L
Journal of Experimental Marine Biology and Ecology 243 (2000) 227–240
www.elsevier.nl / locate / jembe
Response of the non-indigenous Caulerpa racemosa
˚
(Forsskal) J. Agardh to the native seagrass Posidonia
oceanica (L.) Delile: effect of density of shoots and
orientation of edges of meadows
a ,* b b
Giulia Ceccherelli , Luigi Piazzi , Francesco Cinelli
a
`
Dipartimento di Botanica ed Ecologia Vegetale, Universita di Sassari, via Muroni 25 Sassari I-07100,
Italy
b
`
Dipartimento di Scienze dell’Uomo e dell’Ambiente, Universita di Pisa, via Volta 6 Pisa I-56126, Italy Received 29 January 1999; received in revised form 19 July 1999; accepted 27 July 1999
Abstract
Caulerpa racemosa is a tropical green alga introduced into the Mediterranean as an immigrant from the Red Sea which has successfully fast-spread in the south-eastern and in the north-western part of the basin. C. racemosa occurs mostly in shallow but also in deep subtidal habitats colonising hard and soft substrata where turfs, erect algae and even seagrasses are present with the potential to profoundly alter indigenous communities. However, the extent to which biotic interactions influence the spread of the alga is not well known. In this study the effects of the presence of the native seagrass Posidonia oceanica on the non-indigenous alga Caulerpa racemosa are examined: a multifactorial experiment was designed to test (1) the importance of the seagrass canopy structure and (2) of orientation of seagrass edge on algal performance along the edge and inside the meadow of P. oceanica and (3) whether patterns of algal growth are consistent at different spatial scales (few centimetres to several metres). The aim of this study is to provide a basis for further experimental investigations of the factors and mechanisms affecting the performance of this alga in the Mediterranean. The results of this study indicated that where Caulerpa racemosa is at the edge of Posidonia oceanica meadow, the vertical growth of the alga (blade length) is sensitive to the combination of time, seagrass density and edge-meadow orientation, that the spread of the alga along the edge of the seagrass meadow is dependent on the characteristics of the area and that the growth of the alga inside the meadow is influenced by seagrass density. The findings of this study suggest that the susceptibility of the indigenous P. oceanica community to invasion of the introduced alga C. racemosa is related to the availability of sand habitat ground created, since low invasion of the very dense edges of the seagrass was observed compared to the less dense ones. 2000 Elsevier Science B.V. All rights reserved.
*Corresponding author.
E-mail address: [email protected] (G. Ceccherelli)
Keywords: Biological invasion; Caulerpa racemosa; Habitat structure; Posidonia oceanica
1. Introduction
Biological invasions are a threat to the integrity of natural communities of plants and animals and to the preservation of endangered species (Walker and Kendrick, 1998; Lodge, 1993; Carlton and Geller, 1993; Ribera and Boudouresque, 1995; Vitousek et al., 1997). However, despite the growing concern over the negative effects of such invasions we still know surprisingly little about the determinants of the distribution and abundance of invading species at both local and regional scales (e.g. Richardson and Bond, 1991;
´
Williams and Black, 1994; Trowbridge, 1995; Rejmanek and Richardson, 1996; ´
Thebaud et al., 1996), about the conditions under which they will successfully invade and establish in new communities (Hobbs and Atkins, 1988), and the vulnerability to invasion of different communities (Elton, 1958; Crawley, 1987; Burke and Grime, 1996; Stapanian et al., 1998).
Intentional or accidental introductions of algal species have been widely reported (Chambers et al., 1993; Ribera and Boudouresque, 1995; Walker and Kendrick, 1998). Coenocytic Chlorophyta, such as Codium fragile ssp. tomentosoides (Van Goor) Silva (Trowbridge, 1995), have also been reported as being highly invasive. In particular, members of the Caulerpales are predominantly tropical and subtropical species which exhibit traits of a particularly invasive species. Most notably, the introduced alga Caulerpa taxifolia (Vahl) C. Agardh has rapidly expanded its distribution in the Mediterranean since 1984 (Boudouresque et al., 1992, 1994; Meinesz et al., 1993), but additionally, in Australia Caulerpa scalpelliformis (R. Brown ex Turner) C. Agardh has been recorded as a fast-spreading alga by Davis et al. (1997).
