PAN-Immobilized PVC-NPOE Membrane
for Environmentally Friendly Sensing of Cd(II) Ions
Moersilah
1,2, Dwi Siswanta
2, Roto Roto
2, and Mudasir
2,* 1Department of Chemistry, Faculty of Mathematics and Natural Sciences, Jakarta State University, Jl. Pemuda No. 10 Rawamangun Jakarta Timur 13220, Jakarta-Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Gadjah Mada, Sekip Utara PO BOX BLS 21 Yogyakarta 55281, Indonesia
Received September 14, 2016; Accepted February 7, 2017
ABSTRACT
A simple, cheap and environmentally friendly analytical method of Cd(II) analysis in the aqueous system has been developed by immobilization of 1-(2-pyridilazo)-2-naphtol (PAN) in polyvinyl chloride (PVC) matrix and nitrophenyl octyl ether (NPOE) as a plasticizer. Upon contact with Cd(II) in the solution, the color of sensor membrane changes from dark yellow to dark red, which is due to the formation of Cd(II)–PAN complex. The best
sensing results were obtained at pH 8.0 and λmax 558 nm. The dimension of the proposed sensor membrane was
0.8 cm x 2 cm with a thickness of 0.05 mm, the volume of sample was 2 mL with the Cd(II) concentration range of 0– 1.2 ppm. The detection limit of the method was found to be 0.048 ppm and molar absorptivity of 4.42 x 105L mol-1cm-1. The proposed methods have been applied in the determination of Cd(II) in water samples after addition of internal standard with recovery 98.88-100.75%.
Keywords:1-(2-pyridylazo)-2-naphtol (PAN; PVC; Cd(II) analysis; chemical sensor
ABSTRAK
Metode analisis yang sederhana, murah dan ramah lingkungan untuk menentukan Cd(II) dalam air telah dikembangkan, dengan immobilisasi 1-(2-piridilazo)2-naftol (PAN) dalam matriks PVC dan nitrofenil oktil eter sebagai pemlastis. Setelah kontak dengan larutan Cd(II), membran sensor berubah warna dari kuning tua menjadi merah tua, karena terbentuknya kompleks Cd(II)-PAN. Respon optimum membran sensor diperoleh pada pH 8,0
dan λmaks558 nm. Ukuran membran sensor yang diajukan 0,8 cm x 2 cm, tebal 0,05 mm dan volume sampel 2 mL
dengan rentang konsentrasi 0-1,2 ppm. Batas deteksi metode 0,048 ppm dan absorptivitas molar 4,42 x 105L mol-1cm-1. Metode yang diajukan telah diaplikasikan untuk menentukan Cd(II) dalam sampel air setelah penambahan larutan standar dengan rekoveri 98,88-100,75%.
Kata Kunci:1-(2-piridilazo)-2-naftol (PAN); PVC; analisis Cd(II); sensor kimia
INTRODUCTION
The presence of heavy metal ions in the water bodies is becoming serious environmental problems due to their potential hazards to living organisms. Therefore, monitoring of those metal ions in the environment such as wastewater, river water, water reservoirs or drinking water source is of crucial issues. Cadmium (Cd) is one of hazardous metal ions in the body of waters. As a pollutant, the high level of Cd(II) in water is dangerous for human health due to its toxic and carcinogenic potentials[1]. Cadmium can affect erythrocyte and kidney [2-3], liver and can cause osteoporosis [4]. The maximum permitted concentration of Cd(II) in waste water and drinking water is 0.1 and 0.05 ppm respectively [5-6].
To monitor the exposure of Cd(II), a simple, fast, safe, cheap, and environmentally friendly analytical method is necessary to be developed. Optical chemical sensor membrane is a suitable and promising alternative for the purposes, especially for the application in field analysis, because it is easy to prepare and can be prepared as a test kit. Furthermore, it has been known that the protocol analysis using optical chemical sensor membrane meets the criteria of ‘green analytical chemistry, e.g. reducing organic solvent consumption (toxic compounds), decreasing workforce and energy, vapors and gas emission, and waste [7-8].
