www.elsevier.com / locate / bres
Research report
Efferent projections of infralimbic and prelimbic areas of the medial
prefrontal cortex in the Japanese monkey, Macaca fuscata
a ,
*
b bTanemichi Chiba
, Tetsuro Kayahara , Katsuma Nakano
a
Department of Anatomy and Neurobiology, Chiba University School of Medicine, Inohana 1-8-1, Chuo-Ku, Chiba 260-8670, Japan b
Mie University School of Medicine, Tsu 514-8507, Japan Accepted 19 September 2000
Abstract
The infralimbic area (IL) and prelimbic area (PL) have been postulated as an autonomic motor region in the medial prefrontal cortex. The present study was conducted to reveal the projection sites of IL and PL of the monkey, Macaca fuscata, using biotinylated dextran amine as an anterograde tracer. IL and PL projected densely to the ventromedial caudate nucleus, the core and shell of the nucleus accumbens (Acb), parvicellular lateral basal and magnocellular accessory basal nuclei of the amygdala, lateral preoptic area, ventromedial hypothalamic nucleus, tubero-mammillary nucleus (TM), medial part of the magnocellular and dorsal part of the parvicellular (MDpc) dorsomedial thalamic nuclei, reunience and medial part of the medial pulvinar nucleus, and dorso-lateral part of the periaqueductal gray (PAGdl) in the mesencephalon. Moderately to weakly projected areas were the intermediate and lateral parts of the agranular insular cortex, orbital part of area 12, agranular and dysgranular part of the temporal pole cortex (TPa-g), auditory temporal cortex, lateral and medial (MS) septal nuclei, bed nucleus of the stria terminalis, diagonal band of Broca, substantia innominata, and medial preoptic area, dorsomedial, lateral, and posterior hypothalamic nuclei, magnocellular lateral basal and lateral amygdaloid nuclei, paratenial, paraventricular (PV), inter-antero-medial (IAM), reticular, central medial (CeM), parafascicular (PF) and limitans nuclei of the thalamus, lateral habenular nucleus, pedunculo-pontine nucleus, dorsal part of the lateral lemniscal nucleus, ventral tegmental area (VTA), dorsal raphe, superior central nucleus, medial and lateral parabrachial nuclei (PBl) and nucleus locus coeruleus (LC). A few scattered terminals were observed in the perifornical nucleus of the hypothalamus and substantia nigra pars compacta. PL and area 24 were characterized by projections to the entorhinal (Ent) and piriform (Pir) cortex as well as to the magnocellular part of the ventral anterior thalamic nucleus (VAmc). The morphology of the terminal arborization in each nuclei was different in appearance, perhaps reflecting the synaptic interaction between the nerve terminals and postsynaptic dendrites. PL projected uniquely to Ent, Pir and VAmc and IL projected uniquely to TPa-g, MS, IAM, CeM, MDpc, PF, PBl and LC. IL projected more strongly than PL to the shell of Acb, amygdaloid nuclei, PV, TM, VTA and PAGdl. The present results support the hypothesis that IL is a major cortical autonomic motor area and PL integrates limbic and autonomic inputs in the primate. 2001 Elsevier Science B.V. All rights reserved.
Theme: Other systems of the CNS
Abbreviations: ABmc, accessory basal amygdaloid nucleus, magnocellular part; Acb, accumbens nucleus; BLmc, basolateral amygdaloid nucleus,
magnocellular part; BLpc, basolateral amygdaloid nucleus, parvicellular part; BST, bed nucleus of stria terminalis; CeM, central medial thalamic nucleus; CDvm, caudate nucleus, ventromedial part; DB, nucleus of diagonal band; DMH, dorsomedial nucleus of hypothalamus; DR, dorsal raphe nucleus; Ent, entorhinal cortex; Hbl, lateral habenular nucleus; Iai, agranular insular cortex, intermediate part; Ial, agranular insular cortex, lateral part; Iam, agranular insular cortex, medial part; IAM, interanteromedial nucleus of thalamus; Iapm, agranular insular cortex, posteromedial part; L, lateral amygdaloid nucleus; LC, nucleus of locus coeruleus; LH, lateral hypothalamic nucleus; Lim, limitans nucleus of thalamus; LLd, dorsal nucleus of lateral lemniscus; LS, lateral septal nucleus; MDmc, dorsomedial nucleus of thalamus, magnocellular part; MDpc, dorsomedial nucleus of thalamus, parvicellular part; MS, medial septal nucleus; NCS, superior central nucleus; NTS, nucleu of tractus solitarius; PAG, periaqueductal gray; PAGdl, periaqueductal gray, dorso-lateral part; PBl, lateral parabrachial nucleus; PBm, medial parabrachial nucleus; PF, parafascicular nucleus of thalamus; PFC, prefrontal cortex; PfH, perifornical nucleus of hypothalamus; PH, posterior hypothalamic nucleus; Pir, piriform cortex; PM, medial pulvinar nucleus; POL, lateral preoptic nucleus; POM, medial preoptic nucleus; PPN, pdunculo-pontine nucleus; PT: pretenial nucleus of thalamus; PV, paraventricular nucleus of thalamus; PVH, paraventricular nucleus of hypothalamus; R, reticular nucleus of thalamus; Re, reuniens nucleus of thalamus; RPC, reticular parvicellular nucleus; SI, substantia innominata; SNc, substantia nigra, pars compacta; TA, temporal auditory cortical area; TM, tubero-mammillary nucleus of hypothalamus; TP, temporopolar cortex; TPa-g, temporopolar cortex, agranular area; TPdg, temporopolar cortex, dysgranular area; VAmc, ventral anterior nucleus of thalamus, magnocellular part; VMH, ventromedial nucleus of hypothalamus; VTA, ventral tegmental area; ZI, zona incerta; 12o, area 12o of prefrontal cortex; 12l, area 12l of prefrontal cortex
*Corresponding author. Tel.:181-43-226-2022; fax:181-43-226-2025.