Caulerpa racemosa is a tropical green alga introduced in the Mediterranean possibly as an immigrant from the Red Sea (Lipkin, 1972; Verlaque, 1994) which was recorded for the first time by Hamel in 1926 along Tunisian coasts and since then has successfully fast-spread in the south-eastern basin (Hamel, 1926; Aleem, 1948; Alongi et al., 1993); now it also occurs even in the north-western part along the Tuscan coasts, where it has been noted since 1994 (Piazzi et al., 1994, 1997a, 1997b). C. racemosa occurs mostly in shallow but also in deep subtidal habitats colonising hard and soft substrata where turfs, erect algae and even seagrasses are present (Piazzi et al., 1997a, 1997b; Piazzi and Cinelli, 1999); its quantitative dominance exhibited in many habitats has the potential to profoundly alter indigenous communities (Piazzi, personal observation). Although C. racemosa is clearly a weedy species exhibiting rapid growth (Piazzi et al., 1997b), predominant asexual reproduction, high dispersal and broad tolerance to physiological conditions (Piazzi, personal observation), the extent to which biotic interactions influence the spread of the alga is not well known.
Several explanations have been given for the loss of seagrass habitat: biological invaders have been evidenced as a great threat. For example, changes to Zostera marina L. meadows have been associated in the Atlantic with the spread of the invading introduced seagrass Zostera japonica Aschers. and Graebn. (Posey, 1988). Furthermore, Den Hartog (1997) documented that the introduced brown alga Sargassum muticum (Yendo) Fensholt settles into Zostera marina meadows and interferes with the regeneration of the bed, and no seagrass germlings were found where the alga was present. Also in the Mediterranean, the successful invasive alga, Caulerpa taxifolia, was invoked as a further cause of loss of meadows of both Cymodocea nodosa (Ucria)
` Ascherson (Ceccherelli and Cinelli, 1997) and Posidonia oceanica (de Villele and Verlaque, 1995). Overall, although many non-indigenous species have been recognised as negatively interacting with native seagrasses, their contribution to declines is poorly known. For Caulerpa racemosa, many indications of impact have been seen in Cymodocea nodosa beds with lower shoot densities or unhealthy P. oceanica meadows displaying yellowish leaves (Piazzi, personal observation). However, the negative effect of this alga on seagrasses has not been experimentally demonstrated.
In this study, which represents a preliminary approach, the effect of the native seagrass Posidonia oceanica on the non-indigenous Caulerpa racemosa is examined: a multifactorial experiment was designed to test the importance of (1) the seagrass canopy structure and (2) orientation of the edges of meadow on algal performance along the
edge and inside the meadow of P. oceanica and (3) whether patterns of algal growth are
consistent at different spatial scales ranging from few centimetres to several metres. Given that seagrass canopy has been shown to structure the entire understory assem-blages (Bell and Westoby, 1986a, 1986b; Orth, 1992; Connolly and Butler, 1996; Ceccherelli and Cinelli, 1998), manipulation of the density of the seagrass, such as reduced shoot density, can be used to examine the influence of the seagrass on size and growth of the alga. In this system P. oceanica provides a subcanopy microhabitat to C. racemosa that is distinctly different from the open substrate because of canopy shading (Ceccherelli and Cinelli, 1999), reduction of water motion (Gambi et al., 1989, 1990) and production of secondary metabolites that can allelopathically interfere with understoried species and grazers (Cuny et al., 1995). Furthermore, orientation of seagrass edges was tested as a potential source of variation on C. racemosa performance since the west edge is seaward and hence more exposed relative to the east one which is faced to the land; thus, given the geographical position of the coast, algal specimens on east-facing edge may be exposed to a different water flow regime, degree of bending of the seagrass leaves, photoperiod and light intensity relative to the west-facing one (Ceccherelli and Cinelli, 1999).
This study represents a useful basis for further experimental investigations on the factors and mechanisms affecting the performance of this alga in the Mediterranean.
2. Methods
along the Tuscan coasts of the north-western Mediterranean (438309N, 108209E). In this area Caulerpa racemosa occurs on sandy and rocky substrate, mixed with C. nodosa and
along the edge of P. oceanica meadows.
