Summary Foliar ozone uptake rates of different-sized black cherry (Prunus serotina Ehrh.) trees were compared within a deciduous forest and adjacent openings in north-central Penn-sylvania during one growing season. Study trees included open-grown seedlings and saplings, forest understory seedlings and saplings, and sunlit and shaded portions of mature canopy tree crowns. Instantaneous ozone uptake rates were highest in high-light environments primarily because of higher stomatal conductances. Low ozone uptake rates of seedlings and sap-lings in the forest understory could be attributed partially to lower average ambient ozone concentrations compared to the canopy and open environments. Among the tree size and light combinations tested, ozone uptake rates were highest in open-grown seedlings and lowest in forest-open-grown seedlings. Despite lower ozone uptake rates of foliage in shaded environments, ozone uptake per net photosynthesis of foliage in shaded environments was significantly higher than that of foliage in sunlit environments because of weaker coupling between net photosynthesis and stomatal conductance in shaded environ-ments. The potential for greater ozone injury in shaded environments as a result of greater ozone uptake per net pho-tosynthesis is consistent with previous reports of greater ozone injury in shaded foliage than in sunlit foliage.
Keywords: air pollution, irradiance, Prunus serotina, shade, stomatal conductance.
Introduction
Ozone (O3) is perhaps the most damaging regionally dispersed air pollutant affecting forest trees (Reich 1987, Lefohn and Runeckles 1987, Hogsett et al. 1988). There is evidence that ozone injury is greater in shaded foliage than in sunlit foliage (Tjoelker et al. 1993, Volin et al. 1993), but the mechanism underlying this difference has not been elucidated.
Ozone uptake can be calculated as the product of stomatal conductance and ozone concentration (Reich 1987, Wieser and Havranek 1993, Winner 1994). Because of the strong
depend-ence of ozone uptake on stomatal conductance, ontogenetic and environmental factors that influence stomatal conductance will also influence ozone uptake. For example, ozone uptake may vary because of differences in stomatal conductance due to tree size (Kozlowski and Constantinidou 1986, Cregget al. 1989, Donovan and Ehleringer 1991, Miller et al. 1992, Sa-muelson and Edwards 1993, Grulke and Miller 1994, Hanson et al. 1994, Fredericksen et al. 1995, 1996) or differences in light environment within the forest (Kozlowski et al. 1991). In addition, potential differences in local ozone concentrations within mature forests or areas of forest regeneration could also influence ozone uptake (Enders et al. 1989, Fuentes et al. 1992). Factors that lead to high ozone uptake rates result in greater exposure of leaf mesophyll cells to ozone which may increase foliar injury.
The degree of foliar ozone injury may also depend on plant resources available for anti-oxidant defenses and repair of damaged tissues. Volin et al. (1993) and Tjoelker et al. (1993) suggested that the ratio of stomatal conductance or ozone uptake to net photosynthesis is closely related to the amount of foliar ozone injury because the availability of photosynthate is probably important in anti-oxidant defenses and repair of tis-sues injured by ozone. Thus, ozone taken up through the stomata in excess of photosynthate available for defense and repair may be an important determinant of foliar oxidant in-jury. This hypothesis suggests that high rates of net photosyn-thesis may balance high ozone uptake and ameliorate foliar injury.
Given the potential importance of ozone uptake or stomatal conductance per net photosynthesis in explaining foliar ozone injury, understanding environmental factors that regulate the degree of coupling of net photosynthesis to stomatal conduc-tance is important for elucidating the impacts of ozone. Al-though net photosynthesis and stomatal conductance have been strongly and positively related in many studies performed under controlled conditions or high light environments (Koz-lowski et al. 1991), tight coupling of these gas exchange parameters may be less common in natural environments
char-Light environment alters ozone uptake per net photosynthetic rate in
black cherry trees
T. S. FREDERICKSEN,
1,2T. E. KOLB,
3J. M. SKELLY,
4K. C. STEINER,
5B. J. JOYCE
5and
J. E. SAVAGE
41
Environmental Resources Research Institute, Pennsylvania State University, University Park, PA 16802, USA 2
Present address: National Audubon Society, 204 Ferguson Building, University Park, PA 16802, USA 3 School of Forestry, Northern Arizona University P.O. Box 15018, Flagstaff, AZ 86011-5018, USA
4
acterized by heterogeneous irradiances. For example, studies of net photosynthetic and stomatal conductance responses to simulated sunflecks (e.g., Knapp 1992, Stickan and Zhang 1992) have shown much faster reductions in net photosynthe-sis in response to shading than in stomatal conductance. Loose coupling between stomatal conductance and net photosynthe-sis suggests the potential for high rates of foliar ozone uptake per unit net photosynthesis for leaves growing in environments characterized by low and heterogenous irradiances.
