Preface
All praises are due to Allah, God Almighty, Who made this annual event of successful. The
“3
rdAnnual Basic Science International Conference (BaSIC-2013)”
is an annual scientific event organized
by the Faculty of Mathematics and Natural Sciences, Brawijaya University. As a basic science conference,
it covered a wide range of topics on basic science: physics, biology, chemistry, mathematics and statistics.
In 2013, the conference took a theme of “
Basic Science Advances in Energy, Health and Environment
”
as those three aspects of life are hot issues.
The conference in 2013 was the continuation of the preceding conferences initiated in 2011 as the
International Conference on Basic Science (ICBS)
, where it was a transformation from the similar
national events the faculty had organized since 2004. What also changed in year 2013 was the use of the
ISSN for the conference proceedings book, instead of an ISBN used in previous proceedings books. The
change was based on the fact that BaSIC is an annual event, and, therefore, the use of ISSN is more
appropriate. The proceedings book was also divided into four books: Physics, Biology, Chemistry and
Mathematics, each with a different ISSN. The proceedings were also published in electronic forms that can
be accessed from BaSIC website. I am glad that for the first time both types of publication can be realized.
This event is aimed to promote scientific research activities by Indonesian scientists, especially
those of Brawijaya University, in a hope that they may interact and build up networks and collaborations
with fellow overseas counterparts who participated in the conference. This is in line with university vision
as a World Class Entrepreneurial University.
I am grateful to all the members of the program committee who contributed for the success in
framing the program. I also thank all the delegates who contributed to the success of this conference by
accepting our invitation and submitting articles for presentation in the scientific program. I am also
indebted to PT Semen Gresik and PT PLN (Persero) for their support in sponsoring this event.
I wish for all of us a grand success in our scientific life. And I do hope that the coming conferences
will pick up similar success, and even better.
Malang, April 2013
Foreword by the Rector of Brawijaya University
First of all I would like to congratulate the Organizing Committee for the success in organizing this
amazing event. I believe all dedicated time and efforts will contribute to the advancement of our beloved
university.
I would like to welcome all participants, domestic and overseas, especially the distinguished invited
speakers, to Malang, to the conference. An international conference is a good means to establish and build
relationships and collaborations among participants. So, I hope this conference will facilitate all of you, the
academicians and scientists, to setup a network of mutual and beneficial collaboration. As a university with
a vision to be “
A World Class Entrepreneurial University
”, Brawijaya University will support all efforts to
realize that dream.
Finally, I do hope that the conference will run smoothly and nicely and is not the last one. I would
like to thank all parties who have lent their hands in making this conference happened.
Malang, April 2013
Table of Contents
Preface ... i
Foreword by the Rector of Brawijaya University ... ii
Table of Contents ... iii
Program Committee ... iv
Scientific Program... vii
Scientific Papers
Invited Papers
Cluster Dynamics by Ultra-Fast Shape Recognition Technique... I01
Nanotechnology Development Strategy for Supporting National Industry in Indonesia ... I02
Role of Atomic Scale Computational Research in the Nanoscale Materials ... I03
Paeonilorin(PF) Strongly Effects Immuno System... I04
Investigating Chlamydia trachomatis using mathematical and computational... I05
Recent Trends in Liquid Chromatography for Bioanalysis ... I06
Submitted Papers
Analysis of Inorganic Compounds Cr, Cd, CN, Mn, and Pb in RAW Water and Water Filtration
Results in Jakarta-Indonesia... C02
Pervaporation through NaA Zeolite Membranes - A Review... C03
Optimization of NaOH as the cleaning of Polyethersulfone (PES) membrane fouled by Palm oil
mill effluent... C08
Room-Temperature Synthesis of TiO2 - Chitosan Nanocomposites Photocatalyst ... C10
Structure of Hf(IV) in aqueous solution - An ab initio QM/MM MD approach ... C15
Molecular Dynamics Simulation of Scandium (I) Singlet In Liquid Ammonia By AB Initio
Author List
Program Committee
Patrons
Rector, Universitas Brawijaya
Dean, Faculty of Mathematics and Natural Sciences, Universitas Brawijaya
Advisory Boards
Associate Deans 1, 2 and 3, Faculty of Mathematics and Natural Sciences, Universitas Brawijaya
Chairperson
Johan A.E. Noor, Ph.D.