Cd2+ + N choice of suitable compounds/ligands that are soluble in
polymer matrix should come into consideration [9]. So far, some polymers have been reported to be used for immobilization of various specific ligands. Among them are cellulose three acetate (CTA) with PAN for Cd(II) analysis [10], 2-amino-cyclopentane-1-dithiocarboxylic acid for Cd(II) analysis [11], dithizone for Cd(II) [12], 5(p-dimethylaminobenzylidene)rhodanine for Ag(I) [13], indophenol for Ni(II) [14], polymethacrylate (PMA) with PAN for Co, Cd, Ni, Cu, Zn, Mn, and Pb [15-16], with dithizone for Ag(I) [17]. Other reports have also used polyvinyl chloride (PVC) with 2-mercaptopyrimidine for Hg [18], with c-methylcalix[4]resorcinarene (CMCR) for Ti(III) [19], 4-(2-pyridylazo)resorcinol (PAR) for thallium [20], bis(thiophenol)-4,4´-methylenedianiline for Hg(II) [21], PAN for Cr(III) [22]. Also, polysulfone membrane with porphyrin for Cd(II) [23], thin layer calix [4] dicyano-diimidazole on the glass surface for Cu (II) analysis [24] has also been reported.
In this study, we proposed an optical chemical sensor membrane by immobilization of PAN in PVC membrane using nitrophenyl octyl ether (NPOE) as a plasticizer. The obtained chemical sensor is intended to be used for Cd(II) determination in aqueous systems. The selection of PAN as a specific ligand is based on the fact that this ligand can coordinate various transition metals especially Cd(II) to form colored metal complexes [25-26]. The hydroxyl group and azo-nitrogen in PAN can build a covalent bond with Cd(II) forming an intense color complex. The produced optical chemical sensor membrane has been characterized using infrared (IR) spectrophotometer and applied for Cd(II) determination in natural water samples spiking with Cd(II) ions.
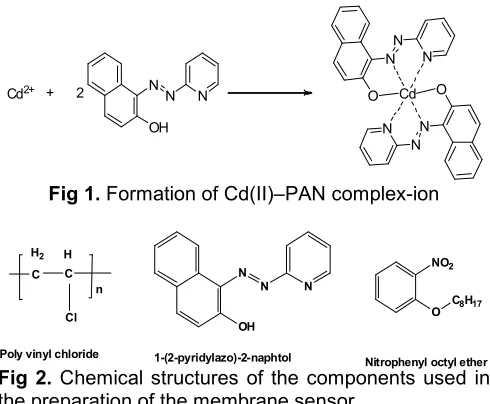
PAN is a complexing agent for some metal ions, such as Cd, Zn, Hg(II), Bi, Cu, Pb, Fe(III), and Th [25-27]. PAN is not a selective reagent, yet its selectivity can be reached by adjusted the pH value appropriate with the tested solution [26]. Stoichiometrically, the molar ratio of the reagents to form Cd(II) – PAN complex in water is 1:2 [25-26]. Reaction equation of formation of Cd(II) – PAN complex is shown in Fig. 1.
EXPERIMENTAL SECTION
Materials
The reagents were of analytical grades, i.e. Cd(NO3)2, NaOH, HCl, THF, CuCl2, Zn(NO3)2, Ni(NO3)2
and FeCl3 (Merck), 1-(2-pyridylazo)-2-naphtol) (PAN)
and NPOE, PVC (molecular weight 5000) (Sigma-Aldrich), and distilled water used as solvent. The pH of the solution was adjusted using a solution of NaOH and HCl 0.1 M. Fig. 2 gives the chemical structures of the components used in the preparation of the sensor membrane.
