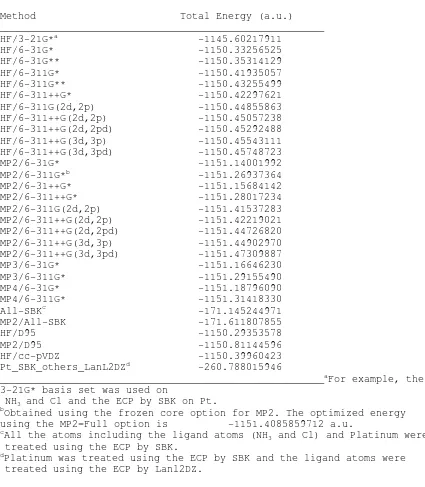
Table I.
Ab Initio
optimized Total energies (in a.u.) for cisplatin
using a variety of basis sets on the ligands (NH
3and Cl) and the
effective core potential (ECP) by SBK basis set on Pt.
______________________________________________________
Method
Total Energy (a.u.)
______________________________________________________
HF/3-21G*
a-1145.60217911
HF/6-31G*
-1150.33256525
HF/6-31G**
-1150.35314129
HF/6-311G*
-1150.41935057
HF/6-311G**
-1150.43255499
HF/6-311++G*
-1150.42297621
HF/6-311G(2d,2p)
-1150.44855863
HF/6-311++G(2d,2p)
-1150.45057238
HF/6-311++G(2d,2pd)
-1150.45292488
HF/6-311++G(3d,3p)
-1150.45543111
HF/6-311++G(3d,3pd)
-1150.45748723
MP2/6-31G*
-1151.14001992
MP2/6-311G*
b-1151.26937364
MP2/6-31++G*
-1151.15684142
MP2/6-311++G*
-1151.28017234
MP2/6-311G(2d,2p)
-1151.41537283
MP2/6-311++G(2d,2p)
-1151.42219021
MP2/6-311++G(2d,2pd)
-1151.44726820
MP2/6-311++G(3d,3p)
-1151.44902970
MP2/6-311++G(3d,3pd)
-1151.47309887
MP3/6-31G*
-1151.16646230
MP3/6-311G*
-1151.29155490
MP4/6-31G*
-1151.18796090
MP4/6-311G*
-1151.31418330
All-SBK
c-171.145244971
MP2/All-SBK
-171.611807855
HF/D95
-1150.29353578
MP2/D95
-1150.81144596
HF/cc-pVDZ
-1150.39960423
Pt_SBK_others_LanL2DZ
d-260.788015946
______________________________________________________
aFor example, the
3-21G* basis set was used on
NH
3and Cl and the ECP by SBK on Pt.
b
Obtained using the frozen core option for MP2. The optimized energy
using the MP2=Full option is -1151.4085859712 a.u.
c
All the atoms including the ligand atoms (NH
3
and Cl) and Platinum were
treated using the ECP by SBK.
Table II.
Ab Initio
optimized Total energies (in a.u.) for cisplatin
using a variety of basis sets on the ligands (NH
3and Cl) and the
effective core potential (ECP) by LanL2DZ basis set on Pt.
_____________________________________________________
Method Total Energy (a.u.)
