www.elsevier.com / locate / bres
Research report
Striatal preprotachykinin mRNA levels are regulated by stimulatory
agents and dopamine D1 receptor manipulation in rodent organotypic
slice cultures
a a,b ,
*
Brian M. Campbell , Paul D. Walker
a
Cellular and Clinical Neurobiology Program, Department of Psychiatry and Behavioral Neurosciences, Wayne State University, School of Medicine,
Detroit, MI 48201, USA b
Department of Anatomy and Cell Biology, Wayne State University, School of Medicine, 540 East Canfield Avenue Detroit, MI 48201, USA
Accepted 19 September 2000
Abstract
We have utilized an organotypic slice culture system to determine factors which directly influence the expression of striatal preprotachykinin (PPT) mRNA. Striatal slices were generated from 3-day-old male rat pups and cultured on Millicell-CM inserts in serum-containing media. Under these conditions, striatal PPT mRNA levels fell significantly (255.766.2%) in slices cultured for 2 days in vitro (2DIV) as compared to slices placed in culture for 3 h (0DIV). However, striatal PPT mRNA expression did not decline further in
1
4DIV cultured slices (259.667.1%). When 2DIV slices were exposed to combined high potassium (K , 10 mM) and forskolin (10mM) stimulation for 3 h, PPT mRNA levels were increased within areas of the brain normally associated with tachykinin production. Application of the dopamine (DA) D1 receptor agonist SKF-38393 (10 mM) at 2DIV for 3 h also increased (1162.9628.9%) PPT mRNA expression, but increases were localized within the striatum. SKF-38393-stimulated increases were completely blocked by the D1 antagonist SCH-23390 (10mM), which alone had no effect on mRNA levels. However, a 3-h incubation with SKF-38393 on 0DIV slice cultures did not affect PPT mRNA expression whereas SCH-23390 decreased PPT message levels (224.565.4%). These findings indicate that tachykinin gene expression is inducible within slice culture preparations and that the maintenance of normal striatal PPT mRNA levels depends on DA D1 receptor tone. 2001 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Basal ganglia
Keywords: Tachykinin; Basal ganglia; In situ hybridization; Gene expression
1. Introduction where variables can be more easily controlled and
manipu-lated. The organotypic slice culture model developed by Our current understanding of striatal tachykinin gene Stoppini et al. [29] provides an additional approach in regulation by trans-synaptic factors is based largely upon which to study how different receptor systems interact to whole animal studies involving systemic drug administra- regulate striatal transmission. This method permits drug tion. Although such approaches have contributed valuable application directly to striatal cells maintained in an in information to the field of basal ganglia research, data vitro environment which retains much of the intrinsic interpretation problems inherent to these models have organization of the intact striatum. For example, choliner-necessitated the need for alternative experimental systems gic interneurons are present in organotypic slice cultures [1,10] indicating that the intrastriatal cholinergic network may still be functional. This is important because
choliner-*Corresponding author. Department of Anatomy and Cell Biology,
gic interneurons may influence how dopamine (DA)
re-Wayne State University, School of Medicine, 540 East Canfield Avenue,
ceptor systems interact to regulate tachykinin biosynthesis
Detroit, MI 48201, USA. Tel.:11-313-5775-678; fax:11-313-5773-125.
E-mail address: [email protected] (P.D. Walker). [32]. Also, at least a portion of the glutamatergic
tion from the cortex remains functionally connected in Media (MEM; Gibco-BRL), and transferred to 0.4-mm slice cultures [30] and glutamate receptor stimulation Millicell-CM (Millipore) tissue culture inserts. The inserts influences the transcription of striatal preprotachykinin were incubated at 378C, 5% CO in media which contained2
(PPT) mRNA [27,28]. Furthermore, D1 receptors have 50% MEM, 25% Hank’s Balanced Salt Solution
(Gibco-been reported to regulate the expression of transcription BRL), 25% heat-inactivated horse serum, 2 mM NaHCO ,3
factors in organotypic slice cultures [17] which are im- 2 mM L-glutamine, and 6.5 mg / ml D-glucose [29]
sup-portant in the activation of striatal tachykinin gene expres- plemented with 10 ng / ml brain-derived neurotrophic factor
sion. (BDNF; Sigma). At 1 day in vitro (1DIV), the media was
How DA receptor populations interact to regulate striatal replaced with fresh media containing 100 U / ml penicillin, tachykinin biosynthesis remains unclear. In the intact 100 mg / ml streptomycin but devoid of BDNF. Slices were striatum, blockade of either D1 or D2 receptors reduces maintained in culture for 2 or 4 days in vitro (DIVs). Some striatal PPT mRNA expression levels [2,3,13] indicating slices were terminated at 3 h after initial culturing (0DIV). that DA transmission is involved in stimulation of tach- All animal use procedures were in strict accordance with ykinin synthesis. Furthermore, in DA-depleted animals, the NIH Guide to the Care and Use of Laboratory Animals PPT message levels are reduced [24,25,33] and stimulation and approved by Wayne State University Animal Inves-with SKF-38393, a D1 receptor partial agonist, reverses tigation Committee.