Deputy-Chair
Dr. Suharjono
Secretary
Agus Naba, Ph.D.
Treasurers
Mrs. Sri Purworini
Mrs. Rustika Adiningrum
Mr. Surakhman
Secretariat & Registration
Dr. Masruroh
dr. Kusharto
Mr. Sugeng Rianto
Mr. Gancang Saroja
Conference Web
Agus Naba, Ph.D.
Publication & Proceedings
Arinto Y.P. Wardoyo, Ph.D.
Mr. Wasis
Public Relations & Sponsorship
Chomsin S. Widodo, Ph.D.
Mr. Moch. Djamil
Mrs. Firdy Yuana
Venue
Mr. Ahmad Hidayat
Dr. Ahmad Nadhir
Mr. Sunariyadi
Mr. Purnomo
Mr. Karyadi Eka Putra
Accommodation & Hospitality
Ms. Siti J. Iswarin
Mrs. Nur Azizah
Mr. Robi A. Indrajit
Mrs. Trivira Meirany
Master of Ceremony
Himafis
Transportation, Excursion & Social Events
Djoko Santjojo, Ph.D.
Dr. Sukir Maryanto
Mr. Wahyudi
Mrs. Arnawati
Workshop, Poster & Scientific Exhibitions
Hari Arief Dharmawan, Ph.D.
Mr. Pudji Santoso
Mr. Sahri
Mr. Murti Adi Widodo
Documentation
Mauludi A. Pamungkas, Ph.D.
Mr. Susilo Purwanto
General Supports
Himafis
Scientific Program
Dr. rer.nat. M. Nurhuda
Dr. Sunaryo
Mr. Agus Prasmono
Local Scientific Committees (Reviewers & Editors)
Physics
Dr. rer.nat. Abdurrouf
Adi Susilo, Ph.D.
Mr. Unggul P. Juswono
Dr.-Ing. Setyawan P. Sakti
Biology
Dr. Moch. Sasmito Djati
Dr. Muhaimin Rifai
Dr. Catur Retnaningdyah
Chemistry
Dr. Masruri
Dr. Ahmad Sabarudin
Dr. Lukman Hakim
International Scientific Committee and Editors
A/Prof. Lilibeth dlC. Coo,
University of the Philippines, the Philippines
Prof. Dr. Gereon Elbers, FH Aachen, Germany
Prof. S.K. Lai, National Central University, Taiwan
Prof. Kwang-Ryeol Lee, Korean Institute of Science and Technology, Korea
A/Prof. Dann Mallet, Queensland University of Technology, Australia
Prof. Lidia Morawska, Queensland University of Technology, Australia
Prof.Dr. Petr Solich, Charles University, Czech Republic
Dr. Michitaka Suzuki, Nagoya University, Japan
Prof. Hideo Tsuboi, Nagoya University, Japan
Scientific Program
Time
Day One – 16 April 2013
Day Two – 17 April 2013
07.30 – 08.00 Registration
08.00 – 08.30 Inaugural Session, Welcome Remarks
and Opening Ceremony Poster Preparation
08.30 – 09.00 Coffee Break
Poster Session (08.30-09.30) (Majapahit Hall)
09.00 – 09.45
Invited Speaker 1
Prof. Lidia Morawska, Queensland University of Technology, Australia
Title: “Emissions to the Air: from Multidisciplinary Science to
Applications” Coffee Break (09.30 – 10.00)
09.45 – 10.30
Invited Speaker 2
Dr. rer. nat. M. Nurhuda, Universitas Brawijaya
Title: “Towards Energy Security for the Poor”
Parallel Session (start at 10.00) 10.30 – 11.15
Invited Speaker 3
Prof. S.K. Lai, National Central Univ., Taiwan
Title: “Cluster Dynamics by Ultra-Fast Shape Recognition Technique”
11.15 – 12.00
Invited Speaker 4
Dr. Nurul Taufiqurrochman*, Indonesian Nanotech Society
Title:”Nanotechnology Development Strategy for Supporting National Industry
in Indonesia”
12.00 – 13.00 Lunch Break
13.00 – 15.00
Parallel Session Parallel Session
15.00 – 16.30
16.30 – 17.00 Closing Ceremony
17.00 – 19.00 Free Time
Parallel Session Day One - 16 April 2013
Majapahit 1 Room: Chemistry
Time Paper ID Author(s) Title Moderator
13.00-13.30 Invited Prof. Petr Solich Recent Trends in Liquid Chromatography for Bioanalysis
13.30-14.30
C01
Saprizal Hadisaputra, Harno Dwi Pranowo, and Ria Armunanto
Liquid-Liquid Extraction of UO2 2+
cation by 18-Membered Crown Ethers: A DFT Study using A Continuum Solvation Model
Akhmad Sabarudin,
D.Sc.