_____________________________________________________
HF/3-21G*
a-1144.98344800
HF/6-31G*
-1149.71700373
HF/6-31G**
-1149.73715318
HF/6-311G*
-1149.80269707
HF/6-311G**
-1149.81485210
HF/6-311++G*
-1149.80852187
HF/6-311G(2d,2p)
-1149.83499309
HF/6-311++G(2d,2p)
-1149.83840967 HF/6-311++G(2d,2pd)
-1149.84084210 HF/6-311++G(3d,3p)
-1149.84522894 HF/6-311++G(3d,3pd)
-1149.84715206
MP2/6-31G*
-1150.45488162
MP2/6-311G*
-1150.58138883
MP2/6-31++G*
-1150.47578644
MP2/6-311++G*
-1150.59899774
MP2/6-311G(2d,2p)
-1150.73830663
MP2/6-311++G(2d,2p)
-1150.74896853
MP2/6-311++G(2d,2pd)
-1150.77514148
MP2/6-311++G(3d,3p)
-1150.78070683 MP2/6-311++G(3d,3pd)
-1150.80546904
MP3/6-31G*
-1150.48780550
MP3/6-311G*
-1150.61033870
MP4/6-31G*
-1150.50445590
MP4/6-311G*
-1150.62802240
All-LanL2DZ
b-260.169929423
MP2/All-LanL2DZ
-260.588801233
HF/D95
-1149.67522946
MP2/D95
-1150.12581446
HF/cc-pVDZ
-1149.78273028
Pt_LanL2DZ_others_SBK
c-170.528172642
____________________________________________________
aFor example, the 3-21G* basis set was used on NH
3
and Cl and the ECP by
LanL2DZ on Pt.
b
All the atoms including the ligand atoms (NH
3
and Cl) and Platinum were
treated using the ECP by LanL2DZ.
Table IV. Ab Initio optimized geometric parameters for cisplatin (using a variety of
basis sets on the ligands, NH3 and Cl, and using the ECP by LanL2DZ basis set on Pt).
Distances in Angstroms, Angles in Degrees. The "∆c-o" columns next to each geometric
parameter correspond to the difference between the calculated value and the experimental value (calculated - observed). The last column refers to the "overall mean % difference" obtained with respect to the experimental values.
_________________________________________________________________________________________ _____________________________________________
Method Pt-Cl ∆c-o Pt-N ∆c-o N-Pt-N ∆c-o
N-Pt-Cl ∆c-o Cl-Pt-Cl ∆c-o difference
_________________________________________________________________________________________ _____________________________________________
HF/3-21G*b 2.373 0.043 2.121 0.111 95.9 8.9 83.7 -6.6
HF/6-31G* 2.363 0.033 2.124 0.114 95.3 8.3 84.5 -5.8 HF/6-31G** 2.364 0.034 2.126 0.116 95.4 8.4 84.4 -5.9 HF/6-311G* 2.368 0.038 2.119 0.109 94.9 7.9 84.9 -5.4 HF/6-311G** 2.367 0.037 2.122 0.112 95.1 8.1 84.8 -5.5 HF/6-311++G* 2.361 0.031 2.118 0.108 95.1 8.1 84.7 -5.6 HF/6-311G(2d,2p) 2.346 0.016 2.110 0.100 95.3 8.3 84.5 -5.8 HF/6-311++G(2d,2p) 2.346 0.016 2.106 0.096 95.4 8.4 84.3 -6.0 HF/6-311++G(2d,2pd) 2.346 0.016 2.103 0.093 95.5 8.5 84.3 -6.0 HF/6-311++G(3d,3p) 2.346 0.016 2.110 0.100 95.8 8.8 84.1 -6.2 HF/6-311++G(3d,3pd) 2.345 0.015 2.110 0.100 95.8 8.8 84.1 -6.2 MP2/6-31G* 2.347 0.017 2.083 0.073 97.4 10.4 83.9 -6.4 MP2/6-311G* 2.347 0.017 2.079 0.069 97.0 10.0 84.4 -5.9 MP2/6-31++G* 2.339 0.009 2.079 0.069 97.7 10.7 83.7 -6.6 MP2/6-311++G* 2.336 0.006 2.079 0.069 97.4 10.4 83.9 -6.4 MP2/6-311G(2d,2p) 2.322 -0.008 2.037 0.027 98.2 11.2 83.1 -7.2 MP2/6-311++G(2d,2p) 2.321 -0.009 2.030 0.020 98.3 11.3 82.9 -7.4 MP2/6-311++G(2d,2pd) 2.323 -0.007 2.016 0.006 98.6 11.6 82.6 -7.7 MP2/6-311++G(3d,3p) 2.320 -0.010 2.027 0.017 98.4 11.4 82.7 -7.6 MP2/6-311++G(3d,3pd) 2.320 -0.010 2.020 0.010 98.5 11.5 82.6 -7.7 MP3/6-31G* 2.358 0.028 2.098 0.088 96.9 9.9 83.9 -6.4 MP3/6-311G* 2.360 0.030 2.092 0.082 96.5 9.5 84.3 -6.0 MP4/6-31G* 2.358 0.028 2.095 0.085 97.1 10.1 84.0 -6.3 MP4/6-311G* 2.359 0.029 2.090 0.080 96.5 9.5 84.5 -5.8 All-LanL2DZc 2.418 0.088 2.125 0.115 95.9 8.9 83.4 -6.9
MP2/All-LanL2DZ 2.407 0.077 2.115 0.105 97.9 10.9 83.2 -7.1 HF/D95 2.422 0.092 2.126 0.116 95.9 8.9 83.4 -6.9 MP2/D95 2.417 0.087 2.116 0.106 98.0 11.0 83.0 -7.3 HF/cc-pVDZd 2.357 0.027 2.131 0.121 95.4 8.4 84.2 -6.1
Pt_LanL2DZ_others_SBKe 2.410 0.080 2.127 0.117 95.2 8.2 84.4 -5.9
DFTf 2.310 -0.020 2.060 0.050 98.0 11.0 83.0 -7.3
Exptg 2.330 0.000 2.010 0.000 87.0 0.0 90.3 0.0
_________________________________________________________________________________________ ______________________________________________
aFor example, using ECP/3-21G* the overall mean % difference is calculated in the
following fashion:
0.043 * 100 0.111 * 100 8.9 * 100 6.6 * 100 4.9 * 100 1
___________ + ___________ + _________ + _________ + _________ * _ = 6.0
2.33 2.01 87.0 90.3 91.9 5
bFor example, the 3-21G* basis set was used on NH
3 and Cl and the ECP by LanL2DZ on Pt. cAll the atoms including the ligand atoms (NH
3 and Cl) and Platinum were treated using the
ECP by LanL2DZ.
dDunning’s correlation consistent - valence double zeta with polarization basis set. ePlatinum atom was treated using the ECP by LanL2DZ and the ligand atoms were treated
using the ECP by SBK.
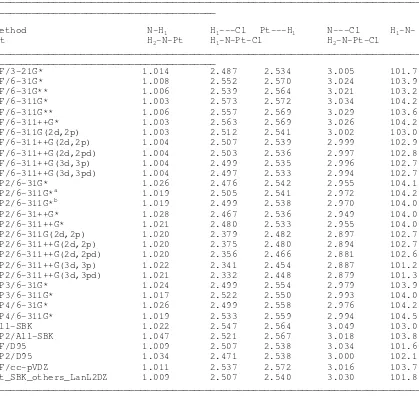
Table V. Ab Initio optimized geometric parameters involving the ligand atoms of
cisplatin (NH3 and Cl, using a variety of basis sets on the ligand atoms and treating Pt using the ECP by SBK basis set). Distances in Angstroms, Angles in Degrees.