The objective of this study was to determine the influence of light environment on ozone uptake in black cherry trees of varying size and to test the hypothesis of Tjoelker et al. (1993) and Volin et al. (1993) that the degree of ozone-induced foliar injury is related to the ratio of ozone uptake to net photosyn-thesis. We present data showing the influence of light environment on coupling of stomatal conductance to net pho-tosynthesis that helps explain the greater ozone-induced injury in shaded leaves than in sunlit leaves of black cherry. We studied black cherry because it is an important commercial timber species in the Appalachian Mountains of the United States and is one of the most ozone-sensitive eastern hardwood tree species (Davis and Skelly 1992, Simini et al. 1992).
Materials and methods
Study site
The study site is located within the Moshannon State Forest in Clearfield County, Pennsylvania (41°08′ N, 78°31′ W, elev. 655 m). The site is occupied by an 80-year-old mixed hard-wood forest stand. Dominant species include northern red oak (Quercus rubra L.), chestnut oak (Q. prinus L.), red maple (Acerrubrum L.), and black cherry. The forest canopy aver-ages approximately 20 m in height and has the physiognomy of a mature stand: rounded crowns and minimal annual height increment. An opening near the forested area was planted with seedlings and saplings. The entire site occupies a ridgetop position within the Allegheny Plateau physiographic province with similar soil properties. Annual average precipitation is 975.4 mm. Total rainfall from April 1 to October 31, 1993 was 767.1 mm. Average yearly minimum and maximum tempera-tures are −10.8 and 27.2 °C, respectively.
The study site has significant deposition of ozone during most growing seasons (Comrie 1994) and foliar ozone symp-toms have been observed on trees of several species growing under ambient conditions (Simini et al. 1992).
Study trees
The black cherry trees selected for intensive study throughout the 1993 growing season included: four canopy trees, five open-grown saplings, five forest understory saplings, twelve open-grown seedlings, and ten forest understory seedlings. Canopy trees (averaging 21.3 m tall) consisted of two pairs of closely spaced dominants that were selected based on their similarity to other mature black cherry trees on the site and the ability to access the crowns of both trees in each pair from a single scaffold tower. Canopy tree pairs were separated by approximately 0.5 km. All saplings ranged in height from 3 to
7 m. Open-grown saplings were 5-year-old trees planted in 1988 in an opening adjacent to naturally regenerated saplings growing under a mature forest canopy. Saplings were located approximately 2 km from the canopy study trees. Forest-grown seedlings were located in the same stand as the forest saplings. Open-grown seedlings were planted at 1.5 m spacing in the fall of 1992 in a forest opening located 0.3 km from the saplings. For the canopy trees, upper (more sunlit) and lower crown (more shaded) positions were delineated based on division of the live crown into equal halves.
Ambient ozone measurement
Ambient ozone concentration was measured with three TECO-49 (Thermoelectron Corporation, Franklin, MA) ozone ana-lyzers positioned at the sites described above. One monitor was used to measure ozone concentrations in the forest opening where open-grown seedlings and saplings were located. An-other monitor was situated in the forest understory with sam-pling positions on the forest floor at seedling height and in the understory foliage layer at the height of sapling crowns. The third monitor was used to measure ozone concentrations within both the sunlit and shaded crown positions of canopy trees. Each monitor was calibrated every two weeks with a TECO ozone calibrator certified for accuracy by the U.S. Environmental Protection Agency. Air samples were drawn through Teflon tubing from locations within the vegetation boundary layer at each location. Sampling was conducted on a 24-h basis from early May until the end of September. On each sample day, ozone concentrations were sampled several times per hour at each location and averaged.