This study was carried out for 1 year (from July 1997 until August 1998) and consisted in a multifactorial experiment testing the importance of seagrass shoot density and orientation of edges (landward / seaward) on algal performance: experimental units
(25350 cm in size) were prepared at the edge of Posidonia oceanica meadow, given
that Caulerpa racemosa fragments establish naturally in this position (Ceccherelli and Piazzi, personal observations). Three different shoot densities of P. oceanica, 10, 50 and 100% of natural density, which corresponded to the categories of very sparse (50–150
2 2 2
shoots / m ), dense (400–700 shoots / m ) and very dense (.700 shoots / m ) meadow
according to Giraud (1977), were obtained by clipping different percentages of shoots of 2
averaged natural density corresponding to 1525 shoots / m . Eighteen areas, nine at the west (exposed, seaward) and nine at the east (sheltered, landward) position, were randomly chosen at least 3 m apart and randomly selected to be assigned to each level of density. Three areas were attributed to each of the six combinations of the levels of shoot density and position (10% E, 10% W, 50% E, 50% W, 100% E and 100% W) and in each area three plots were positioned 50 cm apart so that a total of 54 experimental units were prepared for the whole experiment.
Transplanting of fragments of the alga was undertaken in July 1997: fragments
(14.3460.65 cm, mean6SE in size, n520) were collected from a nearby established
meadow at the same depth. Three fragments per unit were fixed to the substratum at the edge of the seagrass using two staples per fragment within an hour of collection. All fragments were taken from the same area and we assumed that removal of effects of differing initial sizes was not needed. Performance of Caulerpa racemosa transplanted at the edge of Posidonia oceanica was assessed estimating size of the specimens and growth both inside and outside the seagrass meadow.
Caulerpa racemosa size (blade length) was sampled in the field using a plastic calliper in 6 times from the start of the experiment (September, October 1997, February, May, June and August 1998). On each sampling time, one quadrat in the north, centre or south position was randomly chosen within each experimental unit and we measured the length of two randomly chosen blades within the quadrat. To obtain independence of time, data relative to three sampling times were randomly chosen for analysis out of those available (those used for analysis are indicated in Fig. 1) so that blade size of different quadrats were analysed. Data were analysed by using a five way-ANOVA with ‘position’ (two levels), ‘density’ (three levels),‘time’ (three levels), ‘area’ (three levels) and ‘plot’ (three levels).
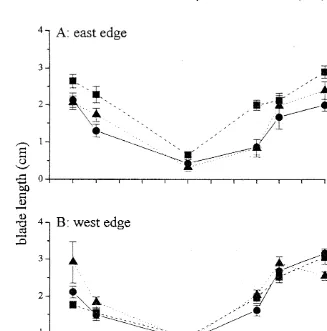
Fig. 1. Temporal variation of mean (6SE) Caulerpa racemosa blade length (cm) at the edge of defoliated
Posidonia oceanica (10, 50 and 100% of shoot density) at the esast end west edge of the seagrass meadow
(n518).
Although patterns of spread of the alga were extremely variable among experimental units, because Caulerpa racemosa elongated in different directions relative to the plot (along the edge of the seagrass as well as outwards the meadow), the density of the alga in the experimental units was estimated measuring the percent cover: on three different
occasions (December 1997, April and August 1998) photographs of a 25325 cm
surface in two plots per area were taken in situ and subsequent analysis of projected images through a transparent grid divided in 130 small quadrats was performed (Foster et al., 1991). Percent of small quadrats in which the alga was present was calculated for each sampling time and only data relative to the last (August 1998) were analysed, as above for the growth of stolons inside of the meadow.
Spread of Caulerpa racemosa outside the meadow along the edge of Posidonia oceanica was assessed on four different times (August 1997, October 1997, April 1998
2
by the alga through the spread originating from the three experimental units; in fact, after spring 1998 the three algal patches belonging to each area were mostly indistinguishable because of edge collapse and thus we decided to measure the total surface coverage per area. As above, data relative to the last sampling time (August 1998) were analysed by a two-way ANOVA with ‘position’ (two levels) and ‘density’ (three levels) as factors.
