Summary Effects of flurprimidol on plant water relations and leaf gas exchange were investigated in one-year-old white ash (Fraxinus americana L.)seedlings subjected to soil water deficits. Flurprimidol (20 mg kg−1 of soil equivalent) was applied to the soil surface of pot-grown seedlings after shoot growth was completed. Two months after flurprimidol appli-cation, water was withheld from one-half of the seedlings. Leaf water relations and gas exchange parameters were measured 5, 7, 10, 14, 18 and 22 days after withholding water. Under both irrigated and nonirrigated conditions, flurprimidol treatment resulted in reduced net CO2 assimilation rate and
transpira-tional water loss of seedlings as a result of decreased stomatal conductance. Consequently, flurprimidol-treated seedlings had higher leaf water potential and relative water content than untreated seedlings. Nonirrigated flurprimidol-treated seed-lings also had greater turgor and sap osmolality and lower osmotic potential at full turgor than seedlings in the other treatments, indicating that flurprimidol increased osmotic ad-justment. Under water-stress conditions, water use efficiency was lower and gas exchange efficiency was higher in flurprimi-dol-treated seedlings than in untreated seedlings, suggesting that flurprimidol treatment enhances survival of plants sub-jected to soil water deficits.
Keywords: osmotic adjustment, plant growth regulators, water stress.
Introduction
Several plant growth regulators (PGRs) are used to suppress shoot growth in horticultural crops and urban trees (Davis and Curry 1991). In addition to controlling growth, PGRs also increase root/shoot ratios (Early and Martin 1988, Numbere et al. 1992), decrease stomatal conductance (Armitage et al. 1984, DeJong and Doyle 1984, Steinberg et al. 1991a), in-crease root hydraulic conductivity and xylem pressure poten-tial (Vaigro-Wolff and Warmund 1987, Rieger and Scalabrelli 1990) and decrease plant water use by reducing transpiration (Atkinson and Chauhan 1987, Schuch 1994). Because all of these effects tend to make plants more drought tolerant and improve their water use efficiency, Frymire and Henderson-Cole (1992) suggested that treatment of container-grown
nurs-ery stock with a growth inhibitor may improve plant survival and quality at times when water is limited.
Flurprimidol (α-(1-methylethyl)-α-[4-(trifluoromethoxy) phenyl]-5-pyrimidine-methanol), a gibberellin synthesis in-hibitor (Rademacher 1991), is used to control shoot growth in trees. Flurprimidol and other pyrimidine PGRs modify the growth of plants by causing shorter internodes and smaller leaves (Davis and Curry 1991). In addition to interfering with gibberellin biosynthesis, pyrimidine compounds also promote the biosynthesis of abscisic acid (ABA) (Cowan and Railton 1987, Grossmann 1992). Both abscisic acid and gibberellins are synthesized via the isoprenoid pathway, and the two plant hormones often exhibit opposing physiological activities (Loveys and Milborrow 1984). The role of ABA in stomatal functioning and plant water relations is well documented (Ko-zlowski et al. 1991). Although the growth regulating effects of flurprimidol have been investigated in several herbaceous spe-cies, little is known about its effects on the physiological processes of tree species. Therefore, we have studied the ef-fects of flurprimidol on the plant water relations, leaf gas exchange, and water use efficiency of irrigated and nonirri-gated white ash (Fraxinus americana L.) seedlings to test the hypothesis that flurprimidol treatment enhances plant toler-ance to water stress.
Materials and methods
One-year-old seedlings of white ash were obtained from the Indiana State Tree Nursery and graded to uniform size. On April 10, 1994, each of 100 seedlings was planted in a 15.2-li-ter plastic pot containing a 7/3/2 (v/v/v) mix of top soil/peat moss/perlite amended with (per m3): 680 g Ca(H2PO4)2, 454 g
KNO3, 454 g MgSO4, 3.6 kg ground limestone and 57 g fritted
trace elements. The seedlings were grown in a greenhouse at Purdue University, West Lafayette, Indiana, USA, in a natural photoperiod at a maximum day/minimum night temperature of approximately 28/23 °C until terminal shoot growth was com-pleted and the leaves were fully expanded.