C02
Heruna Tanty,
Margaretha Ohyver, Tati Herlina, and Nurlelasari
Analysis of Inorganic Compounds Cr, Cd, CN, Mn, and Pb in RAW Water and Water
Filtration Results in Jakarta-Indonesia
C03 Subriyer Nasir, Anthony
B. Hamzah
Pervaporation through NaA Zeolite Membranes – A Review
C04 S.Muryanto and E.
Supriyo
Inhibition of citric acid on the precipitation of calcium sulphate dihydrate (CaSO4.2H2O)
C05
Hermin Sulistyarti, Atikah, Sita Febriyanti, Asdauna
A New Spectrophotometric Method for Iodide Determination
Discussion/Question/Answer
14.30-15.30
C06
Chandrawati Cahyani, Edi Priyo Utomo, and Wa Ode Cakra Nirwana
Optimum Condition for Separation of Two Immiscible Liquids,Patchouli Oil and Water, and the Design of Separator
Masruri, PhD
C07
Rurini Retnowati, Unggul Pundjung Juswono, Oktawirandy Rajaki
Free Radical Scavenging Ability of Xanthone Isolated from the Mangostene Pericarp (Garcinia MangostanaL.) by Electron Spin Resonance (ESR)
C08
Muhammad Said, Abdul Wahab Mohammad, Akil Ahmad
Optimization of NaOH as the cleaning agent of Polyethersulfone (PES) membrane fouled by Palm oil mill effluent
Parallel Session Day Two 17 April 2013
Majapahit 1 Room: Chemistry
Time Paper ID Author(s) Title Moderator
10.00-11.00
C10 Imelda Fajriati, Mudasir,
Endang Tri Wahyuni
Room-Temperature Synthesis of TiO2–
Chitosan Nanocomposite Photocatalyst
Akhmad Sabarudin,
D.Sc.
C14 Masruri and Malcolm D.
McLeod
Amino acid-based ligand for the osmium catalyzed asymmetric aminohydroxylation reaction in styrene
C15
Suwardi,Harno Dwi Pranowo dan Ria Armunanto
Structure of Hf(IV) in aqueous solution – An
ab initioQM/MM MD approach
C16
Crys Fajar Partana, Ria Armunanto, Harno Dwi Pranowo, M Utoro Yahya
Molecular Dynamics Simulation of
Scandium(I) Singlet in Liquied Ammonia by
ab initio QM/MM MD
Discussion/Question/Answers
11.00-12.00
C11
Rosenani A. Haque, Choo Sze Yii and Srinivasa Budagumpi
Silver(I) and mercury(II) complexes derived from nitrile-functionalized N-heterocyclic carbene: Synthesis, crystal structure, DNA binding and nuclease studies
Lukman Hakim,
D.Sc.