For all analyses ‘position’ and ‘density’ were treated as fixed and orthogonal, while
‘area’, ‘time’ and ‘plot’ random, where ‘area’ is nested in the ‘position3density’
interaction, ‘plot’ is nested in ‘area’ and ‘time’ is orthogonal. Cochran’s test was used prior to the analyses to remove heterogeneity of variances when necessary and SNK test to compare levels of significant factors.
3. Results
Vertical blade length of Caulerpa racemosa at Posidonia oceanica edge was greatly affected by the season; minimum size was found in winter while maximum was observed in summer (Fig. 1). Variable size was also evident at a small spatial scale, as
there were significant differences among time3plot combinations (Table 1). Vertical
blade length underwent great fluctuations through time also depending on shoot density and position (significant ‘position3density3time’ interaction, Table 1): smaller blades
were found at the edge of higher P. oceanica shoot density (50 and 100%) with respect
to the less dense treatment areas, especially at the east position (Table 1A SNK test). Despite the very large size of blades found along 10% shoot density areas at the east
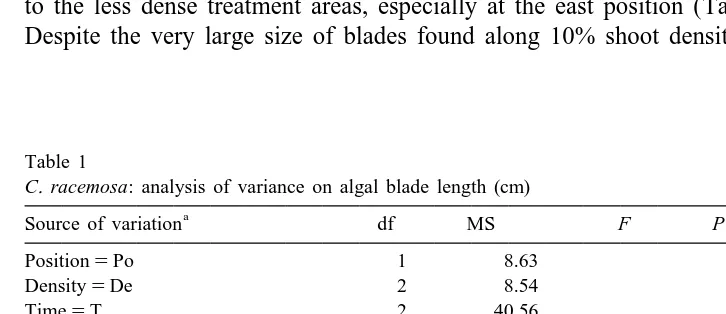
Table 1
C. racemosa: analysis of variance on algal blade length (cm) a
Source of variation df MS F P
Position5Po 1 8.63
Density5De 2 8.54
Time5T 2 40.56
Area (Po3De)5A 12 2.75
Plot (A(Po3De))5Pl 36 1.04
Po3De 2 6.13
Po3T 2 4.59
De3T 4 0.85
T3A(Po3De) 24 0.39 0.58 0.9317
T3Pl (A(Po3De)) 72 0.68 3.21 0.0000*
Po3De3T 4 1.40 3.53 0.0209*
Error 162 0.21
Cochran’s test C50.0638 P.0.05
a
Po5position, De5density, T5time, A5area and Pl5plot. *
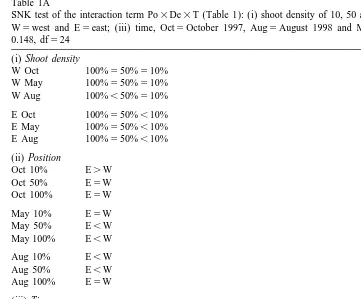
Table 1A
SNK test of the interaction term Po3De3T (Table 1): (i) shoot density of 10, 50 and 100%; (ii) position, W5west and E5east; (iii) time, Oct5October 1997, Aug5August 1998 and May5May 1998, SE5
0.148, df524 (i) Shoot density
W Oct 100%550%510%
W May 100%550%510%
W Aug 100%,50%510%
E Oct 100%550%,10%
E May 100%550%,10%
E Aug 100%550%,10%
(ii) Position
Oct 10% E.W
Oct 50% E5W
Oct 100% E5W
May 10% E5W
May 50% E,W
May 100% E,W
Aug 10% E,W
Aug 50% E,W
Aug 100% E5W
(iii) Time
W 10% AUG.MAY5OCT
W 50% AUG.MAY5OCT
W 100% AUG.MAY5OCT
E 10% AUG.MAY5OCT
E 50% AUG.OCT.MAY
E 100% AUG.OCT.MAY
position, taller blades were found at the west position with respect to the east either in August or May, (10 vs. 50% and 50 vs. 100% densities, respectively). Blade length was significantly greater in August relative to October and May of all position–density combinations, except at the east edge of the seagrass where the short-term effect (October 97) of higher densities (50 and 100%) was intermediate than the longer-term one (May 98).