E-mail address: [email protected] (T. Chiba).
84 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Topic: Limbic system and hypothalamus
Keywords: Infralimbic area; Prelimbic area; Medial prefrontal cortex; Monkey; Biotinylated dextran amine; Efferent connection
1. Introduction used BDA as a marker useful for precisely identifying the
injection sites and also followed very thin axons of the The medial and ventral parts of the frontal lobe of the autonomic neurons in the central nervous system for a monkey can modulate autonomic parameters [21,41,59] considerable distance.
and the role of the ventromedial frontal lobe in autonomic function is that individuals with lesions of this area are
unable to generate autonomic responses to emotional 2. Materials and methods
stimuli. Remarkably, they are also impaired in making
judgments about the consequences of their actions in social Ten adult Japanese monkeys, Macaca fuscata, of both situations, despite possessing the knowledge necessary to sexes and 3.5–5.5 kg body weight were used in the present make the correct decision [9,18,19]. Damasio and his study. All animal protocols were reviewed and approved co-workers [19] have suggested that these two deficits are by the Animal Studies Committee of Mie University. The related and that the sociopath-behavior of these patients is animals were anesthetized with intramuscular injection of due to their inability to generate ‘somatic markers’ that tag ketamine hydrochloride (10 mg / kg) and then with pen-behavioral options as desirable or not [10]. The frontal tobarbital (25 mg / kg). The animals were fixed on stereo-lobe damage would lead to loss of affective responsiveness taxic apparatus, and 0.1–1.0 ml31–3 times of 5% and foresight arising from interoceptive agnosia [32]. biotinylated dextran amine (BDA, 10 000 MW, Molecular Damasio [18] extended this concept to explain the ‘ac- Probes Inc.) in physiological saline was injected through a quired sociopathy’ of patients with bilateral orbitofrontal glass micropipette (inner diameter of the tip was 30–50 damage. mm) using a pneumatic picopump, Model PV800, under an The viscerosensory and visceromotor areas in the frontal operation microscope. A period of 14–21 days later, the lobe are suggested to be localized in the agranular insular, animals were anesthetized again and fixed by perfusion infralimbic and prelimbic cortex (IL and PL). The vis- with 1 l / kg body weight of 8% formalin (3.2% formalde-cerosensory inputs reach specific areas within the agranular hyde), 0.2% glutaraldehyde in 0.1 M phosphate buffer (pH insula in primates as in rodents [14]. Thus, IL and PL have 7.4) and 1000 ml of 10% sucrose in phosphate buffer. been postulated to be an autonomic motor area in the After the perfusion, the brain was removed and placed in medial prefrontal cortex (PFC), as efferent and afferent the 25% sucrose in phosphate buffer at 48C for a few days. connections of IL in the rat were examined by several Serial coronal sections were then cut at 50 mm thickness workers and IL and PL were found to be reciprocally with a freezing microtome. The serial sections were connected with most central autonomic nuclei as far as the classified into three groups and processed as follows. (1) spinal cord in the rat [5,4,27,54]. The sections were rinsed with 0.1 M phosphate buffered-As recent studies revealed, the cytoarchitectonic map saline (PBS) and incubated in a solution composed of and histochemical characteristics of the monkey PFC 15–17ml of streptavidine, 1 ml of 2.5% Triton X-100, and including IL and PL [14], we designed the present study so 4 ml of 0.1 M PBS overnight. After rinsing five times with as to reveal the projection sites of the medial PFC of the PBS, the sections were incubated for 3–5 h with monkey, Macaca fuscata, using biotinylated dextran amine diaminobenzidine solution composed of 50 ml 0.05 M (BDA) as an anterograde tracer. The injection sites covered Tris–HCl buffer (pH 7.6), 10 mg diaminobenzidine and PL, IL and the adjacent medial PFC including area 14, and 125 mg nickel ammonium, and added with 80–100ml of area 24b. We concentrated on the projection pattern of IL 0.3% H O . (2) The sections were processed as in (1), but2 2 compared with PL in this study. Part of the present studies nickel ammonium was omitted from the final reaction were presented as preliminary reports on several occasions solution and they were observed under-dark field optics. [17,38,39]. During the preparation of this manuscript, two (3) The sections were rinsed with the phosphate buffer, important results of projection studies of the medial processed for Nissl staining, and observed by microscope prefrontal cortex of macaque monkeys, Macaca fas- and used for reference to identify nuclear orientation. A
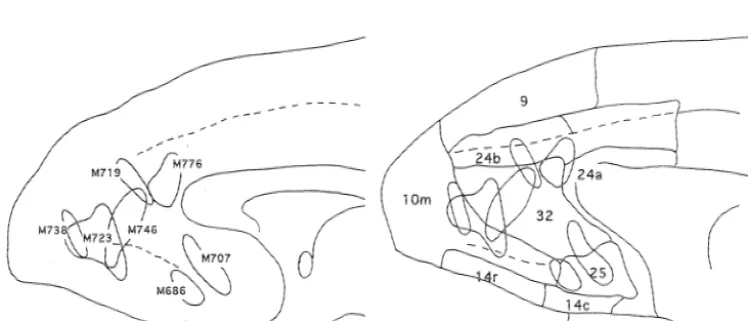
the serial frontal sections and were projected to the saggital Animal Sex Body Injection method of BDA
plane of the medial prefrontal cortex as illustrated in Fig.
No. weight
1A and B. The injection area of each animal was de-(kg)
termined by referring to the atlas of the medial prefrontal plane by Carmichael and Price [14] to identify the approxi-M686 Female 4.7 Injected 3 times in adjacent sites with 1.0, 1.0
mate cortical area. The injection sites of cases M686 and and 1.0ml while withdrawing the micropipette.
