Indonesian Journal of Chemistry, 2002, 3 (2), 98-101
Triyono
98
EFFECT OF IMPREGNATION PROCEDURE OF Pt/
γ
-AL
2O
3CATALYSTS
UPON CATALYTIC OXIDATION OF CO
Triyono
Chemistry Department, Faculty of Mathematics and Natural Sciences Gadjah Mada University, Yogyakarta
ABSTRACT
The oxidation of carbon monoxide by oxygen using two catalysts prepared by two different methods has been investigated. In the first method, catalyst prepared by immersing γ-Al2O3 into the
hexa-chloroplatinic acid solution at 80oC for 4 h, resulted Pt/γ-Al2O3 catalyst having platinum highly dispersed on the support. While that of immersing γ-Al2O3 in the hexa-chloroplatinic acid solution at room temperature for
12 h, produced Pt/ γ-Al2O3 catalyst where platinum dispersion was much lower. Catalytic activity test showed that platinum well dispersed on the support enhanced the activity for oxidation of carbon monoxide. The platinum impregnated at room temperature resulted in the poor activity.
Keywords: platinum catalyst, alumina, supported material, oxidation
INTRODUCTION
Improvement, or at least limitation of damage, to the environment will be the focus of greater public awareness, political debate and increasingly more stringent legislation [1]. Users of fuel oil may need, or choose, to invest in post-combustion clean up of emissions or innovative conversion and combustion processes, such as conversion of CO to CO2 in exhausted emissions.
Carbon monoxide (CO) is one of the most dangerous pollutant gases in the atmosphere. This colorless gas which usually produce from the combustion system has damaging effect toward human and animal life [2]. Environmental Protection Agency has determined that the environmental quality standard is limited to about 9 ppm for CO in the atmosphere. One of the alternative efforts to manage and treat the pollutant gases produced by transportation activities is conversion of those gases [3], especially for CO gas, it can be converted to CO2 by catalytic process [4]
Development of catalysts for automotive emission control is a great challenge because of the extreme operating condition variation encountered. While the most industrial catalytic operations involves continuous and steady-state conditions, automobile operations are intermittent and transient, with wide fluctuations of flow rate and temperature that depend on driving conditions and cold or warm engine starts. Exhaust gas-flow rate can vary from 20 to more than 200 SCF/min, and exhaust temperatures from 250 to 1100oC [5].
Only precious metal catalysts have thus far been used commercially to meet the many difficult
specifications. In most cases, the metal is dispersed as small crystallites on thermally stable, chemically inactive supports such as alumina.
The rate per unit area of catalytically-active material may be small. Thus it becomes necessary to produce high surface areas of catalyst per unit weight of the material. Very small particle sizes are thus indicated. In such a case the catalyst may be finely dispersed on the surface of the pores of a support material such as alumina.
EXPERIMENTAL
Catalysts used here were 0.5 wt% Pt supported on alumina, prepared by two different impregnation methods according to the procedures given below. First method was the conventional impregnation; immersing alumina (γ-Al2O3) powder in aqueous solution of platinum chloride at room temperature while stirring for 12 h. Second method was dipping-impregnation methods, that is immersing alumina powder in aqueous solution of platinum chloride at 80oC while stirring for 4 h.γ-Al2O3 used as support material has surface area 198 m2/gram, average pore radius 26.2 Angstrom and pore volume 0.46 cc/gram.
Indonesian Journal of Chemistry, 2002, 3 (2), 98-101
Triyono
99
All the catalysts were dried and calcined at 500oC or 800oC for 3 h before submitting to the oxidation reaction. These four kinds of the catalysts were submitted to the oxidation reaction using gases consisting 1000 ppm CO, 500 ppm oxygen with W/F being 0.2 g.s.cm-3, in order to see which was the more effective factor, physical parameters or dispersion states. The reaction temperature was 500oC, and the conversion of CO was measured by using non-dispersive infrared spectroscopy.
RESULT AND DISCUSSION
Physical properties of the catalysts.
In Table 1 are given the physical parameters of four catalysts prepared in the laboratory, indicating that those of the room temperature impregnated catalysts were similar to those of the 80oC impregnated catalysts.