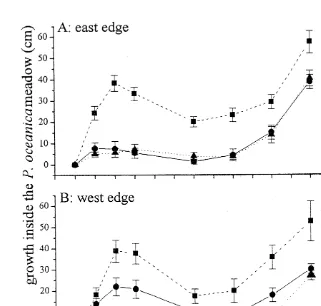
Fig. 2. Temporal variation of mean (6SE) Caulerpa racemosa stolon penetration or growth (cm) into the
Posidonia oceanica meadow to treatments of 10, 50 and 100% of shoot density at the east and west edge of the
seagrass meadow (n59).
penetration was higher (53%) at the west position where shoot density was 50% (Fig. 2). However, stolon elongation of the alga was highly dependent on the experimental area. Percent cover of Caulerpa racemosa in the experimental units was significantly
Table 2
C. racemosa: analysis of variance on penetration distance of stolons into the meadow (cm)
Source of variation df MS F P
Position5Po 1 1075.57 1.67 0.2205
Density5De 2 2634.02 4.09 0.0442*
Area(Po3De)5A 12 643.96 5.79 0.0000*
Po3De 2 82.57 0.13 0.8808
Error 36 111.29
Cochran’s test C50.276 P.0.05
*
Table 3
C. racemosa: analysis of variance on algal percent cover in each experimental unit
Source of variation df MS F P
Position5Po 1 146.61 0.46 0.5080
Density5De 2 462.84 1.47 0.2685
Area(Po3De)5A 12 314.91 8.58 0.0000
Po3De 2 609.02 1.93 0.1870
Error 18
Cochran’s test C50.318 P.0.05
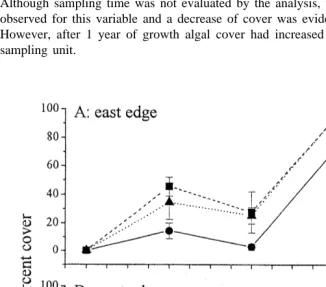
affected by the characteristics of the area and not by other sources of variation (Table 3). Although sampling time was not evaluated by the analysis, temporal fluctuations were observed for this variable and a decrease of cover was evident during winter (Fig. 3). However, after 1 year of growth algal cover had increased to more than 80% of the sampling unit.
Fig. 3. Temporal variation of mean (6SE) percent cover of the experimental unit substrate by Caulerpa
racemosa at the edge of defoliated Posidonia oceanica (10, 50 and 100% of shoot density) at the east and west
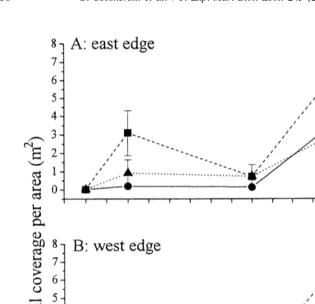
Fig. 4. Temporal variation of mean (6SE) total coverage of the experimental area substrate by Caulerpa
racemosa at the edge of defoliated Posidonia oceanica (10, 50 and 100% of shoot density) at the east and west
edge of the seagrass meadow (n53).
The estimation of total area of substrate affected by Caulerpa racemosa in each experimental area has shown a substantial growth after 9 months of study from spring to
2
summer 1998, since coverage reached more than 6 m per experimental area (Fig. 4). The effect of neither position nor density was highlighted by the analysis (Table 4).
Table 4
2
C. racemosa: analysis of variance on algal total cover per experimental area (m )
Source of variation df MS F P
Position5Po 1 7.220 2.248 0.1596
Density5De 2 9.757 3.038 0.0855
Po3De 2 1.145 0.35 0.7073
Error 12 3.211
4. Discussion
The results of this study indicated that where Caulerpa racemosa is at the edge of Posidonia oceanica (1) the vertical growth of the alga (blade length) is sensitive to the combination of time, seagrass density and location on the east or western edges of the meadow (2) the spread of the alga along the edge of the seagrass meadow is characterised by high degree of spatial heterogeneity and (3) the growth of the alga inside the meadow is greatly influenced by seagrass density.