M707 corresponded approximately to area 25 and caudal M707 Female 4.5 Injected 2 times, 1.0 ml in one site and 0.810.2ml area 32. Those of case M723 corresponded to rostral area in the adjacent site. 32 and caudal area 10m and case M746 to rostral area 32. The injection sites of case M719 and M776 corresponded M719 Female 3.9 Injected 3 times in three adjacent sites, 0.610.6ml, to area 24b and case M738 to the middle of area 10m. The 0.5 and 0.6ml. cortical area was identified by referring to the cytoarchitec-tonic and immunohistochemical map of the rhesus monkey M723 Female 6.8 Injected 2 times in the same site with 0.610.6ml. by Carmichael and Price [14]. Cases M707 and M686 were used for analysis of the projection sites of area 25 and M738 Female 3.0 Injected 2 times in two adjacent sites, 0.310.3 and
M723 and M746 for that of the projection sites of area 32. 0.310.25ml.
The results of cases M719, M738 and M776 were used as control injections for areas 24b, 10m and 24a respectively. M746 Male 4.0 Injected 2 times in the same site with 0.210.25ml
and 0.3ml in the adjacent site.
3.2. Case M686 M776 Female 2.9 Injected 3 times in the same site with 0.110.111.15
ml.
DAB was injected into three adjacent locations of area 25 in this case as illustrated in Fig. 1. The distribution of projection nerve terminals was illustrated in Fig. 2. The
3. Results densest network of nerve terminals with many varicosities
was observed in the ventro-medial caudate nucleus 3.1. Injection sites (CDvm), core and shell of the nucleus accumbens (Acb), magnocellular accessory basal and parvicellular lateral From a series of BDA injections into the medial basal (ABmc, BLpc) nuclei of the amygdala, and the prefrontal cortex of monkeys, seven cases were selected magnocellular part of the mediodorsal (MDmc) nuclei of for the present analyses of the projection pattern of the the thalamus. Moderately to weakly projected areas were medial prefrontal cortex including the anterior cingulate the lateral septum (LS), bed nucleus of the stria terminalis cortex (area 24b), IL (area 25) and PL (area 32). Injection (BST), diagonal band of Broca (DB), substantia in-sites were identified by the distribution of pyramidal cells nominata (SI), dorsomedial, ventro-medial, lateral and labeled by the uptake of injected BDA, reconstructed from posterior hypothalamic nuclei (DMH, VMH, LH, PH),
86 T. Chiba et al. / Brain Research 888 (2001) 83 –101
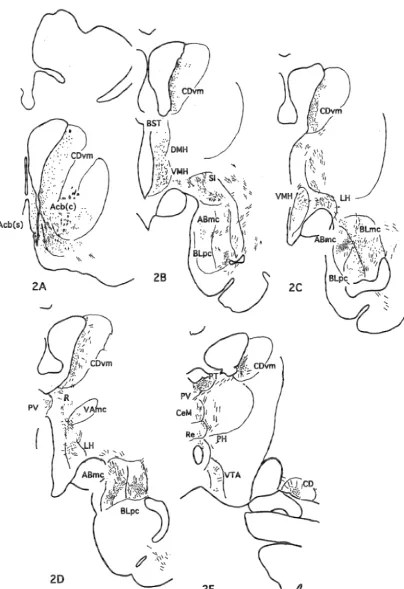
Fig. 2. A series of frontal sections of M686 demonstrating the distribution of nerve terminals labeled by BDA.
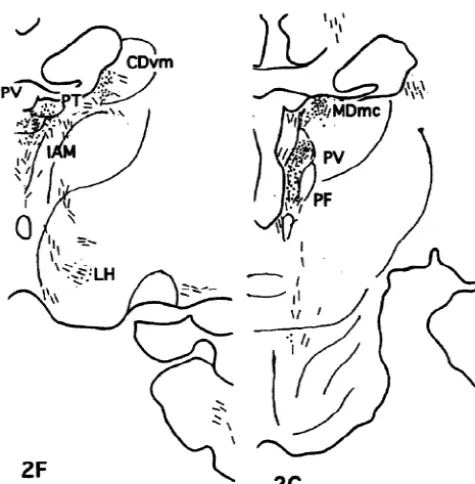
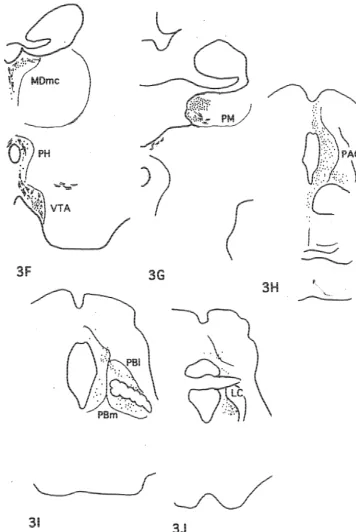
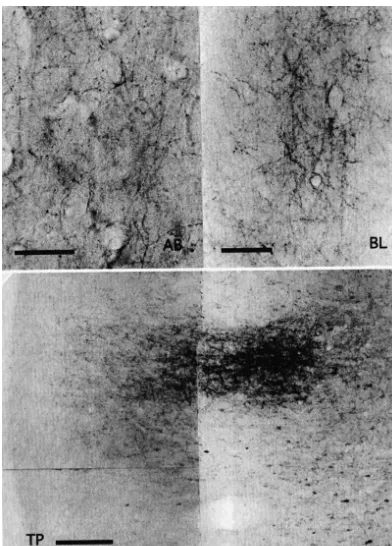
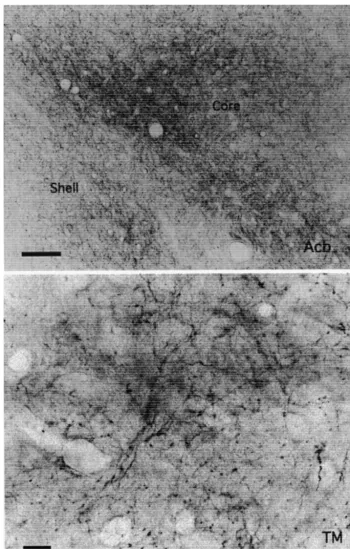
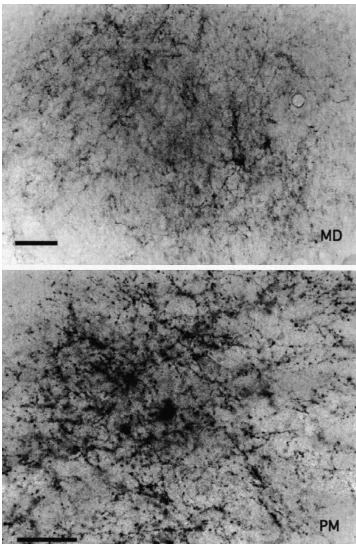
between the nerve terminals and postsynaptic dendrites may have been responsible. Typical photomicrographs of such terminals were observed in the shell and core of Acb (Fig. 4B), BL and AB amygdaloid nuclei (Fig. 4A), TM hypothalamic nucleus (Fig. 4B), MDmc (Fig. 4C) and PM thalamic nuclei (Fig. 4C) and TP (Fig. 4A).