After leaf growth ceased in early June, one half of the seedlings were randomly selected for flurprimidol treatment. On June 14, 1994, flurprimidol was applied to the soil at the rate of 20 mg kg−1 of soil (312 mg flurprimidol per15.6 kg soil in each pot). No additional leaf flushes occurred in either
Gas exchange and water relations of
Fraxinus americana
affected by
flurprimidol
GNANASIRI S. PREMACHANDRA, WILLIAM R. CHANEY and HARVEY A. HOLT
Department of Forestry and Natural Resources, Purdue University, West Lafayette, IN 47907, USA
Received August 30, 1995
flurprimidol-treated (flur(+)) or untreated (flur(−)) seedlings. On August 11, 1994, two months after flurprimidol applica-tion, one half of the flurprimidol-treated and untreated seed-lings were randomly selected for water stress treatment. They were not watered again for the 22-day duration of the study. The other half of the treated and untreated seedlings continued to receive regular watering to resaturation every other day and served as irrigated controls.
Leaf water relations and gas exchange parameters were measured to compare flurprimidol-treated and untreated seed-lings under irrigated and nonirrigated conditions 5, 7, 10, 14, 18 and 22 days after water was withheld. Before dawn on the day of measurement, 20 seedlings (five replicates from each of the four treatments) were moved to a large growth chamber where sampling could be carried out under conditions of uni-form light, temperature and humidity. Light from six 1000-W high-pressure sodium vapor lamps (Energy Technics, York, PA) was filtered through a 3-cm layer of water, providing an incident photosynthetic photon flux to the sample leaves of approximately 900 µmol m−2 s−1. Temperature and relative humidity were 25 ± 2 °C and 40 ± 5%, respectively, and ambient CO2 concentration was 340--360 µl l−1. Plants were
equilibrated under these conditions for 3 h before measure-ments were initiated.
Measurement of leaf gas exchange
Net CO2 assimilation rate (A), stomatal conductance (gs),
tran-spiration rate (E) and internal CO2 concentration (Ci) were
measured between 1000 and 1200 h on two leaflets of the two uppermost, fully expanded leaves with an ADC portable pho-tosynthetic and transpiration measurement system (Analytical Development Company Limited, Hoddesdon, England). In-stantaneous water use efficiency (WUE) was calculated as the ratio of A/E (mmol mol−1). Intrinsic gas exchange efficiency (GEE) was calculated as the ratio of A/gs (µmol mol−1).
Measurements of leaf water potential, osmotic potential and relative water content
Discs (0.5-cm diameter) were cut from the same leaflets used for gas exchange measurement and immediately put in thermo-couple psychrometer sample chambers (Wescor C-52; Wescor Inc., Logan, UT) and allowed to equilibrate for 4 h. Leaf water potential (Ψw) was then measured psychrometrically with a
microvoltmeter. The Ψw values were accurate to ± 0.05 MPa
and were comparable to Ψw values measured with a pressure
chamber. Afterwards, the leaf discs were frozen and thawed in the psychrometer sample chambers and their osmotic potential (Ψπ) was measured psychrometrically. Leaf Ψπ was corrected for the dilution of symplastic sap by apoplastic water, which occurs when cell walls are broken during freezing and thawing of leaf discs (Tyree 1976); a constant apoplastic fraction of 0.16 was assumed (Parker and Pallardy 1987). Turgor (Ψp) was
calculated by subtracting the corrected Ψπ from Ψw. Relative
water content (RWC) was computed as RWC = (FW--DW)/ (TW--DW) (where FW = fresh weight, DW = dry weight and TW = turgid weight), using 1-cm-diameter discs excised from leaflets of the same two leaves used for previous
measure-ments. Osmotic potential at full turgor (Ψπ(100)) was calculated
as Ψπ(100) = Ψπ (RWC − AWC)/(1.0 − AWC), where AWC is the
apoplastic water content (Wilson et al. 1979). Osmotic adjust-ment (OA) was estimated as the difference between the Ψπ(100)
values estimated in leaves of irrigated and nonirrigated plants.