C12 Nurul Filzah Ghazali
and Ibrahim Baba
Synthesis and Spectroscopy of Dibutyltin (lV) Dithiocarbamates Compounds
C13 Nur Fariza Abdul
Rahman, Mahiran Basri
Studies of Parameter Effects on Lipase-catalyzed Synthesis of Engkabang Fat Esters
C17
Abdolhamid Ansari, Zahra Sajadi and Jaber Mozafarizadeh
Assessment of Hydrochemical Interactions between Galendar's Aquifer and Geological Formations
Discussion/Question/Answers
Abstract—Study of structural properties of Sc+singlet in liquid ammonia has been carried out by means of the ab initio QM/MM molecular dynamics simulation approach. Structural properties of Sc+ in liquid ammonia have been evaluated on the basis of a molecular dynamics (MD) simulation by the ab initio quantum mechanical/molecular mechanical (QM/MM MD) method at Restricted Hartree–Fock (RHF) level using LANL2DZ ECP basis sets for Scndium and Dunning double-ζ plus polarization (DZP) for liquid ammonia, respectively. Solvation structure of Sc+ in liquid ammonia was characterized using RDF, CND, and ADF data obtained from trajectory files. The first solvation shells consist of 6 liquid ammonia molecules, with Sc+_N distance of 2.197 Å.
Keywords: ab initio, liquid ammonia, Sc+ singlet, Solvation
QM/MM MD simulation
I. INTRODUCTION
candium (Sc) is one of the transition metal plays an important role in the metabolism of living things. The research on scandium metal function as in suppressing the formation of harmful bacteriostatic in Klebsiella pneumoniae is present in serum have been carried out [1]. Scandium complex of enterochelin promote bacteriostasis P.aeruginosa in serum and also provide a therapeutic effect against infection with P. aeruginosa in living organisms. Scandium can also function as antibodies [2].
Structure and dynamics of ions dissolved by the solvent can be determined in two ways: by experiment and computer simulation. Determination of structure and dynamics of ion solvation through experiments require some equipment, such as: X-ray diffraction, neutron diffraction, electron diffraction, spectroscopic methods, NMR and some of the equipment based on the method of scattering the others. Determination of structure and solvation dynamics through computer simulations performed by Monte Carlo simulation (MC) and Molecular Dynamics (MD) [3].
Ray diffraction techniques (X rays, neutrons, electrons) give information about the structure of complex compounds such as ligand bond distance and coordination number of ion-ligand complex, while the NMR provides information on the nature of dynamics known as residence time of the average ligand in the solvation layer. NMR technique provide the solvation number (if ion strongly bound to the ligand), but
1)Departement of Chemistry, Faculty of mathematics and natural sciences, States University of Yogyakarta
2)Departement of Chemistry, Faculty of mathematics and natural sciences Gadjah Mada University, Yogyakarta
NMR technique can not follow the process of fast ligand exchange [4]. It also can not detect the dynamics of condensation occurring in unit time under a 10-9 second. Similar situation for a femtosecond (10-15second) laser pulse spectroscopy which can not describe accurately the nature of the dynamics of the solution. This information indicates that the way the experiment has the weakness in the detection limit the movement of molecules in solution. This experimental weaknesses can be solved by computer simulation [5].
This research is using quantum mechanical/molecular mechanical mechanics dynamics (QM/MM MD) method. This method was chosen because it takes relatively quick and fairly accurate results, provides the proper basis set is used and involves many body potential.
Electron configuration of scandium (Sc) in the ground state is 1s22s22p63s23p63d14s2. Sc+initial electron configuration (low spin/triplet) is 1s22s22p6 3s23p63d1 4s1 whereas high-spin configuration of Sc+ (singlet) is 1s2 2s2 2p6 3s2 3p6 3d2 4s0.
II. EXPERIMENTAL SECTION
A.
Materials
This research is a theoretical study of metal ion interaction Sc+ singlet in liquid ammonia as a ligand by using ab initio calculation method. Sc+ as central metal ion is surrounded by as many as 215 molecules of NH3.
B.