3.4. Case M746
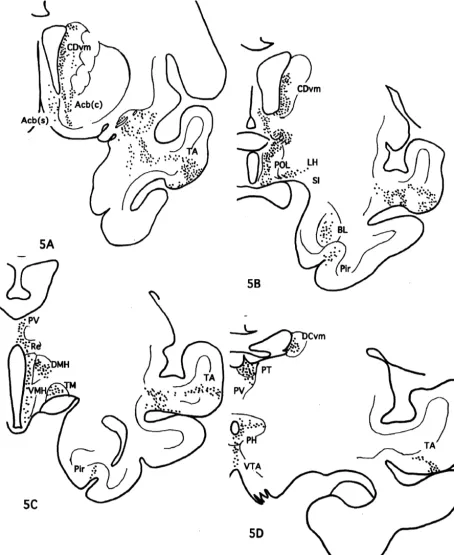
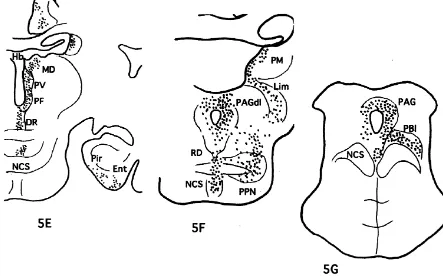
The injection site in this case was localized in the rostral part of area 32 as shown in Fig. 1. Moderately to weakly labeled networks of nerve terminals were observed in Iai, 12o of the frontal cortex, TA of the temporal cortex, core and shell of Acb, DB, LS, CDvm, SI, as well as Ent and Pir cortex. The nerve terminals were also seen in PT, PV, R, Re, MDmc, PM and Lim of the thalamus, POL, DMH, VMH, LH, PH and TM of the hypothalamus, Hbl, PPN, LLd, VTA, DR, NCS and PAGdl of the midbrain (Fig. 5).
Fig. 2. (continued )
3.5. Case M723
DAB was injected into the rostral part of area 32 and caudal part of area 10m as illustrated in Fig. 1. Moderately Lim), ventral tegmental area (VTA, A10), substantia nigra to weakly labeled nerve terminals with varicosities were pars compcta (SNc), dorsolateral part of the periaqueductal distributed in Iai, 12o and the medial part of the agranular gray (PAGdl) and nucleus locus coeruleus (LC). insular cortex, TPdg and TA of the temporo-polar cortex, the core of Acb, DB, BST, LS, CDvm, SI, as well as Ent 3.3. Case M707 and Pir in the frontal cortex, ABmc, BLmc, BLpc and L of the amygdala, PV, R, Re, VAmc, MDmc, PM and Lim of DAB was injected into area 25 and the caudal part of the thalamus, POL, DMH, VMH, LH, TM, PfH of the area 32 as seen in Fig. 1. Projection sites of the case M707 hypothalamus, Hbl, VTA, DR, NCS, and PAGdl of the the were schematically demonstrated in Fig. 3. The densest midbrain (Fig. 6).
network of nerve terminals with many varicosities was observed in the CDvm, core and shell of Acb, BLpc and
ABmc, lateral preoptic area (POL), VMH, TM, medial part 3.6. Case M719 of MDmc and dorsal part of MDpc, Re and PM of the
thalamus, PAGdl, pedunculo-pontine nucleus (PPN) and DAB was injected almost exclusively in area 24b in this dorsal lateral lemniscal nucleus (LLd). case as illustrated in Fig. 1. Medium to weakly labeled Moderately to weekly projected areas were the lateral networks of nerve terminals were observed in Iai, Ial and part of the agranular insular cortex (Ial), orbital part of areas 14 and 13 of the frontal cortex, TA of the temporo-area 12 (12o), agranular and dysgranular part of the polar cortex, core and shell of Acb, CDvm, DB, BST, LS, temporal pole cortex (TPa-g, TPdg), auditory temporal MS, SI and Pir in the cortex, ABmc, BLmc and BLpc of cortex (TA), medial septal nucleus (MS), BST, DB, SI, the amygdala, PV, R, IAM, CeM, Re, VAmc, MDmc. medial preoptic area (POM), DMH, LH, PH of the MDpc, Lim of the thalamus, POL, POM, DMH, VMH, hypothalamus, Blmc, L of the amygdala, and magnocellu- LH, PH, TM, and PfH of the hypothalamus, Hbl, VTA, lar part of the ventral-anterior (VAmc), parafascicular (PF) SNc, DR, NCS, PAGdl, PBl and LC of the midbrain (Fig. and PT, PV, R, IAM, CeM and Lim of the thalamus, lateral 7).