Fig 1.Formation of Cd(II)–PAN complex-ion
Fig 2.Chemical structures of the components used in the preparation of the membrane sensor
Instrumentation
Instrumentation of pH meter (Thermo SCIENFIC ORION 4 STAR, pH*ISE portable) for measuring the pH of the analyte solution. UV-Vis spectrophotometer Shimadzu (Japan) UV-2450 models to measure the absorbance of the Cd(II) solution and FTIR Shimadzu (Japan) spectrophotometer model IR Prestige-21 for the characterization of membrane sensors.
Procedure
Preparation of sensor membrane
The sensor membrane was prepared by dissolved 0.4 g PVC, 0.01 g PAN, and 90 µL NPOE in 10 mL THF, stirred in constant speed at room temperature for 5 h. Then the reaction mixture was poured in a glass cast, kept at room temperature for 5–7 h until all the solvent evaporated. Hereafter, the membrane was taken off from the cast, washed with distilled water, dried at room temperature, and then cut 0.8 x 2 cm with a thickness of 0.05 mm, so that the membrane is ready to be used.
Characterization of sensor membrane
Determination of optimum pH
The optimum pH was determined by soaking the membrane in a 2-mL solution containing 2 ppm Cd(II) for 240 min, and the absorbance of the membrane was measured using a UV-Vis spectrophotometer (Shimadzu
- UV 2450) at λ 558 nm. The studied range of pH was
7.5–9.0, where the highest absorbance was chosen as the optimum pH.
Calibration curve
The range of calibration curve of absorbance of Cd(II) – PAN complex was studied at a concentration of Cd(II) between 0.0–1.2 ppm. The procedure followed as the determination of optimum pH.
Interference of ions
To study the interference of other ions, the following ions were used, i.e. Cu(II), Zn(II), Ni(II), and Fe(III). It was done as the optimum condition obtained earlier. The solution of interfering cations was added to Cd(II) solution in concentration ratio 1:1, and the
absorbance was measured at λ 558 nm and pH 8.0.
Application in water sample
For the application of the procedure, a water sample was obtained from River Code, a river that flows across Yogyakarta City, Indonesia. The water sample was acidified with 1.0 M HNO3 until pH about 3, filtered
off and kept in a propylene bottle at room temperature. For Cd(II) determination, the sample was adjusted to pH
8.0 by adding NaOH 0.1 M solution. Furthermore, the sample is ready to be analyzed quantitatively using sensor membrane, and the absorbance was measured using UV-Vis spectrophotometer.
RESULT AND DISCUSSION
Membrane Sensor Characterization by IR
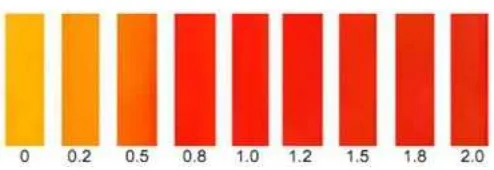
The infrared spectra indicate that immobilization of PAN in the membrane matrix is successful as displayed in Fig. 3 (d). A band at 3310 cm-1suggests a stretching vibration of –OH group of a naphthol, while a band shows the existence of N=N group attached to the aromatic ring at 2338 cm-1. An intense band at 1605 cm-1 is attributed to the N=O group, whereas a band at 1257 cm-1shows the existence of Caryl-O-Calkyl
group. Both functional groups suggest the presence of NPOE as a plasticizer of the sensor membrane.
Influence of pH
The pH of Cd(II) solution was measured in the range of 7.0–9.0 to obtain the better response of the sensor membrane, where pH 8.0 gave the optimum pH. The absorbance of the membrane at various pH is showed in Fig 4. The noticeable decrease in the absorbance was observed, which is believed to be due to the leaching of the immobilized ligand from the sensor membrane.
