Sulphur Isotopes, Trace Elements and Mineral Stability
Diagrams of Waters from the Abandoned Fe
–
Cu Mines
of Libiola and Vigonzano (Northern Apennines, Italy)
Gianni Cortecci&Tiziano Boschetti& Enrico Dinelli&Roberto Cabella
Received: 5 November 2007 / Accepted: 27 January 2008 / Published online: 17 February 2008
#Springer Science + Business Media B.V. 2008
Abstract The geochemical characteristics of rills draining pyrite-chalcopyrite tailings impoundments and of bordering streams were investigated at the ophiolite-hosted Libiola and Vigonzano abandoned massive sulphide mines, northern Apennines Italy.
Water samples were analysed for major and trace chemical composition, hydrogen and oxygen isotope composition, and sulphur isotope composition of aqueous sulphate. Sulphur isotope composition was determined also for some samples of ore sulphides. At Libiola, the newly acquired chemical results on waters corroborate those from previous investigations, thus providing additional support to existing geo-chemical models in terms of metal distribution, solid phases precipitation, reaction path modelling and mixing reaction paths, and environmental problems. At Vigonzano, the chemical characteristics of waters are similar to those at Libiola. In both localities, solution-secondary phase equilibria estimated using an updated thermodynamic dataset account for min-eralogy in the field, including poorly crystalline phases like jurbanite and hydrowoodwardite. The hydrogen and oxygen isotope composition of waters at Libiola and Vigonzano agrees with their meteoric origin. Acid to neutral mine waters do not show any significant isotope shift with respect to the initial water, in spite of the oxidation of even large amounts of pyrite/chalcopyrite ore. The sulphur isotope com-position of aqueous sulphate in mine rills at Libiola (δ34S=5.6 to 8.5‰; mean 6.5‰) matches that of
massive sulphide ore (δ34S=−0.5 to 6.7‰; mean
5.8‰), in keeping with the supergenic origin of the
sulphate and related isotope effects in the sulphide oxidation process. Sulphate in mine waters at Vig-onzano displays lowerδ34S values in the range 0.6 to DOI 10.1007/s11270-008-9637-8
G. Cortecci (*)
Istituto di Geoscienze e Georisorse, Area della Ricerca-CNR,
Via Moruzzi 1, I-56124 Pisa, Italy e-mail: g.cortecci@igg.cnr.it
T. Boschetti
Dipartimento di Scienze della Terra, Università di Parma, Viale Usberti 157a,
I-43100 Parma, Italy
E. Dinelli
Centro Interdipartimentale di Ricerca per le Scienze Ambientali, Alma Mater Studiorum-Università di Bologna, Centro di Ravenna, Via Sant’Alberto 163,
I-48100 Ravenna, Italy
E. Dinelli
Dipartimento di Scienze della Terra e Geologico-Ambientali, Alma Mater Studiorum-Università di Bologna,
Piazza Porta San Donato1, I-40126 Bologna, Italy
R. Cabella
Dipartimento per lo Studio del Territorio e delle sue Risorse, Università di Genova,
1.5‰. Theδ34S signature of massive ore specimens is
within the range reported for most volcanic-hosted massive sulphide deposits, including Cyprus-type deposits.
Keywords Apennine ophiolites . mine water . sulphidic mine tailings . sulphur isotopes . water isotopes
1 Introduction
Massive sulphide ore deposits have great potential environmental concerns, which include human health risks, ecosystem risks and physical hazards (Seal et al.
2002; Seal and Hammarstrom 2003). The ecosystem risks are related to the acid mine drainage (AMD) especially in abandoned mine sites. AMD is originat-ed by the oxidation of sulphides, mostly pyrite, and leads to the mobilization of metals. This process has been recognized as a major environmental issue, and geochemical, pollution, health and management issues have been summarized in several important reference works dealing with geoenvironmental mod-els of mineral deposits or mine wastes (Alpers et al.