habenular nucleus (Hbl), VTA, dorsal raphe (DR), superior central nucleus (NCS), medial and lateral parabrachial
nuclei (PBm and PBl) and LC. 3.7. Case M776 Only a few scattered terminals were observed in the
88 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Fig. 3. (continued )
CDvm, core and shell of Acb and rostral cingulate cortex. as well as ABmc, BLmc, BLpc in the amygdala. Medium The distribution of labeled terminals was more intense in intensity of labeling was also seen in DR, NCS and PAGdl the core than the shell of Acb. Moderately labeled termi- of the midbrain.
nals were seen in the Iai, Ial, and 12o of the prefrontal
cortex, and TPa-p segment of the temporo-polar cortex. 3.8. Case M738 Medium density of nerve terminals was seen in DB, LS,
90 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Fig. 4. (continued )
prefrontal cortex as Iai, Iam, IL and 12o as well as in TA 3.9. A summary of the efferent projections of the IL and of the temporo-polar cortex. Weakly labeled nerve termi- PL from the seven cases examined in the present study
nals were scattered in the core and shell of Acb, CDvm,
92 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Fig. 4. (continued )
observations of M686 and M707 versus M746 and M723. hand, projections to Ent, Pir and VAmc were observed Projections to TPa-g, MS, IAM, CeM, MDpc, PF, PBl, and from area 32 but not from area 25. Projection sites of area LC were observed from area 25 but not from the area 32. 24 seemed to be similar but much weaker than that of area Further, projections to TPdg, TA, CDvm, DB, BST, LS, 25.
tem-Fig. 5. A series of frontal sections of M746 showing the distribution of nerve terminals labeled by BDA.
94 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Fig. 5. (continued )
and noradrenergic LC that are most likely concerned with 14 of mPFC as shown in Fig. 1. The areas in the mPFC the regulation of the synaptic activity in various target were determined by referring to the map of the monkey nuclei of these amine neurons. described by Carmichael and Price [14]. The actual shape of the brain of the monkeys used in this study varied from animal to animal; also, the species used in this study was
4. Discussion the Japanese monkey, Macaca fuscata, which belongs to
the same class but in different subclass from Macaca 4.1. Methodological considerations nemestrina, fascicularis and mulatta used by Carmichael
and Price [14]. The location maps of the areas were not as The present study using BDA as an anterograde tracer exact as those determined cytoarchitectonically or histo-clearly demonstrated the distribution of labeled perikarya chemically. The results, however, clearly showed the and dendrites in the injection sites of mPFC, and axons and difference in projection patterns after the tracer was terminal arborizations with varicosities in the projected injected in area 25 compared to the other cases in which areas. The axons of autonomic and limbic nervous systems the marker was injected in rostral area 32, areas 10, 24b were extremely thin and it was difficult to identify the and 14.
labeled ones with a low magnification microscope, but
were able to observe them at higher magnifications even if 4.2. Projection to prosencephalon they were scattered in a few fiber bundles or a network of
axon terminals. Dark-field optics also helped the observa- Projections from IL and PL were observed in Iai, Ial and tion of thin bundles and terminal axon networks. The 12o of the frontal cortex and TPa-g, TPdg and TA of TP. optimal duration of survival time after the injection of Iai and Ial have been determined as presser related areas of BDA was presumed to be 2 or 3 weeks, although success- PFC in the rat [60] and have reciprocal connections with ful labeling seemed to be intimately related to the amount IL and PL of mPFC [54,60]. IL projects to area 14 (Gyrus of uptake of tracers rather than the survival period of the rectus) [37] and PL projects to IL, and areas 14, 24, 9 and
animals. We could follow projection axon terminals as far 10 [42].
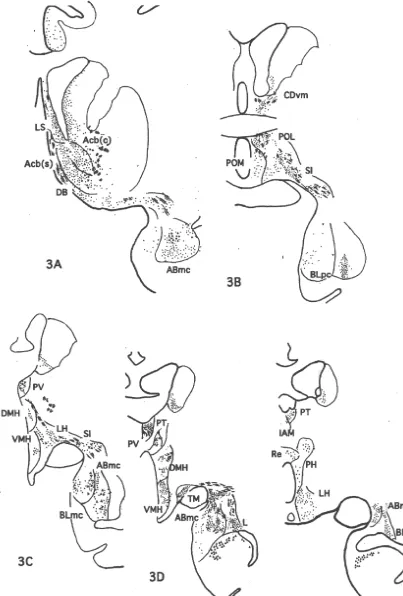
Fig. 6. A series of frontal sections of M723 showing the distribution of nerve terminals labeled by BDA.
96 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Fig. 6. (continued )
the parahippocampal cortex [6,56]. The mPFC reciprocally limbic, autonomic and motor behavioral outputs in re-connects with the piriform cortex in the rat [20] and sponse to sensory inputs [22,34,35].
projects to the parahippocampal cortex in the monkey [53]. Thus, the temporal pole is most probably a site where
sensory and limbic inputs converge, sending integrated 4.3. Projection to diencephalon outputs to mPFC which also sends feedback projections to
TP reciprocally. The medial part of PM is connected reciprocally with IL projects to the lateral capsular subdivision of the PFC including areas 9, 12l, 10, 24, 25, 32 as well as with central amygdaloid nucleus, corticomedial amygdaloid TP, rostral superior temporal gyrus and sulcus, amygdala nuclei, medial, anterior subdivision of the cortical- and anterior cingulate cortex. Medial PM with connections periamygdaloid cortex, ventromedial subdivision of L, with dorsomedial PFC, auditory cortical regions of the accessory basal amygdaloid nucleus and anterior superior temporal gyrus, polymodal processing areas of the amygdaloid area, while PL projects to the lateral capsular superior temporal sulcus and the amygdala might play a subdivision of the central and medial portion of BLmc and role in auditory, auditory-spatial, or other attentional the adjacent portions of L in the rat [11,33]. Porrino et al. processes [49]. Projection from mPFC to PM was strong [46] concluded that the ventromedial region of the frontal and indicated that this area of the pulvinar is intimately cortex receives both direct amygdalo-cortical and indirect related with autonomic and limbic functions in connection amygdalo-thalamo-cortical input from the amygdala in the with integrated audio–visual information.