this trend [5,11]. However, stimulation of D1 receptors
within the intact striatum with the full agonist, SKF-82958, 2.2. Drug treatments has been reported to increase PPT message levels [32]
while partial agonists do not [13]. Also, stimulation of D2 At 2DIV, slices were exposed to vehicle or drug 1
receptors may positively regulate PPT mRNA expression, treatment for 3 h. For high K / forskolin (K / For) stimula-1 but this mechanism seems to be dependent on D1 receptor tion, slices received a final concentration of 10 mM K
tone [13]. and 10mM forskolin (Sigma) [31] dissolved in a vehicle of
In the present study, we have examined the relationship MEM–dimethylsulfoxide. SKF-38393 (Sigma) and SCH-between D1 receptor activation and the maintenance of 23390 (RBI) were each administered at 10 mM [17] in a tachykinin gene expression within striatal organotypic slice vehicle of 0.9% saline. Cultures were harvested by immer-cultures. The use of this system is advantageous in that it sion in 4% paraformaldehyde. Where possible, control and allows brain tissue to be exposed to a known concentration drug treatments were performed on contralateral hemi-of drug without the pitfall hemi-of diffusion which occurs spheres from individual animals. All other experiments following direct brain injections. Also, it limits the in- were carried out on hemispheres from littermate brains. fluence of indirect mechanisms where D1 receptor
stimula-tion could regulate PPT message levels such as activastimula-tion 2.3. ‘Free-floating’ in situ hybridization of distant anatomic circuits that innervate the striatum. The
results of this study are consistent with the idea that D1 PPT cRNA probes were generated from the plasmid receptor stimulation is necessary for basal tachykinin gene pGEM2-31-1 containing the full-length 1100 nt g-PPT
35
expression within the striatum. Furthermore, it indicates cDNA [15]. The resulting S-UTP labeled antisense that cultured striatum responds similar to DA lesion probes were subjected to alkaline hydrolysis to produce models in that PPT message levels decline and D1 receptor ,200 nt probe fragments which were phenol–chloroform
activation reverses this trend. extracted and ethanol precipitated.
Prior to hybridization, tissue slices were partially di-gested by 10 mg / ml proteinase K and acetylated with 2. Materials and methods 0.25% acetic anhydride. The sections were then incubated in hybridization buffer [50% deionized formamide, 53
2.1. Organotypic slice cultures saline sodium phosphate EDTA, 10% dextran sulfate, 53
Denhardt’s solution, 100mg / ml denatured single-stranded Postnatal day 3 male Sprague–Dawley rat pups (Charles (ss) DNA, 100mg / ml Escherichia coli tRNA, and 10 mM River Laboratories) served as the origin for organotypic dithiothreitol] without radio-labeled probe for 60 min at slice cultures in all experiments with the exception of one 528C followed by hybridization in the same buffer
over-6 35
in which postnatal day 5 animals were used as age- night with|1?10 cpm S-UTP labeled PPT cRNA probe
matched controls. Three to four coronal sections (300mm) at 528C.