Leaf gas exchange
From full leaf expansion in early June until leaf senescence in mid-September, leaf gas exchange (stomatal conductance to water vapor and net photosynthesis) and incident photosyn-thetically active radiation (PAR) were measured in situ on trees of all size and light environment combinations with two cross-calibrated LI-6200 portable photosynthesis systems (Li-Cor, Inc., Lincoln, NE) equipped with 0.25-l cuvettes. A fixed area of attached leaves was used for all measurements. Because of logistical constraints, gas exchange measurements were not always made for all combinations on the same sampling day. Measurements were typically made two days per week. For each tree size class × light environment combination, 10--30 randomly selected leaves from 4--10 trees were measured at least once every two weeks. All measurements were restricted to mostly sunny days with two or three 1.5-h sampling periods spaced evenly between 0900 and 1600 h EST.
Ozone uptake
the surrogate water vapor technique provides an accurate measure of U (Winner 1994). Hourly stomatal conductance values were multiplied by matching mean ozone concentration during each 1.5-h sample period for each size class--light environment combination to calculate average U for that sam-ple period. These U values were then corrected to conductance to ozone by dividing by 1.68, the ratio of diffusivities between ozone and water vapor.
Data analysis
For leaf physiological parameters, U, and U per net photosyn-thesis (U/Pn), unpaired t-tests were conducted on data aver-aged for each tree size class over sample periods for each date and individual tree to determine if means were significantly different between light environments. Tests for differences among tree sizes and the interaction between tree size and light environment were not conducted because light environment was nested within canopy trees, but independent of tree size for seedlings and saplings. Regression analysis was used to deter-mine the relationship between instantaneous measurements of stomatal conductance and net photosynthesis for each size class on data averaged by sample date and sample time for individual trees. All statistical analyses were performed using Statistical Analysis System software (SAS Institute, Cary, NC). Differences were considered significant at α = 0.05.
Results
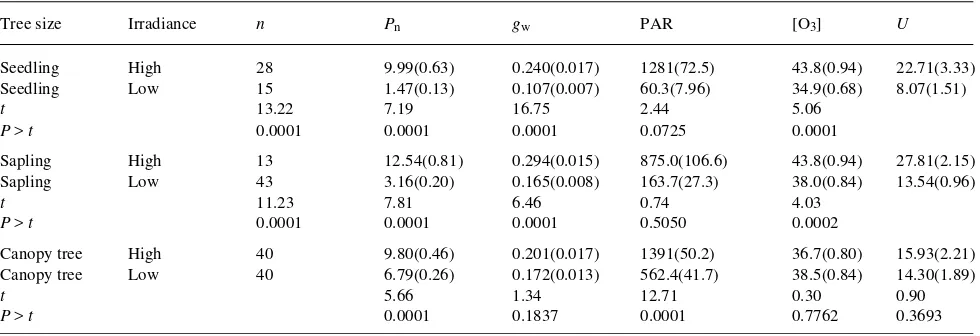
Throughout the growing season, mean hourly ambient ozone concentrations were consistently highest near open-grown saplings and seedlings and lowest near forest understory seed-lings and sapseed-lings (Table 1); however, ozone concentrations did not differ significantly between high- and low-light envi-ronments for any tree size class. For all tree size classes, stomatal conductances and net photosynthetic rates were
con-sistently higher in high-light environments than in low-light environments (Table 1), although differences were relatively small and differences in stomatal conductance were not statis-tically significant for canopy trees. For both seedlings and saplings, U was significantly higher in high light than in low light (Table 1). For canopy trees, U was not significantly different between sunlit (upper crown leaves) and shaded (lower crown) leaves.