Fig. 7. A series of frontal sections of M719 showing the distribution of nerve terminals labeled by BDA.
nuclei [6]. TP projects to the ventral part of the mid- and also has reciprocal connections with the amygdala and rostrocaudal level of MDmc in the monkey [50]. ‘hypothalamic area controlling emotional responses’,
cor-¨ ¨
98 T. Chiba et al. / Brain Research 888 (2001) 83 –101
Fig. 7. (continued )
4.4. Projection to mesencephalon midbrain aversive system, and stimulation in this area produces intense fear with autonomic activation in man DA inhibits pyramidal neurons of IL and PL of mPFC [40,61] and aversive behavioral responses with sympatho-that project to subcortical targets through D DA receptor2 activation in animals ([12] for reviews, [30,31]).
[51], and in addition to direct inhibition of cortical IL, insular cortex, BST, perifornical region, TM, dorsal projection neurons, DA inhibits pyramidal cells indirectly hypothalamic area, VMH, PVH, POL,M, ZI, raphe nucleus,
¨
by augmenting GABA release from interneurons [48]. cuneiform nucleus, Kolliker–Fuse nucleus, PBl, PBm, An et al. [3] reported that PFC projected densely to the NTS and ventrolateral part of PAG project to corticotropin-periaqueductal gray (PAG) of Macaque monkeys, and that releasing hormone-rich neurons of the pontine micturition areas 25, 32 and 10m projected predominantly to the center, known as Barrington’s nucleus in the rat [55]. bilateral dorsolateral columns of PAG. The present results
were similar, showing dense bilateral projections to PAGdl 4.5. Projection to medulla and spinal cord with ipsilateral predominance. PFC projects to PAG which
intermedio-Fig. 8. A schematic diagram summarizing the results of the present study showing nuclei which received projections from the infralimbic area (IL, area 25) of the medial prefrontal cortex.
lateral nucleus of the spinal cord in the rat [27]. The that IL and PL projected indirectly to the spinal cord in the vasomotor center in the mPFC of the rat corresponded to monkey.
the PL by Krettek and Price [29] or Cg3 by Paxinos and
Watson [45] projecting bilaterally and directly to the 4.6. Structure and function of mPFC central autonomic area of the thoracic spinal cord through
the dorsal cortico spinal tract [5]. The cytoarchitectonic and histochemical atlas of the As a control study to reveal projections to the spinal prefrontal cortex of the monkey has clearly identified areas cord from IL or PL, we injected CTb into the caudal 25, 32 and 24 of mPFC [14]. Gabbott and Bacon [24,25] medulla oblongata of the monkey (unpublished data). We further showed histochemical characteristics in relation to could observe retrogradely labeled neuronal perikarya in calcium binding proteins, GABA and nitric oxide in mPFC the cingulate cortex, area 14, and latero–dorsal aspect of and differentiated IL, PL and anterior cingulate cortices of the frontal cortex. Many labeled neurons were also found the monkey.
100 T. Chiba et al. / Brain Research 888 (2001) 83 –101
the orbital and medial prefrontal cortex of Macaque Monkeys, J. yet, the quite similar projection pattern of mPFC in the
Comp. Neurol. 363 (1995) 642–664. monkey as seen in this study to that of rodents strongly
[16] D.F. Cechetto, C.B. Saper, Role of the cerebral cortex in autonomic suggests that mPFC is an autonomic motor area in this function, in: A.D. Loewy, K.M. Spyer (Eds.), Central Regulation of species too. Autonomic Functions, Oxford Univ. Press, Oxford, 1990, pp. 208–
223.
[17] T. Chiba, K. Nakano, Projection sites of infralimbic cortex (area 25) of the monkey, Neurosc. Res. Suppl. 21 (1997) S281.
[18] A.R. Damasio, Descartes’ Error, Avon Science Publishing, New
Acknowledgements
York, 1994.
[19] A.R. Damasio, D. Tranel, H. Damasio, Individuals with sociopathic We are grateful to Ms. E. Takahashi, Dr. T. Takeda and behavior caused by frontal damage fail to respond autonomically to Ms. K. Kitajo for their excellent technical assistance. The social stimuli, Behav. Brain Res. 41 (1990) 81–94.
present study was supported by the grant-in-aid from the [20] F. Datiche, M. Cattarelli, Reciprocal and topographic connections between the piriform and prefrontal cortices in the rat: a tracing Ministry of Education, Science, Sports and Culture of
study using the B subunit of the cholera toxin, Brain Res. Bullet. 41 Japan to T. Chiba.
(1996) 391–398.
[21] J.M.R. Delgado, Circulatory effects of cortical stimulation, Physiol. Rev. 40 (Suppl. 4) (1960) 146–171.
[22] A.Y. Deutch, Prefrontal cortical dopamine systems and the
elabora-References tion of functional corticostriatal circuits: implications for
schizo-phrenia and Parkinson’s disease, J. Neural Trans. (Gen. Sect.) 91 [1] J. Aggleton, M.J. Burton, R.E. Passingham, Cortical and subcortical (1993) 197–221.
afferents to the amygdala of rhesus monkey (Macaca mulatta), [23] H. Ericson, A. Blomqvist, C. Kohler, Origin of neuronal inputs to Brain Res. 190 (1980) 347–368. the region of the tuberomammillary nucleus of the rat brain, J. [2] D.G. Amaral, J.L. Price, A. Pitkanen, S.T. Carmichael, Anatomical Comp. Neurol. 311 (1991) 45–64.
organization of the primate amygdaloid complex, in: J.P. Aggleton [24] P.L.A. Gabbot, S.J. Bacon, Local circuit neurons in the medial (Ed.), The Amygdala: Neurobiological Aspects of Emotion, Mem- prefrontal cortex (areas 24a,b,c, 25 and 32) in the monkey: I. Cell ory, and Mental Dysfunction, Wiley-Liss, New York, 1992, pp. 1-66. morphology and morphometrics, J. Comp. Neurol. 364 (1996a) [3] X. An, R. Bandler, D. Ongur, J.L. Price, Prefrontal cortical 567–608.
projections to longitudinal columns in the midbrain periaqueductal [25] P.L.A. Gabbot, S.J. Bacon, Local circuit neurons in the medial gray in Macaque monkeys, J. Comp. Neurol. 401 (1998) 455–479. prefrontal cortex(Areas 24a,b,c, 25 and 32) in the monkey: II. [4] M. Azuma, T. Chiba, Afferent projections of the infralimbic cortex Quantitative areal and laminar distributions, J. Comp. Neurol. 364
(area 25) in rats: A WGA-HRP study, Acta Anatom. Nippon 71 (1996b) 609–636.