per hemisphere were obtained using a Campden Instru- Following hybridization, sections were treated with 10 ments vibratome starting at 22.0 mm from Bregma [23] mg / ml RNAse A. Slices were mounted onto glass slides and collected in ice cold oxygenated Ringer’s solution and exposed to autoradiographic film (Amersham bmax) containing (in mM): 119 NaCl, 2.5 KCl, 1.3 MgSO , 2.54 for 5 days. Developed films were analyzed as scanned CaCl , 1.0 NaH PO , 26.2 NaHCO , and 2.0 g / l2 2 4 3 D- images using a Molecular Dynamics Personal
changes between sections were calculated by measuring cificity for PPT gene regulation within the striatum (Fig. the average optical density of the entire striatal region, the 3C). However, acute stimulation with SKF-38393 did not ventral thalamus (ventrolateral, ventromedial, ventral post- alter PPT mRNA expression in 0DIV brain slices, whereas erolateral, and ventral posteromedial), the ventromedial blockade of D1 receptors by SCH-23390 produced a small hypothalamus and a fixed circular area within the frontal but significant decrease in PPT mRNA expression in the cortex. Background noise was determined by measuring striatum with (225.964.4%) or without (224.565.4%) optical density values in the corpus callosum and sub- the addition of SKF-38393 (Fig. 4). We also analyzed
tracted from all data. Significance (P,0.05) was deter- PND5 slices at 0DIV to compare PND3 2DIV slice
mined by Student’s t-test or one-way analysis of variance cultures with age-matched controls. Application of
SKF-(ANOVA) with Tukey-HSD post hoc using SPSS 8.0 38393 and SCH-23390 to PND5 0DIV cultures produced
statistical software. results identical to PND3 0DIV cultures (data not shown)
suggesting that the differences in D1 receptor stimulation at 0DIV and 2DIV in PND3 animals were not a result of a
3. Results developmental change in PPT mRNA expression.
To determine the extent to which D1 receptor
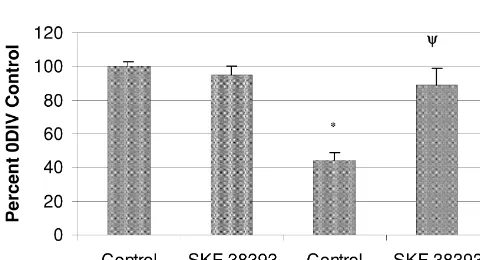
stimula-Slice cultures were analyzed for PPT mRNA content tion was able to rescue PPT mRNA expression, 0DIV
from 0DIV to 4DIV. At 0DIV PPT mRNA expression was cultures were directly compared to 2DIV cultures with or observed in the striatum, thalamus, hypothalamus, without the addition of 38393. Stimulation with
SKF-amygdala, and areas of the cortex. Expression was also 38393 in 2DIV cultures increased PPT mRNA in the
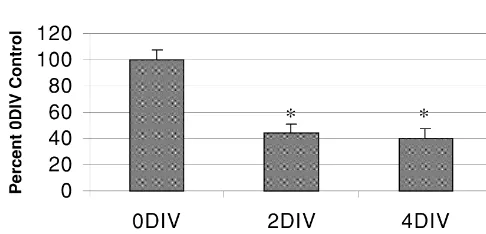
seen to a lesser extent in the hippocampus. Levels of PPT striatum to nearly (89.1610.1%) 0DIV control levels (Fig. mRNA were reduced to 44.366.2% by 2DIV in the 5). Taken together, the results of these experiments suggest striatum, but these levels were maintained at 40.467.1% at that an existing level of D1 receptor tone is necessary to
4DIV (Fig. 1). maintain basal expression of striatal tachykinin production.
