes. For instance, epiphytes residing on leaves of deciduous trees are joined by other fungi and become saprophytes when the leaves drop and start to decay in the upper layer of the forest floor. The subsequent process of nutrient recycling in the saprophytic channel of the ecosystem is an important indirect role of many fungi that are associated with leaves. Fungal decomposers are thought to drive the global carbon cycle and phylloplane yeasts ultimately participate in that process in one way or another. However, the role of phylloplane yeasts in soil processes may not be significant because many only join the upper layers of soil and do not persist in soil after leaves fall (Lanchane et al. 2011).
4
al. 2005; Lee, 2009 a,b). Indonesia is a tropical nation comprised of over 17,000 islands, located at the intersection of multiple tectonic plates. It comprises five main islands: Sumatra, Java, Borneo (known as "Kalimantan" in Indonesia), Sulawesi, and Papua; two major archipelagos (Nusa Tenggara and the Maluku Islands); and sixty smaller archipelagoes, and thus are rich in biodiversity, having unique flora and fauna (ICBG, 2011), and presumably microbes as well (Kanti et al. 2013). Rifai (1995) estimated Indonesia has more than 200,000 species of fungi. However, little information on the species diversity of Indonesia indigenous yeasts and yeasts-like fungi has been generated.
Studies of Indonesian yeasts primarily related to their role in fermented foods (Abe et al. 2004; Kuriyama et al. 1997). Early studies of yeasts from natural environments in Indonesia include that by Deinema in 1961, who found Candida bogoriensis from the surface of leaves of the flowering shrubs Randia melleifera (Rubiaceae) in Bogor. In recent years, studies had been performed to explore yeasts diversity in Indonesia, (Nakase, 2005; Sjamsuridzal et al. 2010; Sudiana and Rahmansyah 2002; Wellyzar et al. 2013).
Sulawesi is one of the five major islands of Indonesia, and is of geographic and biological interest because it lies at the intersection of the Australian and Southeast Asian tectonic plates, and having rich biodiversity occupying the Wallace and Weber line. Sulawesi has high biodiversity, and reported to have high biodiversity of flora (Cannon, 2005; Cannon, 2007) and fauna (Noyes, 2008; Koch, 2011; Kimsey and Ohl, 2012). At the microbial diversity level, further study is needed to verify the species richness of this area.
Other than Sulawesi, Raja Ampat, located on the north-western tip of Papua, eastern Indonesia, is well known for island with high endemicity of plants and animals. Located on East side of Walace line having tropical climate with wet season throughout September to February, having calcareous soil. Intensive survey on marine ecosystem showing high fish diversity representing more than 75 % of tropical fish are found in this marine ecosytem. Ecological surveys in the Raja Ampat archipelago found 1320 species of coral reef fish (Allen et al. 2009) and 553 species of scleracti- nian corals which is around 75% of the world’s total (Veron et al. 2009). Though the important of this area as a biodiversity hot spot, very few studies on microbiology, partcularly soil microbiology studies are carried out in this area, and none dealt with diversity of oleaginous yeasts.
Crossing this island to the west side, still in the eastern part of the Wallace line, East Nusa Tenggara Timur having dry climate with water scarcity. The soils are dominated by litosol, rigosol, aluvial and mediteran, and having savanah ecosystem are unique places that would harbour yeasts having unique physiological characters. Again to the west side of the Wallace line, Bali island has varying ecosystem type from dry in Eastern Part, and wet in the middle island having Piperaceae plant used as traditional medicine (Aravind et al. 2010) that would harbour oleaginous yeasts (Kanti et al. 2013). This phenolic rich plant also found in Java, and has been used widely for medicinal herb on oral hygiene.
To obtain microorganisms from the last of submontain forest in West Java, Gunung Salak and Cibodas Botanical Garden are selected as sampling site.
5 controversy due to resource competition between food and energy sufficiency. Microbial based biofuel through exploiting Single Cell Oils (SCOs), popular as microbial oil, produced by oleaginous yeasts, could offer an alternative for renewable energy sources since consuming less space, and produce biomass rapidly.