Table shows that the difference of surface area, average pore radius and pore volume are more prominently due to catalyst calcination temperatures compared with that of by impregnation temperatures. However, the physical properties of the support are significantly different with those for metal-support catalysts produced. The difference in physical properties of those catalysts can be explained as follows: Interaction between metal and support occurs when the solute to be deposited establishes a bond with the surface of the support at the time of wetting. Such interaction results in a near-atomic dispersion of the precursors active species. The interaction between alumina and chloroplatinic acid (H2PtCl6) is an exchange of the anion PtCl6
=
in the solution for two OH- ions of alumina. This anion exchange is a veritable neutralization of a weak base (alumina) by a strong acids (H2PtCl6); and for this reason it proceeds to a complete exchange of all the surface hydroxyl ions and proceeds very rapid. In this case the rate of diffusion becomes limiting for the overall processes, therefore, during impregnation the colloidal system
must be stirred. Considered kinetically, the rate of the exchange reaction was in this case much greater than the rate of diffusion of solute in the pores, as shown by the preferential deposition of the PtCl6
=
ion in the periphery of the granules. Considering this standpoint, reducing surface area and pore volume together with increasing average pore diameter resulted by impregnation of precursor solution onto the support material are reasonable and accepted. Data also shows decreasing surface area while pore volume and average pore increase. Those changes were caused by disappearance of small pore due to pore collapse and attrition of small pores to be wider.
Dispersed state of the active agent
It is well understood, although the catalyst compositions are the same, the catalytic activity happen to depend upon the catalyst preparation routes. This may be ascribed to the difference in the dispersion state of active species or in the physical properties of the support used [6]. In this paper, the dispersion state figured as percent dispersion of active species was measured in order to explain the difference the catalytic activities of the catalysts prepared in difference routes.
Platinum dispersion in these catalysts were measured by oxygen adsorption at room temperature. Dispersion was calculated based on the oxygen adsorption capacity by assuming that oxygen is adsorbed dissociatively and one platinum atom on the catalyst surface is able to bind one oxygen atom. The number of platinum surface atom Pts, can be derive from the following equations:
Pts = (V/VM) . NA . n
Where V = volume of gas chemisorbed, VM = molar volume of oxygen,
n = stoichiometry of chemisorption reaction, NA = Avogadro number.
Based on the calculation, the dispersion of platinum an the catalyst are summarized in table 2.
Table 1 Physical properties of the catalysts
Catalyst Calcination temp (oC)
Surface area (m2/g)
Av.pore radius (Angst)
Pore volume (cc/g)
1 500 152 28 0.22
Impregnation at
room T for12 h. 2 800 137 32 0.27
3 500 148 29 0.21
Impregnation at
80oC T for 4h. 4 800 129 34 0.22
Indonesian Journal of Chemistry, 2002, 3 (2), 98-101
Triyono
100
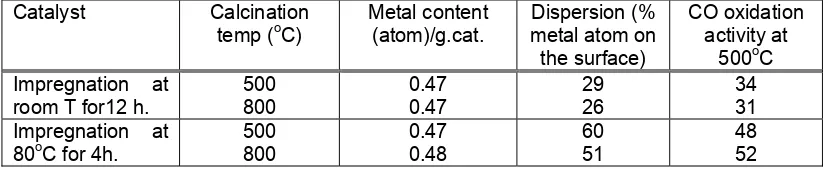
Table 2 Metal content, hydrogen adsorption and dispersion of catalysts*.
Catalyst Calcination temp (oC)
oxygen uptake (1020 molecule)
Dispersion (% metal atom on the surface) Impregnation at
*) The platinum atom content in all catalyst are identical., i.e. 1.45.1021 atom/g.cat.
Table 3 CO oxidation activity and dispersion of catalysts.
Catalyst Calcination
Those data indicating that the platinum is highly dispersed on alumina supports of catalyst prepared by dipping impregnation. Calcination at 800oC producing lower dispersion than at 500oC. The metal sites on a catalysts are found on its surface, however heating catalysts results in agglomeration of the small particulates into larger, less efficient, entities. Heat promotes the coalescence into increasingly larger entities and a corresponding loss of platinum atoms on the surface. The extent of agglomeration is a function of the particular catalyst, the temperature and the time the catalyst is exposed to the heat. For most catalytically active metal particles, agglomeration or sintering may be observed at temperatures above 450oC. For Platinum supported on γ-Al2O3 does not usually sinter at temperature under 500oC [7]. In this investigation, sintering was occured at 800oC as shown by decreasing metal dispersion .