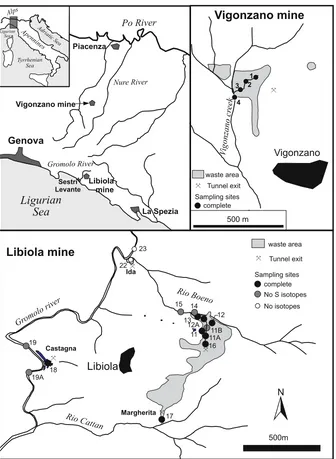
1994; Plumlee and Logsdon 1999; Filipek and Plumlee1999; Jambor et al.2003; Lottermoser2003). The study sites are located in the northern Apennines (Italy). In particular the Libiola mine is located in the drainage basin of the Gromolo River, about 8 km NE of the town of Sestri Levante on the Ligurian Sea coast (Fig. 1). The ore deposit is stratabound, and occurs in pillow lavas and pillow breccias of the Internal Ligurides (Eastern Liguria province) at the floor of the sedimentary cover (late Jurassic-Cretaceous) (Ferrario and Garuti 1980, and references therein). The lavas are partially over-thrusted by serpentinites with local intercalations of rodingitized gabbros. The deposit consists mostly of massive lenses of pyrite and chalcopyrite (and minor sphalerite) nearly concordant with the pillow layering. Gangue minerals are scarce and include mainly carbonates and some quartz (Bertolani1952). Ore also exists as disseminations (small aggregates) in pillows and host volcanic breccia, and as stockwork discordant with respect to the lava beds. No primary sulphate minerals were identified in the deposit (Bertolani1952; Ferrario 1973). The presence in the ore of strongly deformed and fractured chalcopyrite crystals testifies
for the Alpine tectonics undergone by the deposit (Bertolani1952).
A submarine hydrothermal origin was suggested by Spooner et al. (1974) and Bonatti et al. (1976) for all the three types of ore occurrences, whereas some doubts were raised by Ferrario and Garuti (1980) for the massive and disseminated ones which they thought may be magmatic, that is closely related to the volcanic products.
The economic exploitation of the Libiola mine started in the XVII century and ended in 1965. The mine tailings, located at 335 and 230 m a.s.l., extend over about 0.5 km2 and are crossed by rills that convey in the Gromolo, Rio Boeno and Rio Cattan creeks (see Fig.1). The latter two are tributaries of the Gromolo creek.
Previous hydrochemical studies in the Libiola mine area were carried out by Cortecci et al. (2001), Dinelli et al. (2001) and Marini et al. (2003) on acid to near neutral waters from the tailings and polluted and unpolluted bordering stream waters. To be also mentioned the geochemical modelling by Accornero et al. (2005), who investigated the fate of major and trace elements in acid water from the Libiola mine after mixing with the Gromolo stream freshwater. These papers deal with sampling campaigns carried out in different seasons, i.e. November 1996, April 1997, May 1997, December 1997, May 1998, October 1998 and May 2000. Additional water samples from acidic discharges and the Gromolo stream were analysed in July to October 2003 and April 2004 (Accornero et al.2005).
The ore deposit of the derelict Vigonzano mine is located at 750–800 m a.s.l. in the vicinity of the homonymous village in the Piacenza province (Emilia-Romagna region), about 30 km inland from the Libiola mine, on the other side of the Apennine divide. As the latter, it consists mostly of massive pyrite and chalcopyrite, hosted within serpentinites and basalt breccias (External Ligurides), at the boundary between ophiolitic rocks and pelitic sediments (Dinelli et al.
1996, and references therein); it should be also related to Jurassic-Cretaceous submarine volcanism, and then underwent intense tectonic deformation during the Alpine orogeny.
ephemeral rill issuing from an elevated exploratory pit, and in June 1998 from only the uppermost site fed by a quite small water flow. In June 1998, a blue deposit was observed along the uppermost part of the rill. The Rio Vigonzano was also sampled just downstream of the rill both in November 1997 and June 1998.
In the present work, water samples (Libiola: December 1997, May and October 1998; Vigonzano:
November 1997, June 1998) were analysed for major and trace chemical elements, and for hydrogen and oxygen isotopes of water and sulphur isotopes of dissolved sulphate (Libiola: December 1997; Vigon-zano: Novembre 1997). In addition, ore samples and vein sulphides in the rocks were also analysed for the sulphur isotope composition. The present work was aimed to (1) complement the extended abstract by Cortecci et al. (2001) on the Libiola mine with a more
complete presentation and a deeper discussion of the isotopic data dealing with water and ore samples, (2) integrate and extend geochemical modelling of acid mine waters and their evolution, (3) compare the original data on the Vigonzano mine with those on the Libiola mine, the ore deposits being nearly coeval and both hosted in basalts, (4) use the hydrogen and oxygen isotopes of water as natural tracers of hydrologic processes in acid mine drainage systems, (5) verify the sulphur isotope fractionation involved in the sulphide mineral supergenic oxida-tion, and (6) provide constraints on the origin of the ore deposits.