The difference in size of the alga depending on time and seagrass habitat manipula-tions suggests that the effects of Posidonia oceanica canopy on algal growth are contrasting and variable on time, and / or that they change depending on variable algal requirements. However, further experiments will be necessary to investigate whether the
vertical growth of Caulerpa racemosa has the potential to affect P. oceanica in the
Mediterranean. In fact, the ability of the alga to grow at the edge of the seagrass meadow forming stratified mats of stolons and blades on longer terms (even 15 cm high) and the observation that in proximity of those mats seagrass leaves become yellow (Piazzi, personal observation), suggest that the vertical algal growth is likely to exert a
negative effect on P. oceanica. However, mechanistic explanations of how seagrass
canopy conditions affect algal performance were beyond the scope of the study. The pattern of colonisation has been found to be highly influenced by spatial heterogeneity suggesting that the horizontal growth of Caulerpa racemosa is affected by processes that change from one area to another. Many factors can determine this small scale variability: the texture of the substratum, nutrient supply and the presence of turf forming algae, are likely to be important regulators of the performance of the alga in the Mediterranean. This result suggest that appropriately well-designed experiments (at different spatial scale), that isolate other sources of variation, are needed in order to completely elucidate causes affecting the response of C. racemosa at different environmental conditions in the Mediterranean.
Growth of Caulerpa racemosa inside the seagrass meadow was greatly affected by seagrass density. Overall, at the end of the experiment, stolon elongation of the alga was higher than the plot size and measures were taken inside the natural meadow. Given the moderate size (only 25 cm large in the meadow) of experimental units, the experiment tested whether the condition of the seagrass edge can determine a different invasibility of the meadow. In the first period of high production (Autumn 1997), C. racemosa had grown inside the unmanipulated seagrass meadow only where shoot density was of 10% with respect to the natural density. However, this difference in colonisation of the meadow decreased through the experiment, especially at the east position.
seagrass condition. In general, initial plant size has been evidenced as an important regulator of species assemblages in many systems (Tilman, 1990; Gerry and Wilson,
1995): in this study P. oceanica density may be invoked as an important factor
controlling the distribution and the composition of understoried C. racemosa as well as of other vegetation assemblages (e.g. Caulerpa taxifolia, Ceccherelli and Cinelli, 1998, 1999).
In general, communities can be said to be invasible when an introduced species is able to establish and persist or expand (Burke and Grime, 1996). As suggested by Crawley
`
(1987) and Rejmanek (1989), a dense cover of established indigenous species can be a major factor reducing the probability of successful invasion. This would be consistent with the finding of this study which suggest that the susceptibility of the indigenous Posidonia oceanica community to invasion of the introduced alga Caulerpa racemosa was related to the availability of sand habitat ground created, since low invasion of very dense edges of the seagrass was observed with respect to the less dense ones.
Acknowledgements
We are sincerely thankful to Davide Campo for valuable help at the field and Ferruccio Maltagliati for comments on the manuscript. Funds for this study were provided by European Community (research program EU-DG XI-LIFE) which also
supported by contract G.C. and L.P. while writing the paper. [AU]
References
Aleem, A.A., 1948. The recent migration of certain Indopacific Algae from the Red Sea into the Mediterranean. New Phytol. 47, 88–94.
Alongi, G., Cormaci, M., Furnari, G., 1993. Prima segnalazione di Caulerpa racemosa (Chlorophyceae Caulerpales) per le coste Italiane. Boll. Acc. Gioenia Sci. Nat. 26, 49–53.
Bell, J.D., Westoby, M., 1986a. Variation in seagrass height and density over a wide spatial scale: effects on common fish and decapods. J. Exp. Mar. Biol. Ecol. 104, 275–295.
Bell, J.D., Westoby, M., 1986b. Abundance of macrofauna in dense seagrass is due to habitat preference, not predation. Oecologia 68, 205–209.
´
Boudouresque, C.F., Meinesz, A., Verlaque, M., Knoeppffler-Peguy, M., 1992. The expansion of the tropical alga Caulerpa taxifolia (Chlorophyta) in the Mediterranean. Cryptogamie Algol. 13, 144–145.
Boudouresque, C.F., Meinesz, A., Ribera, M.A., Ballesteros, E., 1994. Spread of the green alga Caulerpa
taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: possible consequences of a major ecological
event. Sci. Marina 59 (1), 21–29.
Burke, M.J.W., Grime, J.P., 1996. An experimental study of plant community invasibility. Ecology 77, 776–790.