Measurement of sap osmolality
Several leaflets from the two leaves that were used for gas exchange and water relations measurements were frozen in sealed polyethylene freezer bags. Leaf samples were thawed and centrifuged at 1000 g for 20 min at 6--8 °C to extract cell sap. Sap osmolality was measured with a Wescor Model 5100C vapor pressure osmometer (Wescor Inc.) and was cor-rected for dilution of symplastic sap by apoplastic water.
Statistical analyis
Data were subjected to analysis of variance. For the first four measurement periods, a 4 × 2 × 2 × 5 factorial design was used, including four sampling periods, two flurprimidol treatments, and two watering treatments with five replications. Because many of the nonirrigated flur(−) seedlings died between sam-pling days 14 and 18, no measurements were made of seed-lings in this treatment for sampling days 18 and 22. Data for flur(+), nonirrigated seedlings were analyzed in a 6 × 5 com-pletely randomized block design with six sampling periods and five replications. The least significant difference (LSD) at the 0.05 level was calculated to determine differences among means. Regression analyses of the various parameters meas-ured as a function of leaf Ψw were performed and best fit curves
plotted.
Results
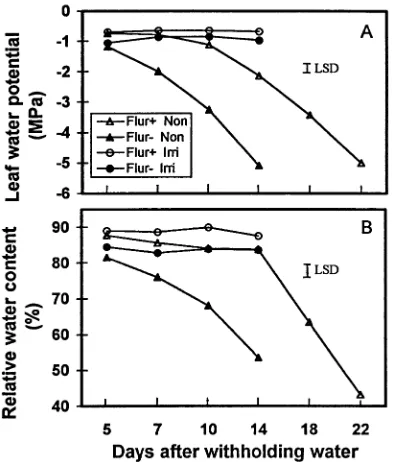
Figures 1 and 2 illustrate changes in leaf Ψw, RWC, Ψp, Ψπ(100),
and OA of flurprimidol-treated (flur(+)) and untreated (flur(−)) white ash seedlings under irrigated and nonirrigated conditions over time. For the first 14 days after withholding water, leaf Ψw
was higher in flur(+) seedlings than in flur(−) seedlings under both irrigated and nonirrigated conditions (Figure 1A). Leaf Ψw of irrigated seedlings was 0.21 to 0.36 MPa higher in the
flur(+) treatment than in the flur(−) treatment during this period. In the nonirrigated treatments, Ψw decreased more
rapidly in flur(−) seedlings than in flur(+) seedlings (−5.09 versus −2.13 MPa on Day 14) (Figure 1A). Leaf Ψw of
nonir-rigated flur(+) seedlings reached −3.43 and −5.00 MPa 18 and 22 days after withholding water, respectively. Leaves for which a Ψw value of −5 MPa was measured were not severely
wilted, presumably because of their high cellulose content, but the leaf margins and small areas of the leaves were beginning to turn brown.
Under both irrigated and nonirrigated conditions, turgor was significantly greater in flur(+) seedlings than in flur(−) seed-lings, except in irrigated seedlings on Days 7 and 14 (Figure 2A). Irrigated flur(+) seedlings exhibited a Ψp of 2.01 to 2.25
MPa compared with 1.69 to 2.13 MPa for irrigated flur(−) seedlings. Turgor of nonirrigated flur(−) seedlings decreased with time and reached zero at Day 14. Nonirrigated flur(+) seedlings maintained higher Ψp at lower leaf Ψw and for a
longer time than flur(−) seedlings, exhibiting a Ψp of 0.4 MPa
even after water was withheld for 22 days (Figures 2A and 3A). In both flur(−) and flur(+) seedlings, withholding water for 14 days resulted in a significant decrease in Ψπ(100)
(Fig-ure 2B). Osmotic potential at full turgor of nonirrigated flur(+) seedlings decreased by 0.6 MPa from Days 5 to 18. At Day 22, Ψπ(100) of nonirrigated flur(+) seedlings was 0.2 MPa above
that at Day 5 (Figure 2B). Nonirrigated flur(+) seedlings main-tained a lower Ψπ(100) at a given leaf Ψw than flur(−) seedlings,
except at Ψw values below −5 MPa (Figure 3B).