Due to the outstanding capacity of Saccharomyces cerevisiae and certain other yeasts to produce ethanol and other organic products, yeasts will continue to be developed as producers of ethanol and other fuels. This process can be achieved by development of strains able to convert low cost substrates, grow quickly to high density, and produce larger quantities of neutral lipid, and development of improved harvesting and dewatering technologies.
Microbial oils do have some inherent advantages over plant oils: microbial biodiesel could be produced year-round (given available feedstock), on land unsuitable for agriculture, with production rates up to 100X that of plant oils in liters/hectare/year (Atabani &Silitonga, 2012). Of the 33.000 known species of algae (Brodie et al. 2007), at least forty are considered oleaginous (Griffiths and Harrison, 2009), i.e. they have been demonstrated to accumulate over 20% lipid by dry weight (Ratledge, 1979). Doubling times vary considerably, depending on species and growth conditions, ranging from hours to days (Sheehan, 1998). Oil and hydrocarbon content of oleaginous species can range from 20-60%, and up to 80% for exceptional genera such as Nannochloropsis, Schizochytrium and Botryococcus (Chisti, 2007). Yeasts are more preferable used as biodiesel since they multiply much faster than algae, and do not require light and large space. Physiologically yeasts accumulate larger amount of lipid than algae (Sitepu et al. 2013). These critical findings reaffirming the urgency of understanding ecological distribution, physiology and genetic chracter of oleaginous yeasts for viable technological development of biofuel industry (Meng et al. 2011; Dai et al. 2007). While oleaginous yeasts are having ability to use varying organic susbstrates included organic acids, simple carbon sources: glucose, fructose, arabinose, xylose and glycerol (Mussatto et al. 2008; Dai et al. 2007), the ability to hydrolize complex carbohydrate included cellulose, hemicellose, major component of agricultural waste woud be of benefit for reducing production cost of industrial-scale biofuel (Choi et al. 2012).
Since several yeasts species are oleaginous and accumulate high concentrations of lipids (Cohen and Ratledge 2005), they warrant evaluation as components for industrial production of oils and biodiesel (Cohen and Ratledge 2005; Dai et al. 2007; Matsumoto et al. 2001; Ratledge 2002). As opposed to culture in terrestrial water bodies, production of single-cell oils could be done in fermentors or in specialized solar reactors, which would be more ecologically sound than certain other technologies. A by-product of biodiesel is glycerol that can accumulate in substantial quantities. Yarrowia lipolytica and other yeasts have been evaluated for the fermentation of glycerol to higher-value products such as the bulk chemical citric acid (Fickers et al. 2005). Study of cellulolytic and oleaginous yeasts from natural habitat particularly tropical region have not been intensively conducted, hence our study will fill this gap of knowledge.
6
substances include cellulose and produce intracellular metabolites including lipid bodies which can be used as biofuel feeding material. This study will comprise: a. Diversity of yeasts isolated from various unique habitats
b. The novel taxa of isolated yeasts
c. Physiological character of cellulolytic yeasts particularly on hydrolyses of cellulose to produce fermentable substances
d. Unique physiological character of oleaginous yeasts especially on their ability to use various carbon sources
Overall objective
To study yeasts diversity in Indonesian resources and verifying its physiological characters especially on hydrolyses of cellulose and lipid accumulation (lipogenesis) on various carbon sources, would be important bio-resources for next generation biofuel research.
Specific objectives
The specific objective of this studies are to:
a. isolate and identify of yeasts from various sources of microorganisms which would include leaf surface, leaf litter, soil, insect, insect larva, insect larva gut, insect tunnel, decay wood and fungal from South East Sulawesi; soil from Papua; soil and leaf litter from East Nusa Tenggara; leaf litter and flower from West Java; and plant material of Piper betle and Piper nigrum from Bali and P. betle from West Java.