Fig 4.Response of the sensor membrane toward 2 ppm Cd(II) solution at various pHs
Fig 5.Calibration curve of the sensor membrane. The absorbance was recorded at 558 nm
Calibration Curve
The experiment to understand the correlation between concentration and absorbance was performed using 2 mL Cd(II) solution with various concentrations (0–1.2 ppm) at 558 nm, pH 8. The results are displayed in Fig. 5. It demonstrated that a linear correlation between concentration of Cd(II) solution and absorbance was obtained following equation of y = 0.7249·x + 0.0781, with coefficient of determination R2 = 0.96 and molar absorptivity 4.42 x 105 L mol-1 cm-1. The linear range was obtained at 0.2–1.2 ppm. The detection limit was calculated by 3x of deviation standard of blank signal and was 0.048 ppm and limit of quantification 0.146 ppm. The detection limits obtained are smaller than the results of previous studies, the immobilization of 2-amino-1-cyclopentene-dithiocarboxylic acid (ACDA) on a cellulose triacetate membrane, which is 0.2 ppm [11].
Response of Sensor Membrane
Absorbance spectra of sensor membrane before and after contact with Cd(II) solution at pH 8.0 is displayed in Fig. 6. Before contact with Cd(II) solution, the sensor membrane showed a maximum absorbance
at λ 470 nm, while after contact at λ 558,40 nm. The
complex of Cd(II) – PAN formed in sensor membrane underwent a relatively large bathochromic shift.
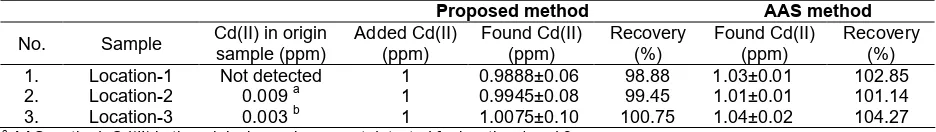
The sensor membrane changed its color from dark yellow to dark red after contact with Cd(II) solution, as showed in Fig. 7. A linearity of the color intensity of the sensor membrane with the concentration of Cd(II) solution was observed.
Influence of Interference Cations
The response of the developed sensor membrane for Cd(II) determination toward interference cations, namely Cu(II), Zn(II), Ni(II) and Fe(III) has been tested.
Fig 6.Absorbance spectra of sensor membrane before (a) and after (b) contact with Cd(II) solution
Fig 7.Color change of the sensor membranes in 2 mL Cd(II) solution at concentration the range of 0-2 ppm and pH 8.0
The observation was conducted at the optimum condition of Cd(II) determination by soaking the sensor membrane in the 2 mL Cd(II) solution added with interference cations for 240 min at pH 8.0 and
λ 558 nm. The ratio of the concentration of Cd(II) to the
interference cations 1:1 and the absorbance were
measured at λ 558 nm. The results of interference test
cations presented in Table 1.
Fig 8. Color change of the sensor membrane in 2 mL solution containing other cations in the absence and the presence of Cd(II)
Table 2. Determination of Cd(II) in river water in the location using the proposed method and AAS as comparison method
Proposed method AAS method
No. Sample Cd(II) in origin
sample (ppm)
Added Cd(II) (ppm)
Found Cd(II) (ppm)
Recovery (%)
Found Cd(II) (ppm)
Recovery (%)
1. Location-1 Not detected 1 0.9888±0.06 98.88 1.03±0.01 102.85
2. Location-2 0.009a 1 0.9945±0.08 99.45 1.01±0.01 101.14
3. Location-3 0.003b 1 1.0075±0.10 100.75 1.04±0.02 104.27
aAAS method, Cd(II) in the original sample was not detected for location 1 and 3 b
The proposed method, Cd(II) in the original sample was not detected for locations 1 and 2
absorbance. Furthermore the presence of Cu(II), Zn(II), Ni(II), and Fe(III) in Cd(II) solution (Cd-mix solution) with ratio 1:1:1:1:1 simultaneously increased significantly the absorbance, namely 24.48%.