In the nonirrigated treatments, osmotic adjustment was evi-dent in both flur(−) and flur(+) seedlings (Figure 2C). The greater osmotic adjustment in flur(−) seedlings than in flur(+) seedlings at 10 and 14 days after withholding water was a result of exposure of flur(−) seedlings to a higher degree of water stress as measured by Ψw. Sap osmolality was greater in
flur(+) seedlings than in flur(−) seedlings over a wide range of leaf Ψw values (Figure 3C).
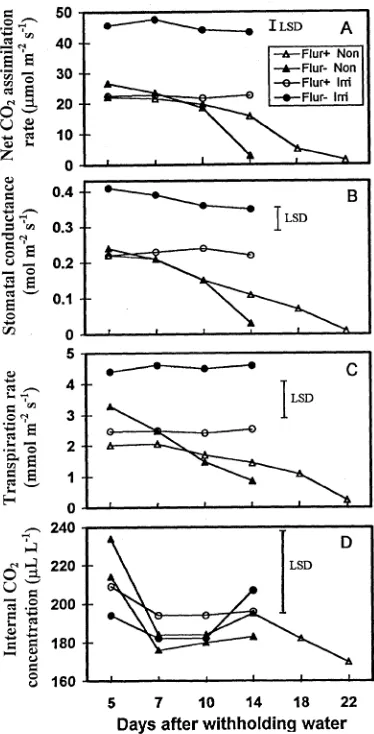
The effects of irrigation treatment on A and gs were observed
as soon as 5 days after withholding water in flur(−) seedlings; however, in flur(+) seedlings, the effects were observed only during the later stages of stress development (Figures 4A and 4B). For example, in flur(+) seedlings, there were no
differ-ences in A and gs between nonirrigated and irrigated seedlings
until Days 14 and 10, respectively. Net CO2 assimilation in
irrigated flur(+) seedlings was about 50% of that in irrigated flur(−) seedlings. Similarly, gs was lower in irrigated flur(+)
seedlings than in irrigated flur(−) seedlings. In flur(−) seed-lings, both A and gs decreased rapidly with increasing water
stress and reached values that were 87 and 89% lower on Days 5 and 14, respectively, than in irrigated flur(−) seedlings. Net CO2 assimilation rate in nonirrigated flur(+) seedlings
de-clined by 93%, but only after withholding water for 22 days. Likewise, stomatal conductance in nonirrigated flur(+) lings declined by 95% between Days 5 and 22. Flur(+) seed-lings exhibited lower A and gs than flur(−) seedlings over a
wide range of leaf Ψw values (Figures 5A and 5B).
Transpiration rate was lower in nonirrigated than in irrigated seedlings in both flur(+) and flur(−) treatments at all sampling periods up to 14 days after withholding water (Figure 4C). Flurprimidol reduced E by 45% in irrigated seedlings. In nonirrigated seedlings, flur(+) seedlings exhibited a lower E than flur(−) seedlings up to 7 days after withholding water, and thereafter, flur(+) seedlings exhibited a higher E than flur(−) seedlings. Transpiration rates of nonirrigated flur(+) seedlings on Days 18 and 22 were approximately 53 and 12%, respec-tively, of those on Day 5. Nonirrigated flur(+) seedlings had a lower E than flur(−) seedlings over a wide range of leaf Ψw
Figure 1. Leaf water potential (A) and relative water content (B) of flurprimidol-treated (Flur+) and untreated (Flur−) white ash seedlings grown in irrigated (Irri) or nonirrigated (Non) conditions at 5, 7, 10, 14, 18 and 22 days after withholding water. The LSD is at 0.05 level.
values (Figure 5C). Neither the flurprimidol treatments nor the irrgation treatments affected Ci (Figure 4D).