Instrumentation
Hardware
A set of complete computer with specs Processor Intel ® Pentium Core 2 Quad 2.4 GHz, Random Access Memory (RAM) 3.34 GB effective, Graphic Array Video Card NVIDIA ® 512 MB, Hard disk with a partition of 120 GB. Software
• Gaussian 2003 is used to obtain the best basis set for the system under study.
• Turbomole version 5.10 is used for collecting energy points on a variety of different points of energy of pair potentials, as well as many body effect of energy correction (three body). • MD simulation programQM/MM MD, which is a special program that is used to simulate the QM/MM MD to obtain energy data systems and time-dependent coordinates data. Procedure
Molecular Dynamics Simulation of Scandium (I) Singlet
In Liquid Ammonia By AB Initio QM/MM MD Methods
S
Determination of coordinates of Sc- NH3in Cartesian
coordinates
Initial geometry of Sc in NH3 is modeled in
[image:14.612.42.305.169.300.2]three-dimensional Cartesian coordinates to adjust the angle and distance between atoms in the system. Based on experiments that the H-N-H angle of 106,68° and N-H bond lengths of 1,0124Å [7]
Table 1
Initial Geometry of Sc in NH3in Cartesian coordinates
Atoms
X (Å)
Y(Å)
Z (Å)
Sc
0,000000
0,000000
1,400000
N
0,000000
0,000000
0,000000
H
0,000000
0,937002
-0,383001
H
0,812002
-0,468001
-0,383001
H
-0,812002
-0,468001
-0,383001
Selection of the best basis set
From several basis pairs that have tested the set of the basis set that does not cause a significant change in the charge of ion scandium (Sc) and has a profile curve of binding energy of Sc-N distance in accordance with the profile curve of Lennard-Jones potential. From the results obtained by the set of the basis set selection Lanl2dz ecp for scandium atoms and DZP for the atoms of hydrogen and nitrogen.
Preparation of Sc-NH3pair potential
In preparation of the pair potential equation, it takes Sc-NH3energy points at various distances Sc against NH3and
at various angles theta (θ) and phi (Φ.). The points of this energy is used to construct pair potential functions.
Pair potential function for Sc-NH3 interaction has been
formulated through the calculation of ab initio methods at the Restricted Hartree-Fock (RHF) for scandium singlet ion (sc+).
The minimum energy system (
E
2b) between Sc and NH3 is calculated by reduction of Sc-NH3 complex energywith the energy of the respective monomers
E
Sc andE
NH3in mathematical form is:
3 3
2b
E
Sc NHE
ScE
NHE
(1)
Data points of energy at various angles theta and phi are obtained, then further processed by fitting two bodies. Fitting the energy conducted to obtain some form of mathematical equations that represent functions that energy with the algorithm. The algorithm used in the preparation of analytical potential functions with the least square method of Lavenberg-Marguart. Potential equation form two bodies Sc+-NH3is as
follows: 3 2 1 M i Mi
i i i i
bd
fit a b c d
i Mi Mi Mi Mi
q q
A
B
C
D
E
r
r
r
r
r
(2)Where a, b, c, d, Ai, Bi, Ci and Di are the optimized
parameters summarized in Table 1, RMidistance of the i-th of
atom of Sc and NH3, qiand qMis the charge of atoms of Sc
and NH3.
Simulation protocol
[image:14.612.320.568.303.453.2]The simulations were performed for one Sc+ and 215 ammonia molecules in a cubic box, at 235.16 K, which corresponds to the experimental density of 0.690 g/cm3. Periodic boundary conditions were applied to the simulation box and the temperature was kept constant by the Berendsen algorithm [8]. A flexible ammonia model which includes an intramolecular term was used [7]. Accordingly, the time step of the simulation was set to 0.2 fs, which allows for explicit movement of hydrogens. A cut-off of 12.0 Å was set except for N–H and H–H non-Coulombic interactions for which it was set to 6.0 and 5.0 Å .