2 Previous Studies
Waters from the Libiola mine tailings include acid terms from oxidative drainage of pyrite and chalco-pyrite ore. These waters are “red”in the most acidic
sites (pH 2.4–2.8), and their colour is due to very fine suspended Fe-particulate. They are oversaturated with respect to jurbanite and close to saturation with respect to ferrihydrite (Marini et al. 2003). The red waters neutralize to “blue” waters (pH 6.5–7.5)
through water–rock interaction (Dinelli et al. 2001),
reaching saturation/oversaturation with respect to gibbsite and basaluminite, and saturation relative to antlerite, brochantite and alunite. Both red and blue waters are slightly undersaturated with respect to gypsum (Marini et al. 2003). However, identified minerals in red waters were amorphous silica, Fe(III)-oxhydroxides, schwertmannite and jarosite (Dinelli et al. 1998; Derron 1999; Dinelli and Tateo 2002), and probably basaluminite, gibbsite and a Cu-Al sulphate hydrate (azure-blue coloured) are the precip-itates in blue waters (Derron1999). Depending on the water discharge, the sediments may change seasonally their colour. In these cases, the water-sediment pairs are located at higher elevation in the mine area and are affected by considerable fluctuations of the water table. The colour of sediments was found to change from ochre (Fe-rich) to blue-green (Cu-rich), follow-ing a pH variation of the water from acid to near neutral (Dinelli and Tateo2002).
Red waters issue from exploratory pits located in the bottom of the Gromolo River valley (Ida and Castagna tunnels, at 106 and 72 m a.s.l., respective-ly), whereas blue waters are discharged by a higher
tunnel near to the Rio Cattan stream (Margherita tunnel; 209 m a.s.l.), a tributary of the Gromolo River. All these sites display a rather constant water flow rate over the year, testifying that the discharge point is close to the intersection of the water table with the topographic surface (Dinelli et al. 2001), and the sediments maintain their colour all over the year. According to Marini et al. (2003), the main process governing the chemical composition of all the mine waters of Libiola (red, blue and intermediate) is their interaction to variable extents with the waste material. Mixing between acid mine waters (red to orange) and local groundwater should be insignificant, that is all mine waters at Libiola derive from the interaction of percolating meteoric water with mine tailings or with ore.
In both red and blue waters, sulphate is the dominant anion; calcium and magnesium are the main cations, reflecting the acid alteration of local rocks (Dinelli et al. 2001; Marini et al.2003). Mg is particularly rich in the acid waters, which greatly alter the mafic silicates present in the tailings and country rocks. At times, iron is the prevailing cation in the red water, due to an appropriate combination of ore oxidation and pH of solution (Dinelli et al. 2001). Finally, red waters are richer in metals (Al, Fe, Cu, Zn and Mn) than blue waters.
Water from the Gromolo River and Rio Boeno upstream of the mined area is Mg (± Ca)-HCO3and
comparatively very diluted for both major and trace constituents (Dinelli et al. 2001; Marini et al.2003). The chemical composition of these surface waters match that of spring water issuing from serpentinites in Liguria and Emilia Romagna regions, its chemical composition varying from Ca-HCO3to Mg-HCO3to
Ca–(Na)–OH (Bruni et al. 2002; Boschetti and
Toscani2008).