The effects of a range of leaf Ψw values on water use
efficiency and gas exchange efficiency of flur(+) and flur(−) white ash seedlings are shown in Figure 6. Water use efficiency of flur(+) seedlings was lower than that of flur(−) seedlings at leaf Ψw values ≤−2 MPa, except at Ψw = −5 MPa, where they
were similar. Flur(+) seedlings had a higher WUE than flur(−) seedlings at high leaf Ψw (−1 MPa). In contrast, GEE was
significantly greater in flur(+) seedlings than in flur(−) seed-lings only when leaf Ψw was less than −2 MPa.
Discussion
Flurprimidol decreased stomatal conductance in white ash seedlings even when soil water was not limiting. On all meas-urement days, stomatal conductance in irrigated flur(+) seed-lings was lower than in irrigated flur(−) seedlings, resulting in lower A and E. In the nonirrigated treatments, gs of flur(+)
seedlings was only reduced below that of flur(−) seedlings
14 days after withholding water. Nonirrigated flur(+) seed-lings had higher Ψw, Ψp, and leaf RWC than nonirrigated
flur(−) seedlings, and only the flur(+) seedlings survived for more than 14 days after water was withheld, presumably as a result of reduced E, reduced plant water use, and increased turgor maintenance. Osmotic adjustment occurred in flur(−) seedlings on Days 10 and 14, and occurred in flur(+) seedlings on Day 14, as indicated by decreased Ψπ(100) and increased sap
osmolality.
The values of Ψw (−5 MPa) determined for leaves of
nonir-rigated seedlings are low compared with published values. The leaves of most vascular plants are irreversibly damaged at such low water potentials as a result of mechanical tearing of proto-plasm and degradation of membranes under the extreme ten-sions developed within cells (Gaff 1980). For example, Cleary and Zaerr (1980) reported that vigor of ponderosa pine seed-lings declined progressively between Ψw values of −2 and
−5 MPa, and the seedlings died when Ψw reached −5 MPa. Figure 3. Regression of turgor (A), osmotic potential at full turgor (B)
and sap osmolality (C) as functions of leaf water potential in flurprimi-dol-treated (Flur+) and untreated (Flur−) white ash seedlings. Vari-ables were measured in leaves of container-grown seedlings during a 22-day period in which water was withheld. The Flur+ and Flur−
There are few studies on the effects of flurprimidol or other pyrimidine growth inhibitors on plant water relations and gas exchange. Pyrimidine growth inhibitors reduce water use (Johnson 1974, Barrett and Nell 1981), and the reduction is partially related to the smaller leaf area of the treated plants (Davis et al. 1986, Vaigro-Wolff and Warmund 1987, Sterrett et al. 1989). Barrett (1983) reported a reduction of 35 and 39% in total plant transpiration in poinsettias treated with EL-500 (flurprimidol) and ancymidol, respectively, whereas on a per leaf basis the difference in transpiration was only 12% for both compounds.
High root/shoot ratios are normally observed in PGR-treated plants (Early and Martin 1988, Numbere et al. 1992), and could lead to improved water absorption and water avail-ability to shoots (Steinberg et al. 1991b). However, in the present study, the flurprimidol treatment was applied after terminal shoot growth was completed and leaves were fully matured. Hence, there was no new shoot growth from the time of flurprimidol treatment to the date of the final measurements.
Accumulation of osmotic solutes may have contributed to the increase in sap osmolality and the decrease in Ψπ(100) in the
nonirrigated flur(+) seedlings (Figures 3B and 3C). Osmotic adjustment usually develops in plants that are stressed slowly; however, it is not detectable in all plant species and is less common in woody plants than in herbaceous plants (Kramer 1983, Kozlowksi et al. 1991). The effects of flurprimidol on osmoregulation or solute accumulation are not known, and studies on solute potential and solute accumulation in plants treated with paclobutrazol, a triazole growth inhibitor, have yielded conflicting results. Mature leaves of paclobutrazol-treated apple seedlings had lower solute potentials than mature leaves of untreated controls, but no differences in Ψp were
observed (Swietlik and Miller 1983). Increased concentrations of non-structural carbohydrates were found in tissues of paclobutrazol-treated apple seedlings (Wang et al. 1985) and paclobutrazol-treated apple trees (Wieland and Wample 1985). However, Vu and Yelenosky (1992) observed lower concentra-tions of soluble sugars in paclobutrazol-treated sweet orange plants than in untreated controls.