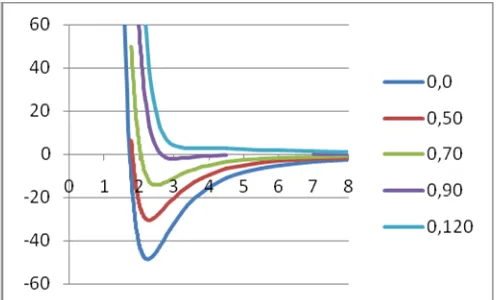
Figure 1. Curve of pair potential function for Sc-NH3with
the basis set LANL2DZ –DZP Simulation QM/MM MD
A classical molecular dynamics simulation was carried out for 100 ps using the pair potential function. The subsequent QM/MM simulation was performed for 10 ps after 20 ps of re-equilibration. The ab initio HF formalism with the same basis sets used for the potential construction was applied to the ion and the full first solvation shell, and for the remaining MM region the same 2-body potential as in the classical simulation was used. According to the Sc–N RDF of the classical simulation, the QM radius had to be set to 3.2 Å in order to include the full first solvation shell. A smoothing function was applied to the transition region between QM and MM regions [8]. The force of the system, Fsystem, is defined as
Fsystem= FMM+ S(FQM- FQM/MM) (4)
where FMM is the MM force of the full system, FQM the QM
force in the QM region and FQM/MMthe MM force in the QM
III. RESULTS AND DISCUSSION
A. Radial Distribution Functions
Radial distribution function (RDF) is distance distribution function of Sc-NH3. RDF of the Sc-N, Sc-H and
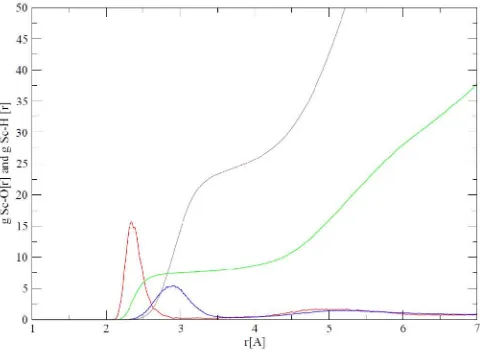
[image:15.612.48.291.190.365.2]the number of its integration obtained from QM/MM MD simulations are shown in Figures 2 and some characteristic value are listed in table 2 and table 3. Figure 1 shows the first shell solvation Sc+ by liquid ammonia is represented by the first peak of RDF Sc-N 2.197Å centered.
Figure 2. Sc-N and Sc-H radial distribution function In figure 2 shows that at a distance of 2.95 peak of RDF Sc+-H reaches a maximum value of the first and was down to a minimum value at a distance of 3.45 Å. This peak shows the first shell solvation of the H atoms of the molecule NH3. RDF integration numbers Sc+-H in the first solvation
shell amounted to 6. The second peak occurs in Sc+-H distance of 5.32 Å and reaches a minimum at a distance of 6.44 Å. RDF integration Numbers Sc+-H in the second solvation shell amounted to ~16.
[image:15.612.316.553.465.637.2]RDF integration Numbers Sc+-H well in the first solvation shell or the shell the second solvation according to the RDF Sc-N. RDF peak of Sc+-H both in the form of ramps (not sharply) suggests that the second shell solvation structure can not be determined precisely.
Table 2
Optimized parameter of the analytical Sc+-H2O
pair potential function A
(kcal mol-1A5) A (kcal mol-1A7)
A (kcal mol-1A9)
A (kcal mol-1 A12)
Sc+- N -7624.28775 41844.02312 -59090.0078 30000.42401 Sc+- H -486.58718 7596.26736 -20518.3923 20472.10809
Distance N and H of Sc+based RDF simulation results in the first solvation shell is 2.197Å and 2.95 Å. This distance difference indicates that the first peak of RDF Sc+-N do not overlap with the first peak of Sc+-H RDF and RDF first peak
of Sc+-N occurred at distances shorter than the first peak of Sc+-H RDF. This phenomenon indicates that the solvation in the first shell has a fixed structure with nitrogen atoms leads to the ions Sc+, while the hydrogen atoms away from Sc+.