b. propose novel taxa of yeasts isolated from Indonesian resources
c. obtain endoglucanase producing yeasts as important physiological character for hydrolyses of cellulose to produce fermentable substances
d. search oleaginous yeasts utilizing various carbon sources
Scope of study
These studies would be deal with the isolation of yeasts from natural habitats in Southeast Sulawesi, Raja Ampat, East Nusa Tenggara, Bali, West Java, Indonesia and their physiology, particullarly lipid accumulation character, carboxymethyl cellulose hydrolyses and their phylogenetic affiliation based on partial 26S ribosomal DNA and ITS sequences. The study covers:
a. Diversity of yeasts isolated from Indonesian resources
b. Proposal of new taxa of isolated yeasts from Indonesian resources c. Diversity of cellulolytic yeasts, and oleaginous yeasts
d. Diversity of cellulo-oleaginous yeasts
7
LITERATURE REVIEW
Yeasts are ecologically distributed from terrestrial marine and fresh water ecosystem (Fell et al. 2000). They have been isolated from numerous biotopes including leaf surface, root, leaf litter, flower, gill of marine fish, insect gut, and event from dessert soil. Land plant tissues (stems, flowers, and fruits) are rich in organic compounds and moisture, and consequently provide a favorable environment for yeasts growth. Likewise, exudates of leaves, roots, flowers, and tree trunks are good habitats in which yeasts flourish. Many yeasts species that are found in live or decaying plant parts are associated with insects that also use these habitats as feeding or breeding sites. In general these three-part associations (insect”yeasts”plant) are maintained by reliance on reciprocal benefits exchanged by the insect”yeasts partners”. Often the yeasts supplies essential nutrients or beneficial supplements to the insect while the insect provides transportation of the yeasts to new habitats. However, when the insect yeasts partners are obtaining their needed nutrients from dead, moist, organic material, the system is part of the saprotrophic channel of the ecosystem and thus the plant is not a part of the symbiosis. Previous reviews (Lachance et al. 1998, Phaff and Starmer 1987) provide additional information on yeasts in soil, tree exudates, tanning liquors, necrotic cactus tissue, flowers and fruits of higher plants, as well as yeasts that are pathogenic to plants.
Soil has been extensively sampled for yeasts because of its general importance in ecosystem processes and because of its easy access. Nevertheless, the diversity of soil types and environmental conditions make soil a complex and difficult habitat to study. Soils occur in diverse climates, have different chemical compositions, can vary across microscopic as well as geographic scales, and are differentially influenced by the activities of local plants and animals that live in them. Capsulated forms of yeasts (Cryptococcus, Lipomyces, and Rhodotorula) survive better than other forms found in soil.
The occurance of yeasts in diverse biotopes, indicating its strong physiological characters including its roles in rich organic subtances (Cadete et al. 2012). Cultivable yeasts by far only 1% of all fungal species. Hence futher work are needed to increase species of cultivable yeasts.
8
RNA gene for all currently accepted ascomycetous and basidiomycetous yeasts (Scorzetti et al. 2002; Kurtzman and Robnett 1998).
The small size of yeasts cells results in a high surface/volume ratio that favors the rapid acquisition of essential nutrients by yeasts. Eventhough the fermentative metabolism of sugars (e.g., fructose, glucose, sucrose, maltose, melibiose, raffinose, lactose) is important to many yeasts, the oxidative utilization of a much broader variety of organic carbon compounds substantially expands the number of dimensions included in the fundamental niche. Commonly non- fermentable organic molecules, for example pentose sugars (xylose, L- and D-arabinose, D-ribose), methylpentoses (L-rhamnose, D-fucose), primary alcohols (methanol, ethanol, propanols), sugar alcohols (glycerol, erythritol, ribitol, D-arabitol, galactitol, mannitol, glucitol), amino sugars (D-glucosamine, N-acetyl-D-glucosamine, D-galactosamine), organic acids (lactic, succinic, citric, malic), and other compounds (e.g., acetone, ethyl acetate, meso-inositol, glucuronic acid), as well as straight or branched chained hydrocarbons and aromatic compounds (phenanthrene, and othercyclic aromatic hydrocarbons, Lahav et al. 2002) are all examples of sources of carbon that yeasts might use (Phaff and Starmer 1987).