Flurprimidol treatment reduced A, probably as a result of the reduction in gs. There are conflicting conclusions about the
effect of flurprimidol and other growth inhibitors on A and gs
depending on whether results are expressed for an entire plant, normalized on a leaf area basis, or expressed as a ratio in terms of water use efficiency (Marquard 1985, Wieland and Wample 1985, Davis and Sankhla 1986, Sterrett et al. 1989, Rieger and Scalabrelli 1990, Steinberg et al. 1991a, Frymire and
Hender-Figure 5. Regression of net CO2 assimilation rate (A), stomatal con-ductance (B) and transpiration rate (C) as functions of leaf water potential in flurprimidol-treated (Flur+) and untreated (Flur−) white ash seedlings. Variables were measured in leaves of container-grown seedlings during a 22-day period in which water was withheld. The Flur+ and Flur− treatment effects were significantly different (P = 0.05).
son-Cole 1992, Vu and Yelenosky 1992, Zhou and Xi 1993). Cathey (1975) observed that ancymidol arrested stomatal opening, but only at a dosage much higher than that adequate for growth control. The concentration of flurprimidol applied in this study (1.0 mM, or 312 mg per pot) is slightly less than the dose recommended for soil application of a similar growth inhibitor to white ash trees.
In other studies, PGR treatment reduced E and water use (Atkinson and Chauhan 1987, Schuch 1994). Total water use was 33% lower in uniconazole-treated hibiscus plants than in control plants; however, WUE was lower in treated plants than in control plants (Steinberg et al. 1991b). We also observed lower WUE (A per unit of H2O transpired) in flur(+) seedlings
than in flur(−) seedlings at leaf Ψw values in the range of −3 to
−5 MPa (Figure 6A); however, GEE (A per unit of gs) was
greater in flur(+) seedlings than in flur(−) seedlings at leaf Ψw
values less than −2 MPa (Figure 6B). A similar response was observed in paclobutrazol-treated nectarine trees and was ac-companied by increases in Ci and mesophyll conductance
(DeJong and Doyle 1984), suggesting an increase in meso-phyll (intracellular) conductance for CO2. In contrast, we
found no significant difference in Ci between flur(+) and flur(−)
seedlings under either irrigated or nonirrigated conditions. The discrepancy between these results may be associated with the determination of Ci. In the conventional calculation of Ci, it is
assumed that photosynthesis and transpiration are uniform throughout a leaf. However, as discussed by Terashima et al. (1988), it cannot be assumed that the stomatal response is uniform over the leaf. Furthermore, variation in stomatal aper-ture could increase as leaf water stress develops.
The decrease in stomatal conductance in response to flur-primidol treatment may be mediated through its effect on ABA metabolism. Ancymidol, an analog of flurprimidol, promotes ABA biosynthesis in the fungus Cercospora rosicola and in the mesocarp tissue of avocado (Norman et al. 1983, Cowan and Railton 1987). Shortly after treatment with pyrimidine com-pounds, increases in ABA concentrations have been observed in cell suspensions, detached leaves, and young plants (Gross-mann 1992). In leaves, the increase in ABA concentration is accompanied by an increase in stomatal resistance and a de-crease in transpiration rate, and in suspension cultures of oilseed rape cells, it is closely correlated with enhanced potas-sium and water content of the cells (Häuser et al. 1990, MacKay et al. 1990). These results are similar to the responses we observed in white ash seedlings treated with flurprimidol.
Flurprimidol treatment improved plant water status of the nonirrigated seedlings by increasing Ψw, RWC, and Ψp, and
decreasing Ψπ(100). Although water use efficiency was not
improved by the flurprimidol treatment, plant water use was reduced as a result of the decline in E; consequently, flur(+) seedlings survived longer than flur(−) seedlings without water-ing. Thus, although total photosynthetic production may be lower in flur(+) seedlings than in flur(−) seedlings, flurprimi-dol enhances plant survival under water stress conditions. The greater sap osmolality and lower Ψπ(100) in flur(+) seedlings
than in flur(−) seedlings suggest that flurprimidol improves plant water status by facilitating osmotic adjustment under water stress conditions.