At Vigonzano, previous studies dealt with the geochemical characterization of the waste rock piles from the mining works (Dinelli et al. 1996), the transfer of metals to vegetation (Dinelli and Lombini
3 Sampling and Methods
The sampling sites at Libiola in December 1997 to October 1998 are shown in Fig. 1. Water samples were collected from rills draining the waste dumps
and the streams bordering the mining area (Gromolo River and Rio Boeno). Temperature, pH, Eh, EC and alkalinity were measured in the field. Water was filtered through 0.45 μm membranes of cellulose acetate, and portions stored for analyses. The portion
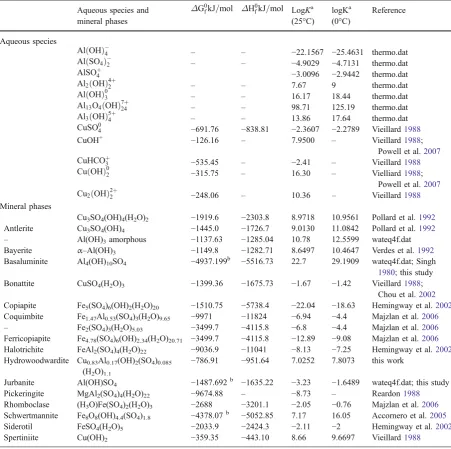
Table 1 Thermodynamic data of aqueous species and mineral phases added to the thermo.com.V8.R6 dataset
Aqueous species and mineral phases
ΔG0
fkJ=mol ΔH0fkJ=mol LogKa
(25°C)
logKa (0°C)
Reference
Aqueous species
Al OHð Þ4 – – −22.1567 −25.4631 thermo.dat
Al SOð 4Þ2 – – −4.9029 −4.7131 thermo.dat
AlSOþ4 −3.0096 −2.9442 thermo.dat
Al2ðOHÞ42þ – – 7.67 9 thermo.dat
Al OHð Þ0
3 – – 16.17 18.44 thermo.dat
Al13O4ðOHÞ724þ – – 98.71 125.19 thermo.dat
Al3ðOHÞ54þ – – 13.86 17.64 thermo.dat
CuSO04 −691.76 −838.81 −2.3607 −2.2789 Vieillard1988
CuOH+ −126.16 – 7.9500 – Vieillard1988;
Powell et al.2007
CuHCOþ3 −535.45 – −2.41 – Vieillard1988
Cu OHð Þ02 −315.75 – 16.30 – Vielliard1988;
Powell et al.2007
Cu2ðOHÞ
2þ
2 −248.06 – 10.36 – Vieillard1988
Mineral phases
Cu3SO4(OH)4(H2O)2 −1919.6 −2303.8 8.9718 10.9561 Pollard et al.1992 Antlerite Cu3SO4(OH)4 −1445.0 −1726.7 9.0130 11.0842 Pollard et al.1992
– Al(OH)3amorphous −1137.63 −1285.04 10.78 12.5599 wateq4f.dat
Bayerite α–Al(OH)3 −1149.8 −1282.71 8.6497 10.4647 Verdes et al.1992 Basaluminite Al4(OH)10SO4 −4937.199b −5516.73 22.7 29.1909 wateq4f.dat; Singh
1980; this study Bonattite CuSO4(H2O)3 −1399.36 −1675.73 −1.67 −1.42 Vieillard1988;
Chou et al.2002 Copiapite Fe5(SO4)6(OH)2(H2O)20 −1510.75 −5738.4 −22.04 −18.63 Hemingway et al.2002 Coquimbite Fe1.47Al0.53(SO4)3(H2O)9.65 −9971 −11824 −6.94 −4.4 Majzlan et al.2006 – Fe2(SO4)3(H2O)5.03 −3499.7 −4115.8 −6.8 −4.4 Majzlan et al.2006 Ferricopiapite Fe4.78(SO4)6(OH)2.34(H2O)20.71 −3499.7 −4115.8 −12.89 −9.08 Majzlan et al.2006 Halotrichite FeAl2(SO4)4(H2O)22 −9036.9 −11041 −8.13 −7.25 Hemingway et al.2002 Hydrowoodwardite Cu0.83Al0.17(OH)2(SO4)0.085
(H2O)1.1
−786.91 −951.64 7.0252 7.8073 this work
Jurbanite Al(OH)SO4 −1487.692b −1635.22 −3.23 −1.6489 wateq4f.dat; this study Pickeringite MgAl2(SO4)4(H2O)22 −9674.88 – −8.73 – Reardon1988 Rhomboclase (H3O)Fe(SO4)2(H2O)3 −2688 −3201.1 −2.05 −0.76 Majzlan et al.2006 Schwertmannite Fe8O8(OH)4.4(SO4)1.8 −4378.07b −5052.85 7.17 16.05 Accornero et al.2005 Siderotil FeSO4(H2O)5 −2033.9 −2424.3 −2.11 −2 Hemingway et al.2002
Spertiniite Cu(OH)2 −359.35 −443.10 8.66 9.6697 Vieillard1988
Last version of the original thermo.com.V8.R6 and thermo.dat files are available in Hydrogeology Program, Department of Geology, University of Illinois (retrieved January 11, 2008, fromhttp://www.geology.uiuc.edu/Hydrogeology/hydro_thermo.htm). See text for details
a
Note that in phreeqc’s datasets the solution species are in the right side of reaction, therefore the sign must be inverted.
b
for determination of metals was added with suprapure HNO3. Within a few days after collection, all water
samples were analysed for Cl (Idrimetric-Kits by Carlo Erba), SO4 (turbidimetry/spectrophotometry;
HACH reagent and instrument), Na, K, Ca, Mg, Fe, and Mn (AAS), Zn, Cu, Cd, Co, Ni, Cr, Ba and Pb (GF-AAS), and Al (turbidimetry/spectrophotometry; HACH reagent and instrument). Precisions were of 3 to 5% for major elements and 5–10% for minor and trace elements. Detection limits were: 1μg/L for Cd and Pb, 2μ/L for Cu, Zn, Cr and Ba, 5μg/L for Ni and Co, and 10 μg/L for Al, Fe and Mn. In the colourless waters at Libiola (see Table3), Cr and Cd were determined after preconcentration by solvent extraction technique (Danielsonn et al.1978).