The estimated remaining oil in Organization of the Petroleum Exporting Countries (OPEC) is around 428.94 billion barrels, and it will potentially trigger oil price crisis in 2020. This is due to rapid world energy consumption, and population growth. The environmental hazard as well as uncertainty of world petroleum reserve triger exploration of sustainable energy sources (Azocar et al. 2010). One economically viable sources is biodiesel (Dai et al. 2007), which has been promoted for their use in both developing and developed countries (Antoni et al. 2007; Hill et al. 2006). Recently growing political concern about the sustainability of feeeding material for biodiesel production since major source of biodiesel is vegetable oils, with 95% of biodiesel produced from edible plant oils (Gui et al. 2008). Rapid land degradation and deforestation result in occurence of sub optimal areas for vegetable oil or palm oil plantation. Economically viable biofuel production are being explored by many scientists ( Saenge et al. 2011; Duarte et al. 2013; Tai & Stephanopoulos, 2013). One possible way to cut production cost is through reducing price of feeding stock for biofuel production, which account for about 70 % total production cost (Gong et al. 2012). Microbial biomass, particularly oleaginous yeasts has been a focus of intensive studies to challenge sustainable biofuel production (Silva Lora et al. 2011). The bottle neck of viable biofuel production is cost for feeding materials (Zhu et al. 2011; Zhang et al. 2010). Reducing cost can be achievable through utilization of agricultural waste, but most oleaginous yeasts utilize slowly cellulose waste (Mussatto et al. 2008). Yeasts capable of hydrolyzing cellulose and accumulate substantial amount of lipid are very useful for biofuel research.
9 or Avicel. Cellobiohydrolases (CBH) are ineffective towards substituted celluloses but are able to degrade crystalline cellulose and produce primarily cellobiose. β-glucosidases hydrolyze cellobiose and other short cello-oligosaccharides produced by the other enzyme to glucose. With the recent development of biotechnology, there has been vast interest to use cellulose digestive microorganisms to convert cellulosic biomass to glucose that can be used in different applications such as production of fuel ethanol, use in animal feed, use in waste water treatment and in brewing industry ( Schneider et al. 2013; Peng et al. 2013).
Although there have been many papers dealing with more efficient cellulose degrading enzyme from various organisms such as Trichoderma reesei, Trichoderma viride, Trichoderma lignorum (Fahrurrozi et al. 2010), Chrysosporium lignorum, Chrysosporium pruinosum and Fusarium solani, only limited research has identified the yeasts as cellulase producer. The cost of production and low yields of this enzyme are the major problems for industrial application. Therefore, investigations on ability of microbial strains to utilize inexpensive substrate and improvement of enzyme productivity are critical. But, the research on bioconversion of agricultural wastes into biodiesel is very few. Utilizing hydrolytic microorganism to convert polymeric substances into fermentable sugar, and convert it into lipid and finally accumulate it as intra cellular lipid stock would be an ideal approach to search potential resources to produce other fuels.
10
The carboxymethyl cellulose (CMC) is used as carbon sources to produce triacilglycerol (TAG) that will forms Fatty Acid Methyl Ester (FAME) through transesterification process as biodiesel.
Lipid production utilizing oleaginous yeasts has been started from the 18th century. An advance chemical and molecular analyses play key central role on technological development of bioindustry utilizing yeasts (Colinet & Renault, 2014). Biomass production is crucial for attaining industrial scale bioindustry. Through aeration technique was developed to stimulate aerobic heterothrophic yeasts since the 19th century, however efficient diffusion technology are still crucial to reduce production cost (Kiraz et al. 2010). High biomass production can be achieved by introducing young actively deciding cell, high nutrient loading and provision of micro nutrients to stimulate production of expected metabolite (Beopoulos et al. 2008).