Carlton, J.T., Geller, J.B., 1993. Ecological roulette: the global transport of nonindigenous marine organisms. Science 261, 78–82.
Ceccherelli, G., Cinelli, F., 1997. Short-term effects of nutrient enrichment of the sediment and interactions between the seagrass Cymodocea nodosa and the introduced green alga Caulerpa taxifolia in a Mediterranean bay. J. Exp. Mar. Biol. Ecol. 217 (2), 165–177.
Ceccherelli, G., Cinelli, F., 1999. Effects of Posidonia oceanica canopy on Caulerpa taxifolia size in a North-Western Mediterranean Bay. J. Exp. Mar. Biol. Ecol. 240 (1), 19–36.
Chambers, P.A., Barko, J.W., Smith, C.S., 1993. Evaluation of invasions and declines of submersed aquatic macrophytes. J. Aquat. Plant Manag. 31, 218–220.
Connolly, R.M., Butler, A.J., 1996. The effects of altering seagrass height on small, motile invertebrates of shallow Mediterranean embayments. P.S.Z.N.I.: Marine Ecol. 17 (4), 637–652.
Crawley, M.J., 1987. What makes a community invasible? In: Crawley, M.J., Edwards, P.J., Gray, A.J. (Eds.), Colonisation, Succession and Stability, Blackwell Scientific, Oxford, pp. 429–454.
Cuny, P., Serve, L., Jupin, H., Boudouresque, C.F., 1995. Water soluble phenolic compounds of the marine phanerogam Posidonia oceanica in a Mediterranean area colonised by the introduced chlorophyte Caulerpa
taxifolia. Aquat. Bot. 52, 237–242.
Davis, A.R., Roberts, D.E., Cummins, S.P., 1997. Rapid invasion of a sponge-dominated deep-reef by
Caulerpa scalpelliformis (Cholorophyta) in Botany Bay, New South Wales. Aust. J. Ecol. 22, 146–150.
`
de Villele, X., Verlaque, M., 1995. Changes and degradation in a Posidonia oceanica bed invaded by the introduced tropical alga Caulerpa taxifolia in the north western Mediterranean. Bot. Mar. 38, 79–87. Den Hartog, C., 1997. Is Sargassum muticum a threat to eelgrass beds? Aquat. Bot. 58, 37–41. Elton, C.S., 1958. The Ecology of Invasions By Animals and Plants, Methuen, London.
Foster, M.S., Harrods, C., Hardin, D.D., 1991. Point vs. photo quadrat estimates of the cover of sessile marine organisms. J. Exp. Mar. Biol. Ecol. 146, 193–203.
Gambi, M.C., Buia, M.C., Casola, E., Scaedi, M., 1989. Estimates of water movement in Posidonia oceanica beds: a first approach. In: Boudouresque, C.F., Meinesz, A., Fresi, E., Gravez, V. (Eds.), International Workshop On Posidonia Beds. Gis Posidonie 2, pp. 101–112.
Gambi, M.C., Nowell, A.R.M., Jumars, P.A., 1990. Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Mar. Ecol. Prog. Ser. 61, 159–169.
Gerry, A.K., Wilson, S.D., 1995. The influence of initial size on the competitive responses of six plant species. Ecology 76 (1), 272–279.
Giraud, G., 1977. Essai de classament des herbiers de Posidonia oceanica (L.) Delile. Bot. Mar. 20 (8), 487–491.
´ ´
Hamel, G., 1926. Quelques algues rares ou nouvelles pour la flore mediterraneenne. Bull. Mus. Nat. His. Nat. Paris 32 (6), 420.
Hobbs, R.J., Atkins, L., 1988. Effect of disturbance and nutrient addition on native and introduced annuals in plant communities in the Western Australian wheatbelt. Aust. J. Ecol. 13, 171–179.
Lipkin, Y., 1972. Contribution to the knowledge of Suez canal migration. Marine algal and seagrass flora of the Suez canal. Israel J. Zool. 21, 405–446.
Lodge, D.M., 1993. Biological invasions: lessons for ecology. Trends Ecol. Evol. 8, 133–137.
Meinesz, A., de Vaugelas, J., Hesse, B., Mari, X., 1993. Spread of the introduced tropical green alga Caulerpa
taxifolia in northern Mediterranean waters. J. Appl. Phycol. 5, 141–147.