Fig. 8 shows the change in the color of the sensor membrane after contact with a solution containing the corresponding interference cation without Cd(II) and interference cation solution with Cd(II) in the ratio 1:1 (ppm). The volume of solution used 2 mL at pH 8.0 and
measured by UV-Vis spectrophotometer at λ 558 nm,
corresponding to the optimum conditions for the analysis of Cd(II).
Application of the Method for Natural Water
The manufactured sensor membrane was applied to the determination of Cd(II) of river water by internal addition method. A defined concentration of Cd(II) solution (1 ppm) was added to the river water sample, and the pH was adjusted at 8.0. The sensor membrane was soaked in 2 mL of sample solution for 240 minutes,
and the absorbance was observed at λ 558 nm. The
results are displayed in Table 2. The results obtained using the proposed method gives a value which is almost equal to AAS method. The performance test of the manufactured sensor membrane demonstrated that the sensor membrane can be used for Cd(II) determination of natural water.
CONCLUSION
An optical chemical sensor membrane for Cd(II) determination in the aqueous system has been developed by PAN immobilization in PVC as matrix and NPOE as a plasticizer. The sensor membrane can be
applied for both qualitative and quantitative analyses of Cd(II) present in environmental samples. The color change of membrane represents the qualitative detection, while absorbance change at 558 nm gives the quantitative one. We believe that it can be used as an alternative method for Cd(II) analysis in water where it offers simple, cheap, safe, energy saving, and environmentally friendly preparation. It is also practical for on-the-spot analyses.
ACKNOWLEDGEMENT
We thank the Directorate General of Higher Education of the Education and Culture Ministry of Indonesia for providing postgraduate scholarships for the first author to study at Universitas Gadjah Mada, Yogyakarta, Indonesia with contract number of 937/D/T/2010, on July 30, 2010.
REFERENCES
[1] Manahan, S.E., 2009, “Water Pollution” in Environmental Chemistry, 9th ed., CRC Press, London, 200–239.
[2] Manahan, S.E., 2011, “Water the Ultimate Green Solvent” in Green Chemistry and the Ten Commandments of Sustainability, ChemChar Research, Inc Publishers, Columbia, 159–176. [3] van der Perk, M., 2006, “Heavy Metals” inSoil and
Water Contamination: From Molecular to Catchment Scale, CRC Press, London, 125–137.
[4] Urek, S.K., Frančič, N., Turel, M., and Lobnik, A.,
[5] Mirzaei, M., and Pili, H.B., 2015, Potentiometric determination of cadmium using coated platinum and PVC membrane sensors based on N,N ′-bis(salicylaldehyde)phenylenediamine (salophen), J. Anal. Chem., 70 (6), 731–737.
[6] Ullah, M.R. and Haque, M.E., 2010, Spectrophotometric determination of toxic elements (cadmium) in aqueous media, J. Chem. Eng., ChE 25 (1), 1–12.
[7] Ivanova, E.H., and Detcheva, A.K., 2012, Green analytical chemistry and its perspectives in Bulgaria, Bulg. Chem. Commun., 44 (1), 5–10.
[8] Tobiszewski, M., Mechlińska, A., and Namieśnik, J.,
2010, Green analytical chemistry—theory and practice,Chem. Soc. Rev., 39, 2869–2878.
[9] Mohr, G.J., 2004, "Polymers for Optical Sensors" in Optical Chemical Sensors, Baldini, F., Chester, A., Homola, J., and Martellucci, S., eds., NATO Science Series II: Mathematics, Physics and Chemistry, vol. 224, Springer, Dordrecht, Netherland, 297–322.
[10] Tharakeswar, Y., Kalyan, Y., Gangadhar, B., Kumar, K.S., and Naidu, G.R., 2012, Optical chemical sensor for screening Cadmium(II) in natural waters,JST, 2 (2), 68–74.
[11] Ensafi, A.A., and Isfahani, Z.N., 2011, A simple optical sensor for cadmium ions assay in water samples using spectrophotometry, J. Anal. Chem., 66 (2), 151–157.