Acknowledgments
This research was funded by a grant from DowElanco, Indianapolis, Indiana, USA. Robert J. Joly provided careful review of the manu-script.
References
Armitage, A.M., Z.P. Tu and H.M. Vines. 1984. The influence of chlormequat and daminozide on net photosynthesis, transpiration, and photorespiration of hybrid geranium. HortScience 19:705--707. Atkinson, D. and J.S. Chauhan. 1987. The effect of paclobutrazol on the water use of fruit plants at two temperatures. J. Hort. Sci. 62:421--426.
Barrett, J.E. 1983. Comparison of growth retardant activities of EL--500 with ancymidol, chlormequat and daminozide on flowering ornamentals. Plant Growth Regul. Bull. 11:8--10.
Barrett, J.E. and T.A. Nell. 1981. Transpiration in growth retardant treated poinsettia, bean and tomato. Proc. Florida State Hort. Soc. 94:167--175.
Cathey, H.M. 1975. Comparative plant growth-retarding activities of ancymidol with ACPC, phosfon, chlormequat, and SADH on orna-mental plant species. HortScience 10:204--216.
Cleary, B.D. and J.B. Zaerr. 1980. Pressure chamber techniques for monitoring and evaluating seedling water status. N.Z. J. For. Sci. 10:133--141.
Cowan, A.K. and I.D. Railton. 1987. Cytokinins and ancymidol inhibit abscisic acid biosynthesis in Persea gratissima. J. Plant Physiol. 130:273.
Davis, T.D. and N. Sankhla. 1986. Soybean photosynthesis and growth as influenced by flurprimidol. Comp. Physiol. Ecol. 11:166--169. Davis, T.D. and E.A. Curry. 1991. Chemical regulation of vegetative
growth. Critical Rev. Plant Sci. 10:151--188.
Davis, T.D., R.H. Walser and N. Sankhla. 1986. Growth and photosyn-thesis of poinsettias as affected by plant growth regulators. J. Curr. Biosci. 3:121--126.
DeJong, T.M. and J.F. Doyle. 1984. Leaf gas exchange and growth responses of mature ‘‘Fantasia’’ nectarine trees to paclobutrazol. J. Am. Soc. Hort. Sci. 109:878--882.
Early, J.D. and G.C. Martin. 1988. Sensitivity of peach seedlings vegetative growth to paclobutrazol. J. Am. Soc. Hort. Sci. 113:23--27.
Frymire, R.M. and J.C. Henderson-Cole. 1992. Effect of uniconazole and limited water on growth, water relations and mineral nutrition of Lalandei Pyracantha. J. Plant Growth Regul. 11:227--231. Gaff, D.F. 1980. Protoplasmic tolerance of extreme water stress. In
Adaptation of Plants to Water and High Temperature Stress. Eds. N.C. Turner and P.J. Kramer. John Wiley & Sons, New York, pp 207--230.
Grossmann, K. 1992. Plant growth retardants: their mode of action and benefit for physiological research. In Progress in Plant Growth Regulation. Eds. C.M. Karssen, L.C. van Loon and D. Vreugdenhil. Kluwer Academic Publishers, The Netherlands, pp 788--797. Häuser, C., J. Kwiatkowski, W. Rademacher and K. Grossman. 1990.
Regulation of endogenus abscisic acid levels and transpiration in oilseed rape by plant growth retardants. J. Plant Physiol. 37:201--207.
Kramer, P.J. 1983. Water relations of plants. Academic Press. Aca-demic Press, Inc., New York, 489 p.
Loveys, B.R. and B.V. Milborrow. 1984. Biosynthesis of abscisic acid.
In The Biosynthesis and Metabolism of Plant Hormones. Eds. A. Crozier and J.R. Hillman. Cambridge University Press, Cam-bridge, England, pp 43--70.