Table 3
Characteristic values of the radial distribution functions for Sc+in liquid ammonia
r
M1r
m1N
m
1r
M2r
m2N
m
2Sc N 1.88 2.74 6 4,03 6.81 ~16
Sc H 2.54. 3,37 18 4.23 6.95
B. Coordination Number Distribution
Based on the analysis of the coordination number or the number of ligands that surround the central atom in both solvation first shell and second solvation on the shell as well as the percentage likelihood that there could be analyzed based on information obtained from the CND. Distribution of coordination number for Sc+-NH3system is shown in Figure 3.
In the first shell solvation solvation numbers indicate the number 6 with an abundance of 90,66% while in the second shell solvation show number ~16 with the accuration of 21,30%
C. Angular Distribution Functions
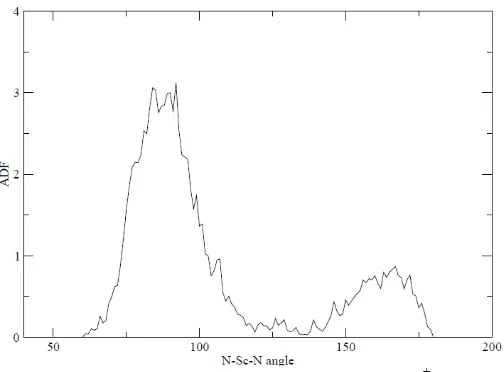
Analysis of solvation structure of Sc+-NH3 is done by
evaluating the angle distribution function (ADF) as result of MM/MD simulation. ADF gives information about the distribution of bond angle formed between the N-Sc+-N. From the angle distribution of N-Sc-N (figure 4) shows a dominant peak at an angle of 85o distance of 2.197 Å. This indicates that the simulation of Sc+ in liquid ammonia show the existence of complexes with a non rigid shape.
[image:15.612.46.301.586.677.2]Figure 4 Angular Distribution Function of O-Sc+-O angles obtained by QM/MM MD simulation
IV. CONCLUSION
QM/MM MD simulation methods is used to study the solvation structure of Sc+ions in liquid ammonia, in order to produce information about the solvation structure of Sc+ ions in liquid ammonia binds six (6) liquid ammonia molecule. The distance between Sc+ with the N of NH3 molecules in first
solvation shell is
2.197
Å. Greatest probability for finding N in the second solvation shell is at a distance of 5.5 Å, with a number of integration in the second solvation shell amounted to ~ 16.REFERENCES
[1] Roger, H.J., Synge, C., Woods, V.E., 1980, Antibacterial Effect of Scandium and Indium Complexes of Enterochelin on Klebsiella pneumoniae,
Antimicrob Agents Chemother, 18, 63-68.
[2] Silva, J. J. R., Williams, R. J. P., 1991, The Biological Chemistry of The Elements, Claredon Press, Oxford.
[3] Pranowo, H.D. dan Hetadi AKR., 2011, Pengantar Kimia Komputasi,
Austrian-Indonesian Centre for Computational Chemistry (AIC), Jurusan Kimia Fakultas MIPA Universitas Gajah Mada, Yogyakarta [4] Armunanto, R., Schwenk, C.F., Rode, B.M., 2004, Gold(I) in Liquid
Ammonia: Ab inito QM/MM Molecular Dynamics Simulations. J. Am. Chem. Soc.,126, 9934.
[5] Rode, B.M., and Hofer, T.S., 2006, How to Access Structure and Dynamics of Solutions: the Capabilities of Computational Methods,
Pure Applied Chemistry, 78, 525–539.
[6] Armunanto, R., Schwenk, C. F., Randolf, B. R., & Rode, B. M. (2004). Ag (I) ion in liquid ammonia. Chemical physics letters, 388(4), 395– 399.
[7] Kheawsrikul, S., Hannongbua, S. V., Kokpol, S. U., & Rode, B. M. (1989). A Monte Carlo study on preferential solvation of lithium (I) in aqueous ammonia. J. Chem. Soc., Faraday Trans. 2, 85(6), 643–649. [8] M.P. Allen, D.J. Tildesley, 1987., Computer Simulation of Liquids,