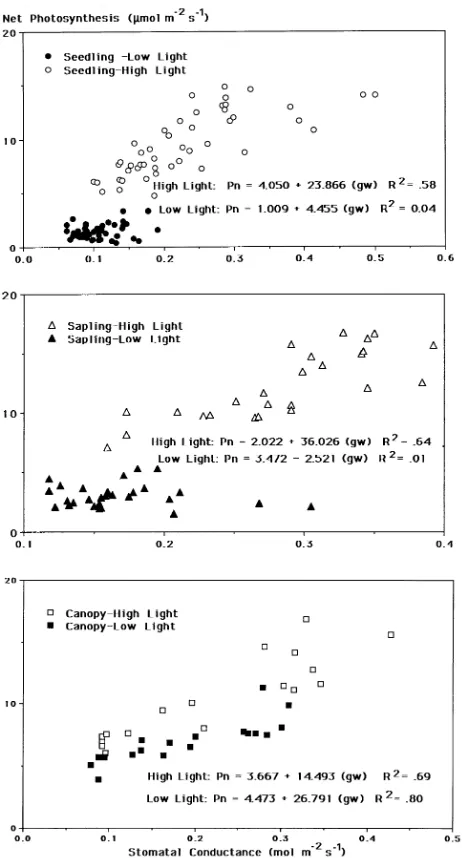
There was a significant, positive relationship between net photosynthesis and stomatal conductance for all tree size classes in high light and for canopy trees in low light (Figures 1a--c). In contrast, net photosynthesis and stomatal conduc-tance were weakly related for seedlings and saplings in low light. For each tree size class, U/Pn was higher in high-light environments than in low-light environments (Figure 2). Dif-ferences in U/Pn between high- and low-light environments decreased with increasing tree size.
Discussion
For saplings and seedlings, higher stomatal conductances and higher average ozone concentrations in high-light environ-ments resulted in higher average rates of foliar instantaneous U than in low-light environments. This pattern also occurred for canopy trees, but differences in U between high- and low-light environments were relatively small. The smaller dif-ference in U between high- and low-light environments in canopy trees compared to saplings and seedlings was likely the result of the smaller difference in PAR between the high- and low-light environments for canopy trees compared to seedlings and saplings. For seedlings and saplings in the low-light envi-ronment, PAR (average of 60.3 and 163.7 µmol m−2 s−1, respectively) was strongly limiting to foliar gas exchange (Harrington et al. 1989).
Despite lower foliar U in low-light compared to high-light
Table 1. Summary statistics, averaged by sample period and date, of physiological variables, ozone concentration, and ozone uptake (U) for seedling, sapling, and canopy black cherry trees growing in high- and low-light environments in the Moshannon State Forest, Pennsylvania during the 1993 growing season. Measurements were made between 0900 and 1600 h EST. Variables include Pn (net photosynthesis, µmol m−2 s−1), gw (stomatal conductance, mol m−2 s−1), PAR (photosynthetically active radiation, µmol m−2 s−1), ambient ozone concentration (nl l−1), and U (µmol
m−2 h−1). Standard errrors of means are presented in parentheses. Student t-tests for differences between light environments were conducted within each size class for all variables. Sample sizes represent each tree × sample period × date combination.
Tree size Irradiance n Pn gw PAR [O3] U
Seedling High 28 9.99(0.63) 0.240(0.017) 1281(72.5) 43.8(0.94) 22.71(3.33)
Seedling Low 15 1.47(0.13) 0.107(0.007) 60.3(7.96) 34.9(0.68) 8.07(1.51)
t 13.22 7.19 16.75 2.44 5.06
P > t 0.0001 0.0001 0.0001 0.0725 0.0001
Sapling High 13 12.54(0.81) 0.294(0.015) 875.0(106.6) 43.8(0.94) 27.81(2.15)
Sapling Low 43 3.16(0.20) 0.165(0.008) 163.7(27.3) 38.0(0.84) 13.54(0.96)
t 11.23 7.81 6.46 0.74 4.03
P > t 0.0001 0.0001 0.0001 0.5050 0.0002
Canopy tree High 40 9.80(0.46) 0.201(0.017) 1391(50.2) 36.7(0.80) 15.93(2.21)
Canopy tree Low 40 6.79(0.26) 0.172(0.013) 562.4(41.7) 38.5(0.84) 14.30(1.89)
t 5.66 1.34 12.71 0.30 0.90
environments, visible foliar ozone injury (dark adaxial stip-pling) was greater in the shaded crown than in the sunlit crown for all sizes of black cherry trees during the 1993 and 1994 growing seasons at the same study site (Fredericksen et al. 1995, 1996). This pattern was consistent despite large differ-ences in the extent of injury between years. The apparent contradictory pattern of lower U but higher injury in low-light environments than in high-light environments may be ex-plained by greater foliar U/Pn in low light than in high light. For trees in all size clases, values of U/Pn were significantly higher in low-light environments than in high-light environ-ments (Figure 2). This finding supports the hypothesis that
foliar ozone injury is related to U/Pn. Injury is likely to result when plant anti-oxidant repair and defense capabilities are overwhelmed by the rate of ozone uptake (Tingey and Taylor 1982, Rennenberg and Polle 1989, Taylor and Hanson 1992). Because these defense and repair processes require energy, net photosynthetic rate may exert strong control over ozone foliar injury.