_______________________________________________________________________________ _________________________________________
Method N-H1 H1---Cl Pt---H1 N---Cl H1
-N-Pt H2-N-Pt H1-N-Pt-Cl H2-N-Pt-Cl
_______________________________________________________________________________ _________________________________________
HF/3-21G* 1.014 2.487 2.534 3.005 101.7 112.7
HF/6-31G* 1.008 2.552 2.570 3.024 103.9 114.7
HF/6-31G** 1.006 2.539 2.564 3.021 103.2 114.5
HF/6-311G* 1.003 2.573 2.572 3.034 104.2 114.5
HF/6-311G** 1.006 2.557 2.569 3.029 103.6 114.4
HF/6-311++G* 1.003 2.563 2.569 3.026 104.2 114.4
HF/6-311G(2d,2p) 1.003 2.512 2.541 3.002 103.0 114.3
HF/6-311++G(2d,2p) 1.004 2.507 2.539 2.999 102.9 114.3
HF/6-311++G(2d,2pd) 1.004 2.503 2.536 2.997 102.8 114.4
HF/6-311++G(3d,3p) 1.004 2.499 2.535 2.996 102.7 114.7
HF/6-311++G(3d,3pd) 1.004 2.497 2.533 2.994 102.7 114.7
MP2/6-31G* 1.026 2.476 2.542 2.955 104.1 114.6
MP2/6-311G*a 1.019 2.505 2.541 2.972 104.2 114.2
MP2/6-311G*b 1.019 2.499 2.538 2.970 104.0 114.2
MP2/6-31++G* 1.028 2.467 2.536 2.949 104.0 114.7
MP2/6-311++G* 1.021 2.480 2.533 2.955 104.0 114.2
MP2/6-311G(2d,2p) 1.020 2.379 2.482 2.897 102.7 114.4
MP2/6-311++G(2d,2p) 1.020 2.375 2.480 2.894 102.7 114.3
MP2/6-311++G(2d,2pd) 1.020 2.356 2.466 2.881 102.6 114.7
MP2/6-311++G(3d,3p) 1.022 2.341 2.454 2.887 101.2 114.3
MP2/6-311++G(3d,3pd) 1.021 2.332 2.448 2.879 101.3 114.4
MP3/6-31G* 1.024 2.499 2.554 2.979 103.9 114.7
MP3/6-311G* 1.017 2.522 2.550 2.993 104.0 114.4
MP4/6-31G* 1.026 2.499 2.558 2.976 104.2 114.8
MP4/6-311G* 1.019 2.533 2.559 2.994 104.5 114.4
All-SBK 1.022 2.547 2.564 3.049 103.0 113.2
MP2/All-SBK 1.047 2.521 2.567 3.018 103.8 113.4
HF/D95 1.009 2.507 2.538 3.034 101.6 112.7
MP2/D95 1.034 2.471 2.538 3.000 102.1 113.0
HF/cc-pVDZ 1.011 2.537 2.572 3.016 103.7 115.2
Pt_SBK_others_LanL2DZ 1.009 2.507 2.540 3.030 101.8 112.7
_______________________________________________________________________________ _________________________________________
a
using the frozen core option for MP2 calculation. b
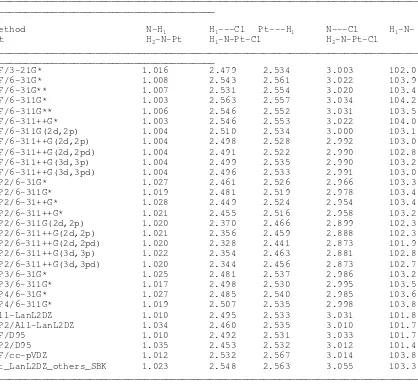
Table VI. Ab Initio optimized geometric parameters involving the ligand atoms
of cisplatin (NH3 and Cl, using a variety of basis sets on the ligand atoms and treating Pt using the ECP by LanL2DZ basis set). Distances in Angstroms, Angles in Degrees.