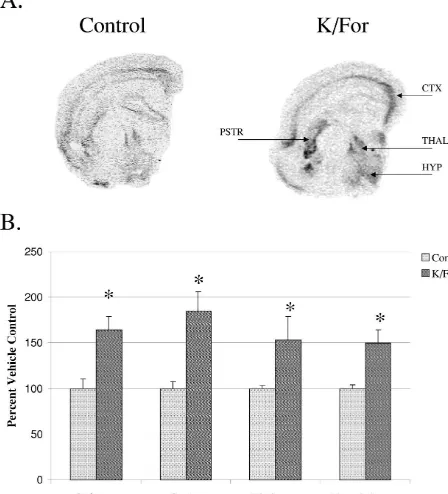
The responsiveness of the slice culture paradigm was tested with K / For at 2DIV. Acute 3 h stimulation resulted
in increased (163.9615.4%) striatal PPT message levels 4. Discussion (Fig. 2). Levels of gene expression were also increased in
the thalamus, hypothalamus, and areas of the cortex The purpose of this study was to examine tachykinin
indicating PPT mRNA expressing cell types in several gene regulation by D1 receptor stimulation within striatal brain regions remain responsive to K / For stimulation at tissue maintained in vitro. Our results agree with previous
2DIV. findings that PPT mRNA expression is reduced under slice
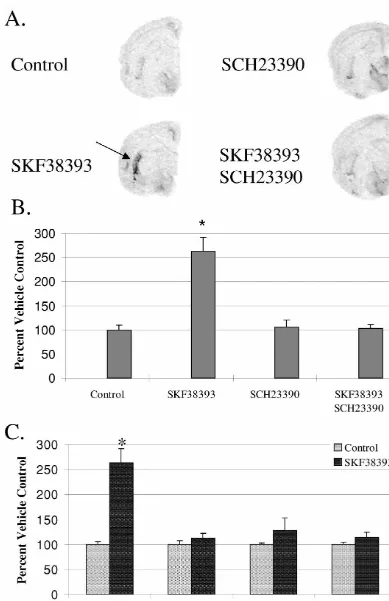
Acute D1 receptor stimulation at 2DIV with SKF-38393 culture conditions [14]. However, application of non-spe-caused a significant increase (1162.9628.9%) in striatal cific stimulating agents (K / For) increased PPT message PPT mRNA expression (Fig. 3). This D1 agonist effect levels at 2DIV indicating that tachykinin neurons remain was completely blocked by SCH-23390, a D1 antagonist viable and responsive to broad stimulating compounds in which alone had no effect on PPT mRNA expression (Fig. culture. It is interesting that while D1 receptor stimulation 3B). SKF-38393 did not significantly alter PPT mRNA was ineffective in raising PPT mRNA levels during the levels in any other region examined indicating its spe- initial hours of culturing, D1 receptor antagonism lowered striatal PPT mRNA expression. However, D1 receptor stimulation significantly increased PPT mRNA levels at 2DIV but the effects were localized within the striatum. Together, these data indicate that D1 receptor tone is necessary for basal expression of PPT mRNA within the striatum.
Why PPT mRNA levels are reduced in culture over time remains to be determined, although several factors may explain this phenomenon. Among these is the loss of monoamine innervation since projections to the striatum from the substantia nigra and dorsal raphe are axotomized during culture preparation. However, the removal of 5-HT
Fig. 1. Densitometric analysis of PPT mRNA levels within the striatum afferents in intact or DA depleted rodents does not appear
maintained in organotypic slice culture. PPT message levels were to significantly alter PPT mRNA expression [4]. Therefore, significantly reduced in striatal tissue at 2DIV and maintained at 4DIV. it is more likely that the loss of DA in our culture system There was no significant difference in message levels between the 2DIV
may produce the observed reduction in PPT mRNA
and 4DIV cultures (*: P,0.05 compared to 3 h (0DIV) control cultures,
content rather than the loss of 5-HT. It is well established
one-way ANOVA with Tukey-HSD post hoc test). All groups represent
Fig. 2. Effects of non-specific stimulating agents on PPT mRNA expression. (A) Autoradiograph representing 2DIV slice cultures with or without K / For
1
stimulation. (B) Densitometric analysis of PPT mRNA levels in the striatum (str) at 2DIV demonstrated that application of high K (10 mM) and forskolin (10mM) resulted in increased gene expression as compared to untreated control cultures (*: P,0.05 compared to untreated control, Student t-test). Each group represents at least five cultures in duplicate. PPT message levels were also significantly increased in the thalamus (152.98626.27%; thal), hypothalamus (149.87614.02%; hyp), and cortex (184.52621.44%; ctx).
ing tachykinin neurons of the striatum. However, it is interesting to note that while application of non-specific stimulating agents increased PPT mRNA in all areas of the brain normally expressing PPT message, D1 receptor-induced stimulation of PPT expression was targeted pri-marily to the striatum. This would be predicted since D1 receptors are located within the striatum with little or no expression in other tachykinin producing regions examined [20,22].