[12] Tavallali, H., and Kazempourfard, F., 2009, Determination of cadmium ions by designing an optode based on immobilization of dithizone on a triacetylecelluose membrane in polluted soil and water samples, J. Korean Chem. Soc., 53 (2), 144–151.
[13] Rastegarzadeh, S., and Rezaei, V., 2008, A silver optical sensor based on 5(p-dimethylamino benzylidene)rhodanine immobilized on a triacetyl cellulose membrane, J. Anal. Chem., 63 (9), 897–901.
[14] Baezzat, M.R., and Karimi, M., 2013, Design and evaluation of a new optode based on immobilization of indophenol on triacetylcellulose membrane for determination of nickel, Int. J. ChemTech Res., 5 (5), 2503–2507.
[15] Gavrilenko, N.A., and Saranchina, N.V., 2009, Analytical properties of 1-(2-pyridylazo)-2-naphthol immobilized on a polymethacrylate matrix, J. Anal. Chem., 64 (3), 226–230.
[16] Gavrilenko, N.A., Saranchina, N.V., and Gavrilenko, M.A., 2015, A colorimetric sensor based on a polymethacrylate matrix with immobilized 1-(2-pyridylazo)-2-naphthol for the determination of
cobalt,J. Anal. Chem., 70 (12), 1475–1479. [17] Gavrilenko, N.A., and Saranchina, N.V., 2010,
Solid phase spectrophotometric determination of silver using dithizone immobilized in a polymethacrylate matrix, J. Anal. Chem., 65 (2), 148–152.
[18] Khezri, B., Amini, M.K., and Firooz, A.R., 2008, An optical chemical sensor for mercury ion based on 2-mercaptopyrimidine in PVC membrane, Anal. Bioanal. Chem., 390, 1943–1950.
[19] Rezayi, M., Heng, L.Y., Kassim, A., Ahmadzadeh, S., Abdollahi, Y., and Jahangirian, H., 2012, Immobilization of ionophore and surface characterization studies of the titanium(III) ion in a PVC-membrane sensor, Sensors, 12 (7), 8806–8814.
[20] Ensafi, A.A., and Fouladgar, M., 2014, A new sensitive optical bulk test-system for thallium based on pyridylazo resorcinol,J. Anal. Chem., 69 (2), 143–148.
[21] Firooz, A.R., Ensafi, A.A., Hoseini, K.S., andKazemifard, N., 2014, Development of a highly sensitive and selective mercury optical sensor
based on immobilization of
bis(thiophenal)-4,4′-methylenedianiline on a PVC membrane, Mater. Sci. Eng., C, 38, 73–78.
[22] Moghimi, A., 2011, Cr(III) Selective PVC membrane electrodes based on Schiff base 1- (2-pyridyl azo)2-naphtol complex as an ionophore, Middle-East J. Sci. Res., 7 (2), 147–152.
[23] Zhao, L., Li, M., Liu, M., Zhang, Y., Wu, C., and Zhang, Y., 2016, Porphyrin-functionalized porous polysulfone membrane towards an optical sensor membrane for sorption and detection of cadmium(II),J. Hazard. Mater., 301, 233–241. [24] Rouis, A., Darbost, U., Bonnamour, I., and Ouada,
H.B., 2015, Development and characterization of a copper ion-selective optical sensor based on a novel calix[4]dicyano-diimidazole thin film, Mater. Chem. Phys., 164, 145–149.
[25] Ueno, K., Imamura, T., and Cheng, K.L., 1992, "Pyridylazonaphthol, Nitrosonaphthols and Nitrosophenols" inHandbook of Organic Analytical Reagents, 2nd ed., CRC Press INC, Tokyo, 209–236, 357–3678.
[26] De, R.A., Khopkar, A.K., and Chalmers, S.M., 1970, Solvent Extraction of Metals, Van Nostrand Reinhold Company, London, 117–124.