Previous studies of light effects on ozone sensitivity of forest trees suggest differences in response between shade-tolerant and shade-intolerant species. Tjoelker et al. (1993) and Volin et al. (1993) reported that ozone reduced the growth of shade-intolerant hybrid poplar (Populus L.) most in high light, whereas the growth of shade-tolerant sugar maple (Acer sac-charum Marsh.) was reduced most in low light. These authors suggested that, in sugar maple, higher U/Pn in low light than in high light might account for the greater ozone-induced growth reduction in low-light environments. Tjoelker et al. (1993) suggested that, in hybrid poplar, the greater ozone-induced growth reduction in high light than in low light was associated with the relatively low PAR of the high-light treatment (350 µmol m−2 s−1), which was below the photosynthetic light saturation for hybrid poplar. Patterns of foliar ozone injury for black cherry (a shade-intolerant species (Marquis 1990)), based on visible foliar symptoms (Fredericksen et al. 1995, 1996) and the U/Pn physiological index of potential injury, suggest that the effect of irradiance on ozone-induce foliar injury was similar to that observed in sugar maple, a shade-tol-erant species. In both species, high sensitivity to ozone was associated with low light and high U/Pn.
High U/Pn in understory seedlings and the lower crown of saplings was promoted by a low degree of coupling between stomatal conductance and net photosynthesis. Uncoupling, which occurred in environments with low PAR (average 200 µmol m−2 s−1), may explain the greater foliar injury in shaded leaves than in sunlit leaves of this species (Fredericksen et al. 1995, 1996). Volin et al. (1993) also reported uncoupling Figure 1. Relationship between mean diurnal net photosynthesis and
stomatal conductance for leaves of (a) seedling, (b) sapling, and (c) canopy black cherry trees growing in high- and low-light environ-ments in the Moshannon State Forest, Pennsylvania. Points represent sampling date × sample period means for individual trees. Regression equations are presented for each light environment.
Figure 2. Mean (± 1 SE) U/Pn of seedling, sapling, and canopy black cherry trees growing in high- and low-light environments in the Moshannon State Forest, Pennsylvania. Results of t-tests for differ-ences in U/Pn between light environments were: seedlings: t = 2.68, P < t = 0.002; saplings: t = 5.62, P < t = 0.0001; canopy trees: t = 2.19,
between net photosynthesis and stomatal conductance of shade-grown sugar maple seedlings exposed to high concen-trations of ozone and also noted the same phenomenon in mature maple trees growing under ambient ozone conditions. Uncoupling of net photosynthesis and stomatal conductance in black cherry in shaded understory environments may result from differences in response of these gas exchange parameters to temporal variation in PAR created by sunflecks. Sunflecks occurred frequently in the understory seedling and sapling environments. Abrams et al. (1992) reported that, in black cherry, decreases in stomatal conductance in response to simu-lated sunflecks occurred over several minutes. In contrast, net photosynthesis should decline within seconds to decreases in PAR below photosynthetic saturation as a result of reduced quantum capture (e.g., Knapp 1992). These factors suggest greater uncoupling of net photosynthesis and stomatal conduc-tance in environments with highly heterogeneous light envi-ronments.
In addition to U/Pn, other factors may also account for greater amounts of foliar ozone injury in low-light compared to high-light environments. Leaves in shaded environments tend to be thinner with fewer palisade cells than those growing in sunlit environments (Boardman 1977). Bennett et al. (1992) showed that mesophyll cells in thin, shade-type leaves of black cherry seedlings tend to be more sensitive to injury than thick, sun-type leaves because of a greater relative exposure of those cells per unit volume of ozone in intercellular air spaces.