Aqueous sulphate for the sulphur isotope analysis was separated as BaSO4, which was thermally
decom-posed to SO3, and then reduced to SO2 for the
spectrometric measurement, using a method similar to that of Holt and Engelkemeir (1970). Ore samples were checked for main mineralogy by XRD. Total ore samples and mineral separates were analysed, the latter being not pure phases, but mixtures in variable proportions of pyrite and chalcopyrite (± sphalerite). Before combustion in a stream of pure oxygen to produce SO2 for the mass spectrometric analysis
(Thode et al. 1961), ore samples were treated with diluted HCl in an ultrasonic bath to remove alteration products, carbonates and oxides. The results are reported in δ34S unit, in per mil, relative to the CDT
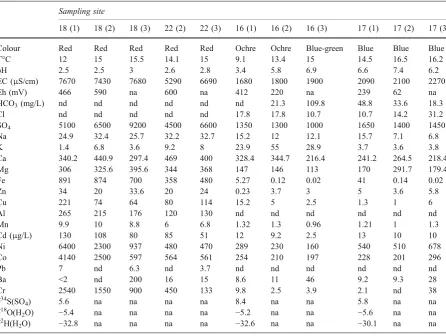
Table 2 Chemical and isotopic composition data on coloured waters from the mine tailings at Libiola
Sampling site
18 (1) 18 (2) 18 (3) 22 (2) 22 (3) 16 (1) 16 (2) 16 (3) 17 (1) 17 (2) 17 (3)
Colour Red Red Red Red Red Ochre Ochre Blue-green Blue Blue Blue
T°C 12 15 15.5 14.1 15 9.1 13.4 15 14.5 16.5 16.2
pH 2.5 2.5 3 2.6 2.8 3.4 5.8 6.9 6.6 7.4 6.2
EC (μS/cm) 7670 7430 7680 5290 6690 1680 1800 1900 2090 2100 2270
Eh (mV) 466 590 na 600 na 412 220 na 239 62 na
HCO3(mg/L) nd nd nd nd nd nd 21.3 109.8 48.8 33.6 18.3
Cl nd nd nd nd nd 17.8 17.8 10.7 10.7 14.2 31.2
SO4 5100 6500 9200 4500 6600 1350 1300 1000 1650 1400 1450
Na 24.9 32.4 25.7 32.2 32.7 15.2 12 12.1 15.7 7.1 6.8
K 1.4 6.8 3.6 9.2 8 23.9 55 28.9 3.7 3.6 3.8
Ca 340.2 440.9 297.4 469 400 328.4 344.7 216.4 241.2 264.5 218.4
Mg 306 325.6 395.6 344 368 147 146 113 170 291.7 179.4
Fe 891 874 700 358 480 5.27 0.12 0.02 41 0.14 0.02
Zn 34 20 33.6 20 24 0.23 3.7 3 5 3.6 5.8
Cu 221 74 64 80 114 15.2 5 2.5 1.3 1 6
Al 265 215 176 120 130 nd nd nd nd nd nd
Mn 9.9 10 8.8 6 6.8 1.32 1.3 0.96 1.21 1 1.3
Cd (μg/L) 130 108 80 85 51 12 9.2 2.5 13 10 10
Ni 6400 2300 937 480 470 289 230 160 540 510 678
Co 4140 2500 597 564 561 254 210 197 228 201 296
Pb 7 nd 6.3 nd 3.7 nd nd nd nd nd nd
Ba <2 nd 200 16 15 8.6 11 46 9.2 9.3 28
Cr 2540 1550 900 450 133 9.8 2.5 3.9 2.1 nd 38
δ34S(SO
4) 5.6 na na na na 8.4 na na 5.8 na na
δ18O(H2O) −5.4 na na na na −5.2 na na −5.6 na na δ2H(H
2O) −32.8 na na na na −32.6 na na −30.1 na na
Colour refers to sediments. Sampling time: (1) 11 December 1997; (2) 18 May 1998; (3) 16 October 1998.
standard. The overall precision of the method is of 0.2–
0.3‰. In the field, the portion of water for the sulphur
isotope analysis was added with calomel (Hg2Cl2) to
prevent bacterial sulphate-reducing activity. For hydro-gen and oxyhydro-gen isotope determination of water, samples were respectively decomposed with metallic zinc at 450°C to give H2 and equilibrated with tank
CO2, and the gases analysed for the 2
H/1H and18O/16O ratios. The results are given in δ2H and δ18O, in per
mil, relative to V-SMOW standard, with reproducibil-ity to 1‰for hydrogen and 0.2‰for oxygen.