As shown in table 1, the physical parameters of the impregnated catalyst calcined at 500oC were similar to those of the dipping-impregnated catalyst calcined at 500oC. However, table 2 suggests that the dispersion state of platinum in the impregnated catalyst were different from those in the dipping-impregnated catalyst calcined at 500oC. Considering the results given for activity, it can be concluded that, for the oxidation reaction on Pt/Al2O3 catalyst, the dispersion states of platinum is more effective factor than the physical parameters of the catalyst.
When the active species are supported, there is no longer a direct relation between the total specific surface area and the observed catalytic performance, particularly the activity; and it is necessary to determine the available active
surface, i.e., the number of molecules or atoms of active agents accessible to the reactants.
Catalytic activity
In order to show the usefulness of oxygen chemisorption, the relation between the rates of various reactions of CO oxidation at several temperature is presented in table 3.
Table 3 shows that all catalysts prepared contain the same amount of platinum. Therefore, impregnation at 80oC for 4 hours was enough to dispersed almost all of the platinum content in the solution. Moreover calcination at two different temperature was not have any effect of the metal content in the catalysts. However, the activity of the catalysts were different significantly. In contrast effect calcination temperature was shown by Okazaki [8], by calcining cobalt or nickel-loaded alumina at high temperature suppressed the oxidation of ethene by oxygen. On the other hand, catalyst prepared by impregnation at 80oC showed higher activity. The different in activity may be addressed to the different state of platinum dispersed on the catalyst. Depending on the impregnation temperature, the exchange reaction between PtCl6
=
ion and OH- ion belong to the equilibrium equation of ionic exchanges:
[PtCl6
Indonesian Journal of Chemistry, 2002, 3 (2), 98-101
Triyono
101
the impregnation at 80oC achieve equilibrium and lead to better distribution of the platinum on the whole surface, because, at higher temperature the platinum were easier to migrate toward the internal pore and resulted better dispersion and smaller platinum particles.
When the platinum species are dispersed on the support, there is no longer a direct relation between the total platinum content and the observed catalytic performance, particularly the activity; it depends on the available active sites or the number of molecules or atoms of active agents accessible to the reactants. Facilitated by smaller platinum particle, at the same platinum content, there are more active platinum accessible to the reactant results higher activity.
CONCLUSION
1. The physical parameters of the impregnated catalysts calcined at 500oC or at 800oC were similar to those of the dipping-impregnated catalysts calcined at 500oC or at 800oC.
2. The activity of the impregnated catalysts were lower than those of the dipping-impregnated catalysts.
3. The dispersion state of platinum in the impregnated catalysts were lower than those in the dipping-impregnated catalysts.
4. For oxidation of carbon monoxide on Pt/Al2O3 catalyst, the dispersion states of platinum is more effective factor than the physical parameters of the catalyst.
REFERENCES
1. Purwono.S, Murachman.B, Triyono.,1999., Prosiding Seminar nasional Kimia IV, Yogyakarta.104-114.
2.
Triyono, Trisunaryanti.W, Purwono.S.,(2002)., Prosiding Seminar Nasional Kimia XI, Jurusan Kimia, FMIPA-UGM, Jogjakarta.3. Canter.L.W., 1996., Environmental Impact Assessment, 2nd Edition, McGraw-Hill, Inc, New York.
4. Murachman.B, Purwono.P, Triyono, Achmad.Z, Mulianti.,1999., Prosiding Seminar nasional Kimia IV, Yogyakarta.71-76.
5. Kanniainen.K.,1994., Catalytic Converters For Gaseous Fuel Vehicles, Emission News.
6. Hamada.H, Haneda.M, Kakuta.N, Miura.H, Inami.K, Nanba.T, Hua.W.Q, Ueno.A, Ohfune.H, Udagawa.Y, (1997).,Chemistry Letters, The Chemical Society of Japan (887-888).
7. Augustine.R.L.,1996, Heterogeneous Catalysis for the Synthetic Chemist, Marcel Dekker, Inc. New York.