In summary, we conclude that irradiance has an important influence on mechanisms of foliar ozone injury. Irradiance affects both stomatal conductance (and hence ozone uptake) and net photosynthesis, and the influence of light environment on the balance of these processes may be important in explain-ing differences in foliar ozone injury between plants grown in disparate light environments. Predictions of ozone damage to forest ecosystems are complicated by heterogeneous light en-vironments that promote uncoupling of net photosynthesis and stomatal conductance.
Acknowledgments
This study was funded by the United States Environmental Protection Agency cooperative agreement CR820417-01-0 with additional coop-eration from the Pennsylvania Department of Environmental Re-sources, Bureau of Forestry Region 9; Penelec Corporation, GPU; and the Pennsylvania Conservation Corps. The authors thank Paul Augustine and Lee Warren of PDER for their support of this study. We also thank Ken Snyder, Kyle Kouterick and Jon Ferdinand for their valuable assistance.
References
Abrams, M.D., B.D. Kloeppel and M.E. Kubiske. 1992. Ecophysi-ological and morphEcophysi-ological responses to shade and drought in two contrasting ecotypes of Prunus serotina. Tree Physiol. 10:343--355. Bennett, J.P., P. Rassat, P. Berrang and D.F. Karnosky. 1992. Relation-ships between leaf anatomy and ozone sensitivity of Fraxinus pennsylvanica Marsh. and Prunus serotina Ehrh. Environ. Exp. Bot. 32:33--41.
Boardman, N.K. 1977. Comparative photosynthesis of sun and shade plants. Annu.Rev. Plant Physiol. 28:355--377.
Comrie, A.C. 1994. A synoptic climatology of rural ozone pollution at three forest sites in Pennsylvania. Atmos. Environ. 28:1601--1614. Cregg, B.M., J.E. Halpin, P.M. Dougherty and R.O. Teskey. 1989.
Comparative physiology and morphology of seedling and mature forest trees. In US--USSR Symp. Air Pollution Effects on Vegeta-tion. Eds. D. Noble, J.L. Martin and K.F. Jensen. U.S. Environ-mental Protection Agency, Corvallis, OR, pp 111--118.
Davis, D.D. and J.M. Skelly. 1992. Foliar sensitivity of eight eastern hardwood species to ozone. J. Air, Water, Soil Pollut. 62:269--277. Donovan, L.A. and J.R. Ehleringer. 1991. Ecophysiolgical differences
among juvenile and reproductive plants of several woody species. Oecologia 86:594--597.
Enders, G., U. Teichmann and G. Kramm. 1989. Profiles of ozone and surface layer parameters over a mature spruce stand. In Mecha-nisms and Effects of Pollutant Transfer into Forests. Ed. H.W. Georgii. Kluwer, Academic Publishers, pp 21--35.
Fredericksen, T.S., B.J. Joyce, J.M. Skelly, K.C. Steiner, T.E. Kolb, K.B. Kouterick, J.E. Savage and K.R. Snyder. 1995. Physiology, morphology, and ozone uptake of black cherry seedlings, saplings, and canopy trees. Environ. Pollut. 89:273--283.
Fredericksen, T.S., J.M. Skelly, K.C. Steiner, T.E. Kolb and K.B. Kouterick. 1996. Size mediated foliar response to ozone in black cherry trees. Environ. Pollut. 96:53--63.
Fuentes, J.D., T.J. Gillespie, G den Hartog and H.H. Neumann. 1992. Ozone deposition onto a deciduous forest during dry and wet conditions. Agric. For. Meteorol. 62:1--18.
Grulke, N.E. and P.R. Miller. 1994. Changes in gas exchange charac-teristics during the life span of giant sequoia: implications for response to current and future concentrations of atmospheric ozone. Tree Physiol. 14:659--668.