The stimulation of striatal PPT mRNA levels in re-sponse to D1 receptor activation at 2DIV indicates that DA receptors remain functionally coupled to tachykinin pro-duction in culture. However, the lack of effect by
SKF-Fig. 4. Densitometric analysis of PPT mRNA expression in response to
38393 in both PND3 and PND5 0DIV cultures suggests
D1 receptor agonism / antagonism in 0DIV cultures. Application of 10mM
that alterations occur in the culture environment over time
SKF-38393 (SKF) for 3 h did not alter PPT mRNA expression. However,
the blockade of D1 receptors with 10mM SCH-23390 (SCH) produced a that are necessary to observe the effects of D1 stimulation. small but significant decrease in striatal message levels with or without An explanation for these results could be that D1 receptor SKF treatment (*: P,0.05 compared to untreated control cultures,
one-tone is necessary for basal expression of PPT mRNA. This
way ANOVA with Tukey-HSD post hoc test). All groups represent
would be consistent with observations from experiments
mean6S.E.M. of five cultures in duplicate.
performed in vivo which show that SKF-38393 does not affect PPT mRNA levels prior to depletion of DA [5,13]. As such, it is possible that 0DIV cultures, unlike 2DIV
of the reduced PPT mRNA content within striatal slice cultures, retain enough endogenous DA to maximally
cultures. stimulate D1 receptors. In support of this theory, DA
Even though PPT mRNA levels decline in the or- content within the striatum of 0DIV cultures is |85% of
ganotypic slice culture environment, tachykinin neurons uncultured tissue [9]. Moreover, PPT message levels are remain responsive to the application of stimulatory agents. reduced following D1 antagonism at 0DIV with no effect
1
Previous studies have shown that K depolarization in at 2DIV. Also, direct comparison of message levels in combination with direct activation of adenylyl cyclase by 0DIV and 2DIV cultures indicates that D1-induced striatal forskolin produced moderate increases in activation of the PPT mRNA expression is not an increase in gene activa-PPT promoter [21,31]. In our experiments, stimulation tion, but instead a return to baseline. Together these data with K / For increased PPT mRNA levels throughout the support the idea that tonic D1 receptor activation is slice culture indicating that transcription of the tachykinin necessary for basal PPT mRNA expression.
gene remained functional in a variety of cell types includ- However, as previously noted, some cell loss is expected in culture. Similar slice culture experiments have estimated that 60–70% of DARPP-32 positive cells survive in culture for 3 days [17]. Unfortunately, it is unknown what portion of these cells are tachykinin producing neurons and which contain enkephalin. As such, it is entirely plausible that a small population of tachykinin producing neurons remains in culture and becomes super-sensitized to D1 stimulation. This would be consistent with whole animal studies in which activation of D1 receptors following DA depletion resulted in increased cAMP and c-fos production without changes in D1 receptor concentration or binding [6–8,19]. Therefore, the response we measured may in fact result from individual cells expressing increased levels of PPT mRNA rather than a regional return to baseline. Whether the effects of D1 stimulation on PPT mRNA expression in
Fig. 5. D1 receptor stimulation rescues PPT mRNA expression in culture.
Densitometric analysis revealed that D1 agonism significantly increased this study were due to receptor plasticity resulting in
PPT mRNA levels in 2DIV cultures while not affecting 0DIV cultures. strengthened gene regulation or simply caused by the The measured increase in message levels at 2DIV with SKF-38393 was replacement of a tonically active stimulatory influence on not significantly different from 0DIV control cultures (*: P,0.05
striatal neurons has yet to be determined. Further studies
compared to 0DIV control, c: P,0.05 compared to 2DIV control;
will be necessary to distinguish between these possibilities.
one-way ANOVA with Tukey-HSD post hoc). All groups represent
gene expression regulated by interaction of D-1 and D-2 dopamine
stimulation is necessary to maintain striatal PPT mRNA
receptors, J. Pharm. Exp. Ther. 248 (2) (1989) 858–862.
expression in organotypic slice cultures.
[14] C. Humpel, J. Marksteiner, A. Saria, Glial-cell-derived neurotrophic factor enhances biosynthesis of substance P in striatal neurons in vitro, Cell Tissue Res. 286 (1996) 249–255.