(1995) 523–540. [26] S.N. Haber, K. Kunishio, M. Mizobuchi, E. Lynd-Balta, The orbital [5] S.J. Bacon, A.D. Smith, A monosynaptic pathway from an identified and medial prefrontal circuit through the primate basal ganglia, J.
vasomotor center in the medial prefrontal cortex to an autonomic Neurosc. 15 (1995) 4851–4867.
area in the thoracic spinal cord, Neuroscience 54 (1993) 719–728. [27] K.M. Hurley, H. Herbert, M.M. Moga, C.B. Saper, Efferent [6] J. Bachevalier, M. Meunier, M.X. Lu, L.G. Ungerleider, Thalamic projections of the infralimbic cortex of the rat, J. Comp. Neurol. 308
and temporal cortex input to medial prefrontal cortex in rhesus (1991) 249–276.
monkeys, Exp. Brain Res. 115 (1997) 430–444. [28] I.A. Ilinsky, K. Kultas-Ilinsky, Sagittal cytoarchitectonic maps of [7] P. Bailey, W.H. Sweet, Effects on respiration, blood pressure and the Macaca mulatta thalamus with a revised nomenclature of the gastric motility of stimulation of orbital surface of frontal lobe, J. motor-related nuclei validated by observations on their connectivity, Neurophysiol. 3 (1940) 276–281. J. Comp. Neurol. 262 (1987) 331–364.
[8] H. Barbas, T.H. Henion, Diverse thalamic projections to the [29] J.E. Krettek, J.L. Price, An autoradiographic study of projections prefrontal cortex in the rhesus monkey, J. Comp. Neurol. 313 (1991) from the amygdaloid complex to the thalamus and cerebral cortex, J.
65–94. Comp. Neurol. 172 (1977) 723–752.
[9] A. Bechara, A.R. Damasio, H. Damasio, S.W. Anderson, Insensitivi- [30] T.A. Lovick, Integrated activity of cardiovascular and pain regula-ty to future consequences following damage to human prefrontal tory systems: Role in adaptive behavioral responses, Prog. Neuro-cortex, Cognition 50 (1994) 7–15. biol. 40 (1993) 631–644.
[10] A. Bechara, H. Damasio, D. Tranel, A.R. Damasio, Deciding [31] T.A. Lovick, V.V. Stenzhka, Neurons in the dorsolateral periaqueduc-advantageously before knowing the advantageous strategy, Science tal gray matter in coronal slices of rat midbrain: electrophysiological 275 (1997) 1293–1295. and morphological characteristics, Exp. Brain Res. 124 (1999)
53–58. [11] M. Beinley-Reed, F. Mascagni, A.J. McDonald, Synaptology of
prefrontal cortical projections to the basolateral amygdala: an [32] A.R. Luria, E.D. Homskaya, Disturbance in regulative role of speech electron microscopic study in the rat, Neurosc. Lett. 202 (1995) with frontal lobe lesions, in: J.M. Warren, K. Akert (Eds.), The
5–48. Frontal Granular Cortex and Behavior, McGraw-Hill, New York,
1964, p. 352. [12] M.M. Behbehani, Functional characteristics of the midbrain
periaqueductal gray, Prog. Neurobiol. 46 (1995) 575–605. [33] A.J. McDonald, F. Mascagni, L. Guo, Projections of the medial and lateral prefrontal cortices to the amygdala: A phaseolus vulgalis [13] S.L. Buchanan, D.A. Powell, Cingulothalamic and prefrontal control
leucoagglutinin study in the rat, Neuroscience 71 (1996) 55–75. of autonomic function, in: B.A. Cogt, M. Gabriel (Eds.),
Neuro-biology of Cingulate Cortex and Limbic Thalamus. A Comprehen- [34] G.J. Mogenson, D.L. Jones, C.Y. Yim, From motivation to action: sive Handbook, Birkhauser, Basel, 1993, pp. 382–414, Chapter 13. Functional interface between the limbic system and the motor
system, Prog. Neurobiol. 14 (1980) 69–97. [14] S.T. Carmichael, J.L. Price, Architectonic subdivision of the orbital
[35] G.J. Mogenson, S. Brudzynski, M. Wu, C. Yang, C. Yim, From and medial prefrontal cortex in the macaque monkey, J. Comp.
motivation to action: a review of dopaminergic regulation of limbic-Neurol. 346 (1994) 366–402.
cuitries involved in limbic-motor integration, in: P.W. Kalivas, C.D. [49] L.M. Romanski, M. Giguere, J.F. Bates, P.S. Goldman-Rakic, Barnes (Eds.), Limbic Motor Circuits and Neruopsychiatry, CRC Topographic organization of medial pulvinar connections with the Press, Boca Raton, 1993, pp. 193–236. prefrontal cortex in the rhesus monkey, J. Comp. Neurol. 379 (1997) [36] M.A. Moran, E.J. Mufson, M.M. Mesulam, Neural inputs into the 313–332.
temporopolar cortex of the rhesus monkey, J. Comp. Neurol. 256 [50] F.T. Russchen, D.G. Amaral, J.L. Price, The afferent input to the (1987) 88–103. magnocellular division of the mediodorsal thalamic nucleus in the [37] R.J. Morecraft, C. Geula, M.M. Mesulam, Cytoarchitecture and monkey, Macaca fascicularis, J.Comp. Neurol. 256 (1987) 175–
neural afferents of orbitofrontal cortex in the brain of the monkey, J. 210.