Hanson, P.J., L.J. Samuelson, S.D. Wullschleger, T.A. Tabberer and G.S. Edwards. 1994. Seasonal patterns of light-saturated photosyn-thesis and leaf conductance for mature and seedling Quercus rubra
L. foliage: differential sensitivity to ozone exposure. Tree Physiol. 14:1351--1366.
Harrington, R.A., B.J. Brown and P.B. Reich. 1989. Ecophysiology of exotic and native shrubs in southern Wisconsin. I. Relationship of leaf characteristics, resource availability, and phenology to seasonal patterns of carbon gain. Oecologia 80:356--367.
Hogsett, W.E., D.T. Tingey and E.H. Lee. 1988. Ozone exposure indices: concepts for development and evaluation of their use. In
Assessment of Crop Loss from Air Pollutants. Eds. W.W. Heck, O.C. Taylor and D.T. Tingey. Elsevier Applied Science, London, pp 107--138.
Knapp, A.K. 1992. Leaf gas exchange in Quercus macrocarpa (Fa-gaceae): rapid stomatal responses to variability in sunlight in a tree growth form. Am. J. Bot. 79:599--604.
Kozlowski, T.T. and H.A. Constantinidou. 1986. Environmental pol-lution and tree growth. Part II. Factors affecting responses to pollu-tion and alleviapollu-tion of pollupollu-tion effects. For. Abstr. 47:105--132. Kozlowski, T.T., P.J. Kramer and S.G. Pallardy. 1991. The
physiologi-cal ecology of woody plants. Academic Press, New York, 657 p. Laisk, A.O., O. Kull and H. Moldau. 1989. Ozone concentration in leaf
intercellular air spaces is close to zero. Plant Physiol. 90:1163--1167.
Lefohn, A.S. and V.C. Runeckles. 1987. Establishing a standard to protect vegetation----ozone exposure/dose considerations. Atmos. Environ. 21:561--568.
Miller, P.M., L.E. Eddleman and J.M. Miller. 1992. The seasonal course of physiological processes in Juniperus occidentalis. For. Ecol. Manage. 48:185--215.
Reich, P.B. 1987. Quantifying plant response to ozone: a unifying theory. Tree Physiol. 3:63--91.
Rennenberg, H. and A. Polle. 1989. Effects of photooxidants on plants.
In Mechanisms and Effects of Pollutant Transfer into Forests.Ed. H. W. Georgii. Kluwer Academic Publishers, The Netherlands, pp 251--258.
Samuelson, L. J. and G. S. A. Edwards. 1993. A comparison of sensitivity to ozone in seedlings and trees of Quercus rubra L. New Phytol. 125:373--379.
Simini, M.J., J.M. Skelly, D.D. Davis, J.E. Savage and A.C. Comrie. 1992. Sensitivity of four hardwood species to ambient ozone in northcentral Pennsylvania. Can. J. For. Res. 22:1789--1799. Stickan, W. and X. Zhang. 1992. Seasonal changes in CO2 and H2O
gas exchange of young European beech (Fagus sylvatica L.). Trees 6:96--102.
Taylor, Jr., G.E. and P.J. Hanson. 1992. Forest trees and trophospheric ozone: role of canopy deposition and leaf uptake in developing exposure--response relationships. Agric. Ecosys. Environ. 42:255--273.
Tingey, D.T. and G.E. Taylor Jr. 1982. Variation in plant response to ozone: a conceptual model of physiological events. In Effects of Gaseous Air Pollutants in Agriculture and Horticulture. Eds.H.M. Unsworth and D.D. Ormond. Butterworth Publ., London, pp 113--138.
Tjoelker, M.G., J.C. Volin, J. Oleksyn and P.B. Reich. 1993. Light environment alters response to ozone stress in seedlings of Acer saccharum Marsh. and hybrid Populus L. I. In situ net photosynthe-sis, dark respiration, and growth. New Phytol. 124:627--636. Volin, J.C., MG. Tjoelker, J. Oleksyn and P.B. Reich. 1993. Light
environment alters response to ozone stress in seedlings of Acer saccharum Marsh. and hybrid Populus L. II. Diagnostic gas ex-change and leaf chemistry. New Phytol. 124:637--646.
Wieser, G. and W.M. Havranek. 1993. Ozone uptake in the sun and shade crown of spruce: quantifying the physiological effects of ozone exposure. Trees 7:227--232.