Intensive exploration of species diversity and metabolic characters of oleaginous yeasts are now conducted in several countries (Wang et al. 1993; Dai et al. 2007; Gen et al. 2014). Up to date much attention has been focused on how oleaginous yeasts produce varying lipid species through exploiting strains primarily isolated from temperate region, which may have distinct physiological character and phylogenetic diversity from their relative isolated from tropical regions (Baek et al. 2012). Strains originated from tropical areas may enrich genetic resources for developing viable biofuel technology (Janso & Carter, 2010). To meet that objective, potential strains are those of having short doubling time, growing very fast, accumulate high lipid content, tolerance to abiotic and biotic stress as well as able to use wide range of carbon sources (Huang et al. 2012). Not only fermentable substances but also lignocelluloses abundant in tropical areas (Zheng et al. 2012).
Oleaginous yeasts are popular as renewable resources for biodegradable oil, oleochemicals including fuels, chemicals and nutritional oils. Dozens of high-oil yeasts species have been described that can accumulate between 20% and 70% intracellular oil in the form of lipid bodies, containing primarily triacylglycerols (Sorger, 2002). Several lipid-metabolizing enzymes are prefer entially localized to the lipid bodies in yeasts, and it has therefore been proposed that they do not solely serve as a depot for lipids but instead may have a more complex role in lipid biosynthesis, metabolism, degradation, and trafficking (Daum et al. 2007). These oils are similar in composition to those of plant oils currently used for human consumption and for biodiesel (Dai et al. 2007). While the most commonly studied yeasts include Yarrowia lipolytica, Rhodotorula glutinis, Lipomyces starkeyi, Cryptococcus curvatus, and Rhodosporidium toruloides, there are dozens of other yeasts species that have been demonstrated to accumulate oil when grown on glucose ( Ladygina et al. 2006; Chang et al. 2013). Some of these less frequently studied species may have superior properties for specific applications such as conversion of a particular feedstock (Mitra et al. 2012).
11 carbohydrates, or tolerance of toxins associated with specific types of lignocellulosic hydrolysates. Because oleaginous yeasts are found in many taxonomic clades (Sitepu et al. 2012; Sitepu et al. 2013), they may utilize different metabolic mechanisms for oil accumulation or for toxin tolerance (Chi et al. 2009).
Compared to plant , microbial is preferable to produce oils due to having short life cycle, able to use organic waste from waste water treatment plan (Joo et al. 2008; Zhou et al. 2013) and agricultural waste (Huang et al. 2011), less labor required, less affected by season and climate, and easier to scale up (Li et al. 2008). Therefore, microorganisms might become one of the promising oil feedstocks for sustainable and environmental friendly future biodiesel production. Oleaginous yeasts are clearly polyphyletic (Kanti et al. 2013), they reside and scatter amongst multiple clades in phyla Ascomycota and Basidiomycota, and they may perform unique lipogenesis pathways depend on strains (Schneider et al. 2013). Obtaining potential oleaginous yeasts for biofuel research can be more efficient using strain indicator. About 3-10% of randomly selected yeasts screened were oleaginous ( Sitepu et al. 2013). As there are currently about 1500 known yeasts species, 50 to 160 of them may be oleaginous. As dozens of new yeasts species descriptions are being published per year, the number of known oleaginous yeasts species will undoubtedly continue to rise.
Methods of visualization, identification and quantification of polar and nonpolar lipids continue to improve. Analytical methods to identify and quantify lipids include gas chromatography-flame ionization detection (GC-FID), gas chromatography-mass spectrometry (GC-MS), thin layer chromatography-flame ionization detection (TLC-FID), matrix-assisted laser desorption and ionization and ionization time-of-flight mass spectrometry (MALDI-TOF-MS), NMR, and HPLC. Some methods are more appropriate for lipid analysis of different types of materials.Volkman et al. 1986, used TLC-FID to identify and quantify non-polar lipids in a variety of marine samples. Sitepu et al. 2013, used GC-FID to identify and quantify fatty acid methyl ester (FAME) from yeasts. Mondello et al. 2004, analyzed different lipid type to evaluate lipid analysis method by GC and GC-MS. Schiller et al. 1999, found MALDI-TOF-MS effective in analyzing mixtures of lipids in biological samples, such as cell membranes. Henderson et al. 2011, used atmospheric pressure ionization ion-trap mass spectrometry to determine the membrane lipid composition of industrial strains of Saccharomyces cerevisiae.