Comp. Neurol. 323 (1992) 341–358. [51] S.R. Sesack, B.S. Bunney, Pharmacological characterization of the [38] K. Nakano, T. Chiba, Afferent connections of the infralimbic area in receptor mediating electrophysiological responses to dopamine in the medial prefrontal cortex (area 25) of the monkey, Macaca the rat medial prefrontal cortex: a microiontophoretic study, J.
fuscata, J. Auton. Nerv. Syst. 65 (1997) 103. Pharmacol. Exp. Ther. 248 (1989) 1323–1333.
[39] K. Nakano, T. Kayahara, T. Chiba, Afferent connections to the [52] O.A. Smith, J.L. DeVito, Central neural integration for the control of ventral striatum form the medial prefrontal cortex (area 25) and the autonomic responses associated with emotion, Ann. Rev. Neurosci. thalamic nuclei in the macaque monkey, in: J.F. McGinty (Ed.), 7 (1984) 43–65.
Advancing from the Ventral Striatum to the Extending Amygdala, [53] W.A. Suzuki, D.G. Amaral, Perirhinal and parahippocampal cortices Vol. 877, Annals New York Acad. Sci, New York, 1999, pp. of the Macaque monkey: cortical afferents, J. Comp. Neurol. 350
662–670. (1994) 497–533.
[40] B.S. Nashold, S.P. Wilson, G.S. Slaighter, The midbrain and pain, [54] M. Takagishi, T. Chiba, Efferent projections of the infralimbic (area Adv. Neurol. 4 (1974) 191–196. 25) region of the medial prefrontal cortex in the rat: an anterograde [41] E.J. Neafsey, Prefrontal cortical control of the autonomic nervous tracer PHA-L study, Brain Res. 566 (1993) 26–39.
system: Anatomicaland physiological observations, Prog. Brain Res. [55] R.J. Valentino, M.E. Page, P.-H. Luppi, Y. Zhu, E. Van Bockstaele, G. 6 (1990) 556–568. Aston-Jones, Evidence for widespread afferents to Barrington’s [42] E.J. Neafsey, R.R. Terreberry, K.M. Hurley, K.G. Ruit, R.J. nucleus, a brainstem region rich in corticotropin-releasing hormone
Frysztak, Anterior cingulate cortex in rodents: Connections, visceral neurons, Neuroscience 62 (1994) 125–143.
control functions, and implications for emotion, in: B.A. Vogt, M. [56] G.W. Van Hoesen, R.J. Morecraft, B.A. Vogt, Connections of the Gabriel (Eds.), Neurobiology of Cingulate Cortex and Limbic monkey cingulate cortex, in: B.A. Vogt, M. Gabriel (Eds.), Neuro-Thalamus. A Comprehensive Handbook, Birkhausser, Boston, 1993, biology of Cingulate Cortex and Limbic Thalamus: A
Comprehen-pp. 206–223. sive Handbook, Birkhauser, Boston, 1993, pp. 249–284.
¨ ¨
[43] D. Ongur, X. An, J.L. Price, Prefrontal cortical projections to the [57] R.M. Vickery, S.H. Morris, L.J. Bindman, Metabotropic glutamate hypothalamus in Macaque monkeys, J. Comp. Neurol. 401 (1998) receptors are involved in long-term potentiation in isolated slices of 480–505. rat medial frontal cortex, J. Neurophysiol. 78 (1997) 3039–3046. [44] D.N. Pandya, E.H. Yeterian, Prefrontal cortex in relation to other [58] H. Wada, N. Inagaki, A. Yamatodani, T. Watanabe, Is the
histaminer-cortical areas in rhesus monkey: Architecture and connections, in: gic neuron system a regulatory center for whole-brain activity?, H.B.M. Uylings, C.G.V. Eden, J.P.C. De Bruin, M.A. Corner, M.G.P. TINS 14 (1991) 415–418.
Feenstra (Eds.), Prog. Brain Res, Vol. 85, Elsevier Sci. Pub, 1990, [59] P.D. Wall, G.D. Davis, Three cerebral cortical systems affecting
pp. 63–94. autonomic function, J. Neurophysiol. 14 (1951) 507–517.
[45] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, [60] Y. Yasui, C.D. Breder, C.B. Saper, D.F. Cechetto, Autonomic Academic Press, San Diego, 1998. responses and efferent pathways from the insular cortex in the rat, J. [46] L.J. Porrino, A.M. Crane, P.S. Goldman-Rakic, Direct and indirect Comp. Neurol. 303 (1991) 355–374.
pathways from the amygdala to the frontal lobe in rhesus monkeys, [61] R.F. Young, Brain and spinal cord stimulation: how and to whom?, J. Comp. Neurol. 198 (1981) 121–136. Clin. Neurosurg. 35 (1989) 429–447.
[47] J.P. Ray, J.L. Price, The organization of projections from the [62] A. Zagon, S. Totterdell, R.S.G. Jones, Direct projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal ventrolateral medulla oblongata to the limbic forebrain: Anterograde cortex in Macaque Monkeys, J. Comp. Neurol. 337 (1993) 1–31. and retrograde tract-tracing studies in the rat, J. Comp. Neurol. 340 [48] S. Reaux, M.J. Besson, J. Penit-Soria, Synergism between D and1 (1994) 445–468.
D dopamine receptors in the inhibition of the evoked release of2 [63] D. Zeng, S.L. Stuesse, Topographic organization of efferent projec-3
[ H]GABA in the rat prefrontal cortex, Neuroscience 43 (1991) tions of medial frontal cortex, Brain Res. Bullet. 32 (1993) 195–