MacKay, C.E., J.C. Hall, G. Hofstra and R.A. Fletcher. 1990. Uni-conazole-induced changes in abscisic acid, total amino acids, and proline in Phaseolus vulgaris. Pestic. Biochem. Physiol. 37:74--82. Marquard, R.D. 1985. Chemical growth regulation of pecan seedlings.
HortScience 20:919--921.
Norman, S.M., S.M. Poling, V.P. Maier and E.D. Orme. 1983. Inhibi-tion of abscisic acid biosynthesis in Cerospora rosicola by inhibi-tion of gibberellin biosynthesis and plant growth retardants. Plant Physiol. 71:15.
Numbere, T.E., F.D. Morrison and R.W. Campbell. 1992. Effects of uniconazole, paclobutrazol and flurprimidol on the control of young apple trees (Malus domestica) growth. Plant Growth Regul. Soc. Am. Quart. 20:65--67.
Parker, W.C. and S.G. Pallardy. 1987. The influence of resaturation method and tissue type on pressure-volume analysis of Quercus alba L. seedlings. J. Exp. Bot. 38:535--549.
Rademacher, W. 1991. Inhibitors of gibberellin biosynthesis: Appli-cation in agriculture and horticulture. In Gibberellins. Eds. N. Taka-hashi, B.O. Phinney and J. MacMillian. Springer-Verlag, New York, pp 296--310.
Rieger, M. and G. Scalabrelli. 1990. Paclobutrazol, root growth, hy-draulic conductivity, and nutrient uptake of nemaguard peach. Hort-Science 25:95--98.
Schuch, U.K. 1994. Response of chrysanthemum to uniconazole and daminozide applied as dip to cuttings or as foliar spray. J. Plant Growth Regul. 13:115--121.
Steinberg, S.L., J.M. Zajicek and M.J. McFarland. 1991a. Short-term effect of uniconazole on water relations and growth of Ligustrum. J. Am. Soc. Hort. Sci. 116:460--464.
Steinberg, S.L., J.M. Zajicek and M.J. McFarland. 1991b. Water relations of Hibiscus following pruning or chemical growth regula-tion. J. Am. Soc. Hort. Sci. 116:465--470.
Sterrett, J.P., T.J. Tworkoski and P.T. Kujawski. 1989. Physiological responses of deciduous tree root collar drenched with flurprimidol. J. Arboric. 15:120--124.
Swietlik, D. and S.S. Miller. 1983. The effect of paclobutrazol on growth and response to water stress of apple seedlings. J. Am. Soc. Hort. Sci. 108:1076--1080.
Terashima, I., S. Wong, C.B. Osmond and G.D. Farquhar. 1988. Characterization of non-uniform photosynthesis induced by ab-scisic acid in leaves having different mesophyll anatomies. Plant Cell Physiol. 29:385--394.
Tyree, M.T. 1976. Negative turgor in plant cells: fact or fallacy? Can. J. Bot. 54:2738--2746.
Vaigro-Wolff, A.L. and M.R. Warmund. 1987. Suppression of growth and plant moisture stress of Forsythia with flurprimidol and XE-1019. HortScience 22:884--885.
Vu, J.C.V. and G. Yelenosky. 1992. Growth and photosynthesis of sweet orange plants treated with paclobutrazol. J. Plant Growth Regul. 11:85--89.
Wang, S.Y., J.K. Byun and G.L. Steffens. 1985. Controlling plant growth via the gibberellin biosynthesis system-II. Biochemical and physiological alterations in apple seedlings. Physiol. Plant. 63:169--175.
Wieland, W.F. and R.L. Wample. 1985. Effects of paclobutrazol on growth, photosynthesis and carbohydrate content of delicious ap-ples. Sci. Hortic. 26:139--147.
Wilson, J.R., M.J. Fisher, E.D. Schulze, G.R. Dolby and M.M. Lud-low. 1979. Comparison between pressure-volume and dewpoint hygrometry techniques for determining the water relations charac-teristics of grass and legume leaves. Oecologia 41:77--88. Zhou, W. and H. Xi. 1993. Effects of mixtalol and paclobutrazol on