[15] J.E. Krause, J.M. Chirgwin, M.S. Carter, Z.S. Xu, A.D. Hershey, Acknowledgements Three rat preprotachykinin mRNAs encode the neuropeptides sub-stance P and neurokinin A, Proc. Natl. Acad. Sci. 84 (1987) 881–885.
We would like to thank Dr. Michael Bannon for his
[16] N. Lindefors, E. Brodin, U. Tossman, J. Segovia, U. Ungerstedt,
helpful comments and suggestions on the manuscript. This
Tissue levels and in vivo release of tachykinins and GABA in
work was supported by grants from Wayne State Universi- striatum and substantia nigra of rat brain after unilateral dopamine ty Internal Research Support Programs and the MI Parkin- denervation, Exp. Brain Res. 74 (1989) 527–534.
[17] F.-C. Liu, H. Takahashi, R.D.G. McKay, A.M. Graybiel,
Dopa-son’s Foundation.
minergic regulation of transcription factor expression in organotypic cultures of developing striatum, J. Neurosci. 15 (3) (1995) 2367– 2384.
[18] J. Luthman, E. Brodin, E. Sundstrom, B. Wiehager, Studies on brain
References monoamine and neuropeptide systems after neonatal
intracereb-roventricular 6-hydroxydopamine treatment, Int. J. Dev. Neurosci. 8 [1] Y. Abiru, C. Nishio, H. Hatanaka, The survival of striatal choliner- (5) (1990) 549–560.
gic neurons cultured from postnatal 2-week-old rats is promoted by [19] J. Luthman, E. Lindqvist, D. Young, R. Cowburn, Neonatal dopa-neurotrophins, Dev. Brain Res. 91 (1996) 260–267. mine lesion in the rat results in enhanced adenylate cyclase activity [2] M.J. Bannon, P.J. Elliott, B.E. Bunney, Striatal tachykinin bio- without altering dopamine receptor binding or dopamine and synthesis: regulation of mRNA and peptide levels by dopamine adenosine 39:59 monophosphate-regulated DARPP-32 immuno-agonists and antimmuno-agonists, Mol. Brain Res. 3 (1987) 31–37. reactivity, Exp. Brain Res. 83 (1990) 85–95.
[3] M.J. Bannon, J.M. Lee, P. Giraud, A. Young, H.U. Affolter, T.I. [20] A. Mansour, J.H. Meador-Woodruff, Q.-Y. Zhou, O. Civelli, H. Akil, Bonner, Dopamine antagonist haloperidol decreases substance P, S.J. Watson, A comparison of D1 receptor binding and mRNA in rat substance K, and preprotachykinin mRNAs in rat striatonigral brain using receptor autoradiographic and in situ hybridization neurons, J. Biol. Chem. 260 (1986) 6640–6642. techniques, Neuroscience 45 (2) (1991) 359–371.
[4] G.J. Basura, P.D. Walker, Suppression of serotonin hyperinnervation [21] C.F. Morrison, J. McAllister, V. Lyons, K. Chapman, J.P. Quinn, The does not alter the dysregulatory influences of dopamine depletion on rat preprotachykinin-A promoter is regulated in PC12 cells by the striatal neuropeptide gene expression in rodent neonates, Neurosci. synergistic action of multiple stimuli, Neurosci. Lett. 181 (1994)
Lett. 274 (1999) 9–12. 117–120.
[5] J.D. Berke, R.F. Paletzki, G.J. Aronson, S.E. Hyman, C.R. Gerfen, [22] C.L. Murrin, W. Zeng, Ontogeny of dopamine D1 receptors in rat A complex program of striatal gene expression induced by dopa- forebrain: a quantitative autoradiographic study, Dev. Brain Res. 57 minergic stimulation, J. Neurosci. 18 (14) (1998) 5301–5310. (1990) 7–13.