_______________________________________________________________________________ _________________________________________
Method N-H1 H1---Cl Pt---H1 N---Cl H1
-N-Pt H2-N-Pt H1-N-Pt-Cl H2-N-Pt-Cl
_______________________________________________________________________________ _________________________________________
HF/3-21G* 1.016 2.479 2.534 3.003 102.0 112.9
HF/6-31G* 1.008 2.543 2.561 3.022 103.9 114.7
HF/6-31G** 1.007 2.531 2.554 3.020 103.4 114.5
HF/6-311G* 1.003 2.563 2.557 3.034 104.2 114.4
HF/6-311G** 1.006 2.546 2.552 3.031 103.5 114.4
HF/6-311++G* 1.003 2.546 2.553 3.022 104.0 114.5
HF/6-311G(2d,2p) 1.004 2.510 2.534 3.000 103.1 114.4
HF/6-311++G(2d,2p) 1.004 2.498 2.528 2.992 103.0 114.5
HF/6-311++G(2d,2pd) 1.004 2.491 2.522 2.990 102.8 114.5
HF/6-311++G(3d,3p) 1.004 2.499 2.535 2.990 103.2 114.5
HF/6-311++G(3d,3pd) 1.004 2.496 2.533 2.991 103.0 114.5
MP2/6-31G* 1.027 2.461 2.526 2.966 103.3 114.5
MP2/6-311G* 1.019 2.481 2.519 2.978 103.4 113.9
MP2/6-31++G* 1.028 2.449 2.524 2.954 103.4 115.0
MP2/6-311++G* 1.021 2.455 2.516 2.958 103.2 114.5
MP2/6-311G(2d,2p) 1.020 2.370 2.466 2.899 102.3 114.8
MP2/6-311++G(2d,2p) 1.021 2.356 2.459 2.888 102.3 114.7
MP2/6-311++G(2d,2pd) 1.020 2.328 2.441 2.873 101.9 115.2
MP2/6-311++G(3d,3p) 1.022 2.354 2.463 2.881 102.8 113.9
MP2/6-311++G(3d,3pd) 1.020 2.344 2.456 2.873 102.7 113.9
MP3/6-31G* 1.025 2.481 2.537 2.986 103.2 114.7
MP3/6-311G* 1.017 2.498 2.530 2.995 103.5 114.2
MP4/6-31G* 1.027 2.485 2.540 2.985 103.6 114.7
MP4/6-311G* 1.019 2.507 2.535 2.998 103.8 114.1
All-LanL2DZ 1.010 2.495 2.533 3.031 101.8 112.9
MP2/All-LanL2DZ 1.034 2.460 2.535 3.010 101.7 112.6
HF/D95 1.010 2.492 2.531 3.033 101.7 112.9
MP2/D95 1.035 2.453 2.532 3.012 101.4 112.6
HF/cc-pVDZ 1.012 2.532 2.567 3.014 103.8 115.3
Pt_LanL2DZ_others_SBK 1.023 2.548 2.563 3.055 103.3 113.5
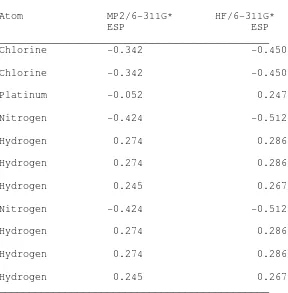
Table VIII. Table of electrostatic potential (ESP) charges for
cisplatin obtained at both MP2/6-311G* and HF/6-311G* basis sets with a
1.80
aÅ radius for Platinum.
_____________________________________________
Atom
MP2/6-311G*
HF/6-311G*
ESP
ESP
_____________________________________________
Chlorine
-0.342
-0.450
Chlorine
-0.342
-0.450
Platinum
-0.052
0.247
Nitrogen
-0.424
-0.512
Hydrogen
0.274
0.286
Hydrogen
0.274
0.286
Hydrogen
0.245
0.267
Nitrogen
-0.424
-0.512
Hydrogen
0.274
0.286
Hydrogen
0.274
0.286
Hydrogen
0.245
0.267
_____________________________________________
a
Based on the van der Waals radius from “J.E. Huheey,
Inorganic
5b
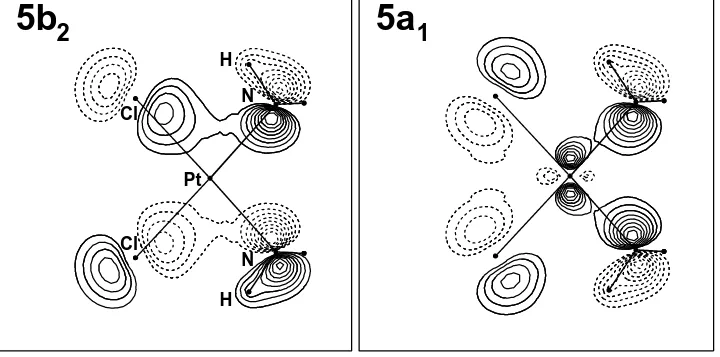
2
5a
1
Pt
Cl
Cl
N
N
H
[image:7.792.31.751.37.394.2]H