Mineral-solution equilibrium in red and blue waters were investigated using activity plots. The calculation of the solute activity in the water samples and of mineral stability diagrams was performed using the PHREEQCI 2.13.2 code (Parkhurst and
Appelo 1999) and the Geochemist Workbench 4.0.3 code (Bethke 2002), both combined with the Law-rence Livermore National Laboratory thermodynamic dataset (thermo.com.V8.R6). Special attention was paid to Al and Cu bearing ions and AMD’s typical
phases, updating the dataset as shown in Table1. New aqueous species were entered only in the PHREEQCI dataset format because in this code the activity coefficient of the charged species is calculated using the Davies equation, thus by-passing the ion size parameter data that are actually unknown for the listed species. Data on schwertmannite and jurbanite minerals were taken from Accornero et al. (2005) and Wateq4f database of PHREEQCI, respectively; data on ferricopiapite, coquimbite and rhomboclase were also included (Majzlan et al. 2006), even if the
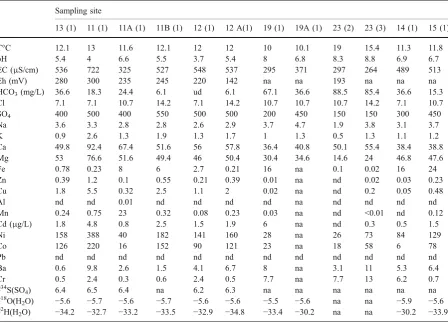
Table 3 Chemical and isotopic composition data on colourless waters from the mine tailings and bordering creeks at Libiola
Sampling site
13 (1) 11 (1) 11A (1) 11B (1) 12 (1) 12 A(1) 19 (1) 19A (1) 23 (2) 23 (3) 14 (1) 15 (1)
T°C 12.1 13 11.6 12.1 12 12 10 10.1 19 15.4 11.3 11.8
pH 5.4 4 6.6 5.5 3.7 5.4 8 6.8 8.3 8.8 6.9 6.7
EC (μS/cm) 536 722 325 527 548 537 295 371 297 264 489 513
Eh (mV) 280 300 235 245 220 142 na na 193 na na na
HCO3(mg/L) 36.6 18.3 24.4 6.1 ud 6.1 67.1 36.6 88.5 85.4 36.6 15.3
Cl 7.1 7.1 10.7 14.2 7.1 14.2 10.7 10.7 10.7 14.2 7.1 10.7
SO4 400 500 400 550 500 500 200 450 150 150 300 450
Na 3.6 3.3 2.8 2.8 2.6 2.9 3.7 4.7 1.9 3.8 3.1 3.7
K 0.9 2.6 1.3 1.9 1.3 1.7 1 1.3 0.5 1.3 1.1 1.2
Ca 49.8 92.4 67.4 51.6 56 57.8 36.4 40.8 50.1 55.4 38.4 38.8
Mg 53 76.6 51.6 49.4 46 50.4 30.4 34.6 14.6 24 46.8 47.6
Fe 0.78 0.23 8 6 2.7 0.21 16 na 0.1 0.02 16 24
Zn 0.39 1.2 0.1 0.55 0.21 0.39 0.01 na nd 0.02 0.03 0.23
Cu 1.8 5.5 0.32 2.5 1.1 2 0.02 na nd 0.2 0.05 0.48
Al nd nd 0.01 nd nd nd nd na nd nd nd nd
Mn 0.24 0.75 23 0.32 0.08 0.23 0.03 na nd <0.01 nd 0.12
Cd (μg/L) 1.8 4.8 0.8 2.5 1.5 1.9 6 na nd 0.3 0.5 1.5
Ni 158 388 40 182 141 160 28 na 26 73 84 129
Co 126 220 16 152 90 121 23 na 18 58 6 78
Pb nd nd nd nd nd nd nd nd nd nd nd nd
Ba 0.6 9.8 2.6 1.5 4.1 6.7 8 na 3.1 11 5.3 6.4
Cr 0.5 2.4 0.3 0.6 2.4 0.5 7.7 na 7.7 13 6.2 0.7
δ34
S(SO4) 6.4 6.5 6.4 na 6.2 6.3 na na na na na na
δ18O(H
2O) −5.6 −5.7 −5.6 −5.7 −5.6 −5.6 −5.5 −5.6 na na −5.9 −5.6 δ2
H(H2O) −34.2 −32.7 −33.2 −33.5 −32.9 −34.8 −33.4 −30.2 na na −30.2 −33.9
Sampling time: (1) 11 December 1997; (2) 18 May 1998; (3) 16 October 1998. Waters 11 to 13 are from mine tailings; 19, 19A and 23 are from the Gromolo River; 14 and 15 are from the Rio Boeno stream.