12
The storage lipids that accumulate in oleaginous yeasts are primarily diacylglycerols (DAGs) and triacyl-glycerols (TAGs). The fatty acid compositions of many yeasts species have been reported (Augustyn and Kock, 1992; Botha and Kock, 1993; Jeffery et al. 1997, Sitepu et al. 2013). Prior to development of ribosomal sequencing methods, fatty acid profiling was one of the methods used to identify yeasts species (Augustyn and Kock, 1992). In fact, companies such as MIDI Labs (www.midilabs.com) still offer microbe identification service based on fatty acid profile.
A small number of yeasts species have demonstrated substantial productivity of both biomass and lipid production. These species of oleaginous yeasts are particularly attractive candidates for lipid production. For example, Cryptococcus curvatus (previously known as Apiotrichumcurvatum or Candida curvata) was reported to accumulate up to 60% lipid (Meesters and Huijberts, 1996; Ratledge and Cohen, 2008) , and Kodamaea ohmeri and Trichosporonoides spathulata accumulated lipid 53.28 % on raw glycerol.This species also grew to a biomass density of 118 g L-1 when cultured on nitrogen sufficient medium with glycerol as a carbon source, with a lipid content of 25% (Meesters and Huijberts, 1996). Similar biomass productivity was seen in cultures of Rhodosporidium toruloides, which reached a culture density of 100 g L-1 and 75% lipid content when cultivated under nutrient stressed conditions (Liu et al. 2009). The lipid content and the total biomass produced are culture dependent and highly variable (Ratledge and Wilkinson, 1988).
Both oleaginous and non-oleaginous yeasts contain lipids such as diacyl and triacylglycerides, free fatty acids, sterols, carotenoids, and phospholipids. Under certain growth conditions, oleaginous yeasts accumulate intracellular triacylglycerols in lipid bodies (Rattray, 1988; Ratledge, 1989; Radulovic and Knittelfelder, 2013). Nitrogen starvation is known to trigger TAG accumulation in most oleaginous yeasts (Rattray, 1988). One notable exception is Cryptococcus terricola, which can accumulate TAGs during logarithmic growth, before nitrogen depletion (Pedersen, 1962). Other yeasts able to accumulate oil during exponential phase are under investigation (Sestric, unpublished data). This is a valuable property for industrial production, allowing shorter production times and lipid production in nitrogen-rich substrates.
13 species, and strain of that species, capable of producing the desired fatty acids, as well as identification of appropriate culture conditions.
In most oleaginous yeasts species, protein synthesis occurs while nitrogen is available, then lipid accumulation dominates when carbon is in excess. During exponential growth phase, the C18:2 FA occurs in higher quantities, but decreases by stationary phase. The C18:0 and C18:1 FA become more prominent in stationary phase, and the addition of the double-bond in from C18:0 transitioning to the C18μ1 occurs by the ∆9desaturase (Ykema
et al. 1988; Hassan et al. 1993; Meesters and Huijberts, 1996,).
The fact that the fatty acid composition varies among oleaginous yeasts species, among strains of the same species, and with the growth conditions has been known for many decades (Viljoen and Kock, 1986; Rattray, 1988). To quantify this effect in newly discovered as well as previously known oleaginous yeasts species, Sitepu et al. (Sitepu et al. 2013) cultured 69 oleaginous and non-oleaginous yeasts in nitrogen-depleted defined medium with excessive glucose (120g/L), and analyzed the fatty acid profile using GC-FID. The major fatty acids in most of the yeasts strains analyzed were oleic (18:19), palmitic (16:0), stearic (18:0), and linoleic (18:26) acids. Minor fatty acids were lignoceric acid (24:0), palmitic (16:17), behenic acid (22μ0), myristic acid (14μ0), α-linolenic (18:33), and arachidic acids (20:0). Other fatty acids were observed in trace amounts. This is in agreement with fatty acid profiles of yeasts species as analyzed by other researchers (Rattray, 1988; Botha and Kock, 1993; Amaretti et al. 2012). Fatty acid profiles vary significantly between species, to a lesser degree among strains of the same species, also for the same strain grown under different growth conditions. For example, total lipid content as well as fatty acid profile of several strains of Rhodotorula glutinis varied considerably between strains, and for the same strain grown under slightly different conditions (Sitepu et al. 2013).