[6] G.R. Breese, A.A. Baumeister, T.C. Napier, G.D. Frye, R.A. [23] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, Mueller, Evidence that D1 dopamine receptors contribute to the Academic Press, New York, 1986.
supersensitive behavioral responses induced by L-dehydrox- [24] S.P. Sivam, G.R. Breese, J.E. Krause, T.C. Napier, R.A. Mueller, yphenylalanine in rats treated with 6-hydroxydopamine, J. Phar- J.S. Hong, Neonatal and adult 6-hydroxydopamine-induced lesions macol. Exp. Ther. 235 (1985) 287–295. differentially alter tachykinin and enkephalin gene expression, J. [7] G.R. Breese, T.C. Napier, R.A. Mueller, Dopamine agonist-induced Neurochem. 49 (1987) 1623–1633.
locomotor activity in rats treated with 6-hydroxydopamine at [25] S.P. Sivam, J.E. Krause, The adaptation of enkephalin, tachykinin differing ages: functional supersensitivity of D1 dopamine receptors and monoamine neurons of the basal ganglia following neonatal in neonatally lesioned rats, J. Pharmacol. Exp. Ther. 234 (1985) dopaminergic denervation is dependent on the extent of dopamine
447–455. depletion, Brain Res. 536 (1990) 169–175.
[8] M. Buonamici, C. Caccia, M. Carpentieri, L. Pegrassi, A.C. Rossi, [26] S.P. Sivam, J.E. Krause, G.R. Breese, J.S. Hong, Dopamine-depen-A. Di Chiara, D-1 receptor supersensitivity in the rat striatum after dent postnatal development of enkephalin and tachykinin neurons of unilateral 6-hydroxydopamine, Eur. J. Pharm. 126 (1986) 347–348. rat basal ganglia, J. Neurochem. 56 (1991) 1499–1508.
[9] B.M. Campbell, P.D. Walker, MK-801 prevents dopamine D1 but [27] D.L. Somers, R.M. Beckstead, Striatal preprotachykinin and pre-not serotonin 2A stimulation of striatal preprotachykinin mRNA, proenkephalin mRNA levels and the levels of nigral substance P and
5
(2000) Neuroreport, submitted. pallidal Met -enkephalin depend on coritcostriatal axons that use the [10] H.P. Fischer, J. Marksteiner, G. Ransmayr, A. Saria, C. Humpel, excitatory amino acid neurotransmitters aspartate and glutamate: NGF but not GDNF or neurturin enhance acetylcholine tissue levels quantitative radioimmunocytochemical and in situ hybridization in striatal organotypic brain slices, Int. J. Dev. Neurosci. 16 (5) evidence, Mol. Brain Res. 8 (1990) 143–158.
(1998) 391–401. [28] D.L. Somers, R.M. Beckstead, N-Methyl-D-aspartate receptor an-5
[11] C.R. Gerfen, T.M. Engber, L.C. Mahan, Z. Susel, T.N. Chase, F.J. tagonism alters substance P and Met -enkephalin biosynthesis in Monsma, D.R. Sibley, D1 and D2 dopamine receptor regulated gene neurons of the rat striatum, J. Pharm. Exp. Ther. 262 (2) (1992) expression of striatonigral and striatopallidal neurons, Science 250 823–833.
(1990) 1429–1432. [29] L. Stoppini, P.A. Buchs, D. Muller, A simple method for or-[12] G.R. Hanson, L. Alphs, W. Wolf, R. Levine, W. Lovenberg, ganotypic cultures of nervous tissue, J. Neurosci. Methods 37
Haloperidol-induced reduction of nigral substance P-like immuno- (1991) 173–182.
reactivity: a probe for the interactions between dopamine and [30] M.P. Thomas, W.W. Webster, R.B. Norgren Jr, D.T. Monaghan, R.A. substance P neuronal systems, J. Pharm. Exp. Ther. 218 (1981) Morrisett, Survival and functional demonstration of interregional 568–574. pathways in fore / midbrain slice explant cultures, Neuroscience 85
[31] P.D. Walker, R. Andrade, J.P. Quinn, M.J. Bannon, Real-time striatum of rats: regulation of D1 / D2 interactions by muscarinic analysis of preprotachykinin promoter activity in single cortical receptors, J. Pharm. Exp. Ther. 281 (1997) 972–982.
neurons, J. Neurochem. 75 (2000) 882–885. [33] W.S. Young III, T.I. Bonner, M.R. Brann, Mesencephalic dopamine [32] J.Q. Wang, J.F. McGinty, The full D1 dopamine receptor agonist neurons regulate the expression of neuropeptide mRNAs in the rat