existence of these phases is unlikely due to low redox potential of the solutions.
In order to obtain the equilibrium constants (K) at T<25°C (Table1), the LogK(25°C) and the enthalpy of the corresponding dissolution reactions were inserted in the Van’t Hoff equation. It is worthy of note that many papers in literature complain the absence of complete thermodynamic data regarding to basic aluminium sulfate hydrates (basaluminite and jurban-ite), particularly for the enthalpy of formation quantity (e.g. Ludwig et al. 2005, Prietzel and Mayer 2005). Thermodynamic properties of basaluminite were in-vestigated by Singh (1980), who calculated a Gibbs free energy and an enthalpy of formation of−6130.606
and −7037.823 kJ/mol, respectively. The free energy
value agrees with that of −4937.199 kJ calculated
by Hemingway et al. (2002) for the anhydrous phase from the logKin Wateq4f dataset, when the contribu-tion due to the crystallizacontribu-tion water is subtracted, i.e.−6130.606−(−238.4*5) =−4938.606 kJ. The
sub-traction of the crystallization water contribution for enthalpy (−304.2*5 kJ; Hemingway et al.2002) yields
a value of ΔH0
f ¼ 5516:73kJ for the anhydrous basaluminite. For jurbanite, the standard enthalpy of formation ΔH0
f ¼ 1635:22kJ
was calculated sum-ming by 1/3 the enthalpies of Al(OH)3(gibbsite) and a
generic Al2(SO4)3*6(H2O), subtracting the enthalpy
due to water (−304.2*2 kJ), and applying a correction
factor of 0.9740 in order to match the Gibbs free energy value of ΔG0
f¼ 1487kJ as calculated from the
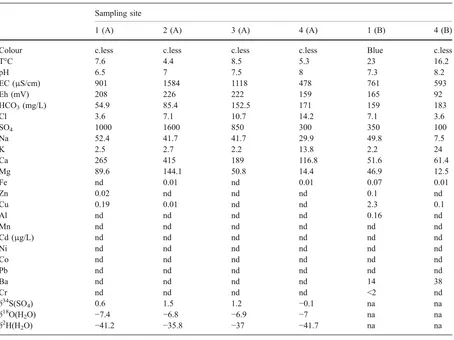
Table 4 Chemical and isotopic composition data on water from a mine pit at Vigonzano and the creek bordering the mine tailings (Rio Vigonzano)
Sampling site
1 (A) 2 (A) 3 (A) 4 (A) 1 (B) 4 (B)
Colour c.less c.less c.less c.less Blue c.less
T°C 7.6 4.4 8.5 5.3 23 16.2
pH 6.5 7 7.5 8 7.3 8.2
EC (μS/cm) 901 1584 1118 478 761 593
Eh (mV) 208 226 222 159 165 92
HCO3(mg/L) 54.9 85.4 152.5 171 159 183
Cl 3.6 7.1 10.7 14.2 7.1 3.6
SO4 1000 1600 850 300 350 100
Na 52.4 41.7 41.7 29.9 49.8 7.5
K 2.5 2.7 2.2 13.8 2.2 24
Ca 265 415 189 116.8 51.6 61.4
Mg 89.6 144.1 50.8 14.4 46.9 12.5
Fe nd 0.01 nd 0.01 0.07 0.01
Zn 0.02 nd nd nd 0.1 nd
Cu 0.19 0.01 nd nd 2.3 0.1
Al nd nd nd nd 0.16 nd
Mn nd nd nd nd nd nd
Cd (μg/L) nd nd nd nd nd nd
Ni nd nd nd nd nd nd
Co nd nd nd nd nd nd
Pb nd nd nd nd nd nd
Ba nd nd nd nd 14 38
Cr nd nd nd nd <2 nd
δ34S(SO
4) 0.6 1.5 1.2 −0.1 na na
δ18O(H2O) −7.4 −6.8 −6.9 −7 na na
δ2H(H
2O) −41.2 −35.8 −37 −41.7 na na
Sampling time:A—21 November 1997;B—07 June 1997
LogK in wateq4f dataset. To be noticed that the above calculated Gibbs free energy for jurbanite is approached when the crystallization water moles of the Al-sulfate precursor are increased, i.e. starting
from Al2(SO4)3*17(H2O) (alunogen) and its
stan-dard enthalpy of −7278.88 kJ (Reardon 1988;
Christov 2003), calculation leads to a standard free energy of −1460.326 kJ for jurbanite, that is Table 5 Main mineralogy and sulphur isotope composition of sulphide minerals from ore specimens and host rocks at Libiola and Vigonzano
Sample Occurrence Mineralogya δ34S
(‰vs. CDT)
Libiola