Orth, R.J., 1992. A perspective on plant–animal interactions in seagrasses: physical and biological deter-minants influencing plant and animal abundance. In: John, D.M., Hawkins, S.J., Price, J.H. (Eds.), Plant–animal Interactions in the Marine Benthos, Clarendon, Oxford, pp. 147–164.
Piazzi, L., Cinelli, F., 1999. Development and seasonal dynamics of a population of the tropical alga Caulerpa
˚
racemosa (Forsskal) J. Agardh in the Mediterranean. Cryptogamie, Algol. in press.
Piazzi, L., Balestri, E., Cinelli, F., 1994. Presence of Caulerpa racemosa in the North-Western Mediterranean. Cryptogamie, Algol. 15, 183–189.
Piazzi, L., Acunto, S., Magri, M., Rindi, F., Balestri, E., 1997a. Preliminary observations on the spread of
˚
Caulerpa racemosa (Forsskal) J. Agardh in Meloria Shoals (Livorno, Italy). Biol. Mar. Medit. 4, 426–428.
Piazzi, L., Balestri, E., Magri, M., Cinelli, F., 1997b. Expansion de l’Algue Tropicale Caulerpa racemosa
˚ ˆ
(Frosskal) J. Agardh (Bryopsidophyceae, Chlorophyta) le long de la cote toscane (Italie). Cryptogamie, Algol. 18, 343–350.
Posey, M.H., 1988. Community changes associated with the spread of an introduced seagrass Zostera
japonica. Ecology 69, 974–983.
´
Rejmanek, M., 1989. Invasibility of plant communities. In: Drake, J., Di Castri, F., Groves, R., Kruger, F., ´
´
Rejmanek, M., Richardson, D.M., 1996. What attributes make some plant species more invasive? Ecology 77 (6), 1655–1661.
Reusch, T.B.H., 1998. Differing effects of eelgrass Zostera marina on recruitment and growth of associated blue mussels Mytilus edulis. Mar. Ecol. Prog. Ser. 167, 149–153.
Reusch, T.B.H., Williams, S.L., 1998. Variable responses of native eelgrass Zostera marina to a non-indigenous bivalve Musculista senhousia. Oecologia 113, 428–441.
Ribera, M.A., Boudouresque, C.F., 1995. Introduced marine plants, with special reference to macroalgae: mechanisms and impact. In: Round, F.E., Chapman, D.J. (Eds.), Progress in Phycological Research, Biopress, Bristol, pp. 187–267.
Richardson, D.M., Bond, W.J., 1991. Determinants of plant distribution: evidence from pine invasions. Am. Nat. 137, 639–668.
Stapanian, M.A., Sundberg, S.D., Baumgardner, G.A., Liston, A., 1998. Alien plant species composition and associations with anthropogenic disturbance in North American forests. Plant Ecol. 139, 49–62. ´
Thebaud, C., Finzi, A.C., Affre, L., Debussche, M., Escarre, J., 1996. Assessing why two introduced Conyza differ in their ability to invade Mediterranean old fields. Ecology 77 (3), 791–804.
Tilman, D., 1990. Mechanisms of plant competition for nutrients: the elements of a predictive theory of competition. In: Grace, J.B., Tilman, D. (Eds.), Perspectives On Plant Competition, Academic Press, San Diego, CA, pp. 117–141.
Trowbridge, C.D., 1995. Establishment of the green alga Codium fragile ssp. Tomentosoides on New Zealand rocky shores: current distribution and invertebrate grazers. J. Ecol. 83, 949–965.
´ ´ ´
Verlaque, M., 1994. Inventaire des plantes introduites en Mediterranee: origines et repercussions sur ´
l’environnement et les activites humaines. Oceanologica acta 17, 1–23. `
Vitousek, P.M., D’Antonio, C.M., Loope, L.L., Rejmanek, M., Westbrooks, M., 1997. Introduced species: a significant component of human-caused global change. NZ J. Ecol. 21, 1–16.
Walker, D.I., Kendrick, G.A., 1998. Threats to macroalgal diversity: marine habitat destruction and fragmentation, pollution and introduced species. Bot. Mar. 41, 105–112.