Several properties must be considered when selecting a yeasts species, and a specific strain of that species, for laboratory research or for commercial development. For instance, an oleaginous yeasts that thrives on corn stover hydrolysate, which is rich in glucose and xylose, may not be appropriate for conversion of dairy waste, which is rich in lactose.
ammonium-14
pretreated hydrolysates provide a usable nitrogen source, but ammonium concentrations should be monitored to ensure it will be depleted at the stage of growth when lipid accumulation is desired.
MATERIALS AND METHODS
Research methodology
To study diversity of oleaginous and cellulolytic yeasts from Indonesian sources, the research design is summarized in Figure 1, and flow chart of experimental work is presented in Figure 2 which include isolation of yeasts from varying resources, species identification, physiology characterization of cellulo-oleaginous yeasts.
15
Figure 2 Flow chart of experimental works
Sampling sites and Samples sources
16
Figure 3 Sampling sites for isolation of yeasts from Indonesian sources, sampling was conducted in 2010, 2011, and 2012. Samples were collected from soil, litter, plant materials, larvae of wood insect, leaf, stem, and fruit of Piper nigrum and Piper betle
Samples types collected were leaf, leaf litter, soil, insect gut, insect larva, insect, insect tunel, fungi, decay wood and plant materials (Table 1) were aseptically collected from different location in Indonesia (Figure 3). A new protocol was introduced to obtain insect larvae from hard wood plants. Six trees belong to Myrtaceae, Meliaceae, Euphorbiaceae were selected. The xyleme parts of stem of selected plants were scratch off about 500 cm long, and plants were let for 6 moths to die. During 6 moths insects attacked the plants and nest and produce larvae in tree. The trees were then cut to remove larvae as microbial sources.
Isolation of Yeasts
Yeasts were isolated from samples using published methods (Boundy-Mills, 2006). One g of soil or leaf litter was added to 25 mL of saline/Tween (0.85% NaCl, 0.01% Tween 80) in a 7 oz.Whirl-Pak filter bag (Nasco) and shaken to suspend the microbes. Two hundred L of liquid from the other side of the filter were plated on agar plates. Leaves were plated using two methods, washing and direct plating. For washing, leaves were added to 10 mL of saline/Tween in a 7 oz. Whirl-Pak filter bag, shaken to wash the surface of the leaf, and liquid withdrawn from the other side of the filter mesh for plating. Aliquots of 200 L of these samples were plated on Rose Bengal Chloramphenicol Agar (RBCA) (OXOID, CM0549), which prevents growth of bacteria and controls the spread of molds. For direct plating, leaves were cut into 3 cm size and surface strelized using alcohol 70 % for 1 minutes. The samples were then plated on the surface of media incubated for 5 days at 30ºC.
17 and yeasts were cultivated. Yeast isolatiuon from insect was performed by taking gut of larvae and crussed it with pestel, and gut liquid were plated on the agar plate. Plates were incubated at ambient temperature at 30oC for two to five days. Typically, up to four morphotypes of yeasts grew per plate. One colony of each morphotype on each plate was selected, assigned a unique identification number (Organism ID), and purified by streaking for single colonies at least twice on potato dextrose agar (PDA, OXOID, CM0139).
Table 1 List of samples collected as biotope for oleaginous and cellulolytic yeasts studies
*Multiple soil cores (15-cm depth by 2-cm diameter) were collected and mixed in a plastic bags from Mekonga, Papalia (Sout East Sulawesi), Batanta Island Raja Ampat, West Papua Kupang (Nusa Tenggara Timur), Indonesia. At the same places litter were collected and placed in paper envelope.