L2 I-A massive Cpy, py,sph;gangue: chlorite, quartz, pumpellyite, oxides 6.5 L2 III-A massive Cpy,py, sph; gangue: calcite, chlorite, quartz, pumpellyite, oxides 1.5
L20 IIA massive Cpy,py, sph; gangue: quartz 6.7
L20 IIB massive Cpy,py, sph;gangue: quartz 5.0
L21 A massive (pillow basalt) Cpy,py, sph; gangue: chlorite, oxides 6.3 L3 A disseminated
(contact basalt–serpentinite)
Cpy,py, sph; 10.1
L6 A disseminated (basalt) Cpy,py, sph; gangue: silicates (clinochlore), spinel 9.7 L6 B disseminated (basalt) Cpy,py, sph; gangue: silicates (clinochlore), spinel 9.4 L17 A disseminated (basalt breccia) Cpy,py, sph; gangue: silicates, spinel, quartz 8.2 L4 A stockwork (basal breccia) Cpy, py,sph;gangue: silicates (clinochlore) 7.2
Lib 1 massive No XRD −0.5
Lib 2 massive No XRD 4.5
Lib 3 massive No XRD 5.6
Vigonzano
Vig A stockwork (basalt) No XRD 6.9
Vig B dissemination (serpentinite) No XRD 7.3
a
Sulphide and gangue main mineralogy; in bold, XRD of analysed sample.
Lib 1, Lib 2, Lib 3, Vig A and Vig B: isotopic analysis after removal of carbonates by HCl treatment.
different by about 27 kJ (1.8%) only. The thermo-dynamic properties of the hydrowoodwardite (mem-ber of the hydrotalcite group), that is the higher hydrated analogue of woodwardite deemed to be responsible for the blue colour precipitates at Libiola (Dinelli et al. 1998), were calculated ap-plying the Bravo-Suarez et al.’s (2004) model to the
formula Cu(1−x)Al(x)(OH)2(SO4)x/2*mH2O with x= 0.17 and m= 1.1. This model was preferred to the Peltier et al.’s (2006) solid-solution non-ideal
model, as the former fits better with the Cu-bearing hydrotalcite minerals with x < 0.3 (Tumiati et al.
2008).
4 Results and Discussion
The chemical data reported in Tables2and3refer to selected red, blue, ochre and colourless samples from the mine rills and bordering streams, and include all the samples analysed for the stable isotope composi-tions of hydrogen, oxygen and sulphur. Additional water samples were analysed, and the complete set of chemical data is available upon request from the authors. The chemical and isotopic data on rill and stream samples at Vigonzano are shown in Table 4. Finally, the sulphur isotope composition values of sulphides from ore and rocks are listed in Table5.
4.1 Chemical Results
At Libiola, the results from the present work corro-borate those from previous investigations, showing basically the same features, thus providing further support to currently accepted hydrogeochemical mod-el of the abandoned mine (metal distribution, solid phases precipitation, water–rock interaction and mix-ing reaction paths, environmental problems). They also corroborate the environmental implications relat-ed to the discharge of mine water rich in potentially toxic metals and the role of secondary mineral phases acting as chemical barriers in mitigating the disper-sion of metals in the nearby waters and soils. However, some aspects merit to be underlined (1) red acid waters from the Ida and Castagna discharges and the blue water from the Margherita discharge maintain their chemical characteristics with respect to prior and later samplings, (2) at the top of the main waste dump, the change of colour from orange (pH
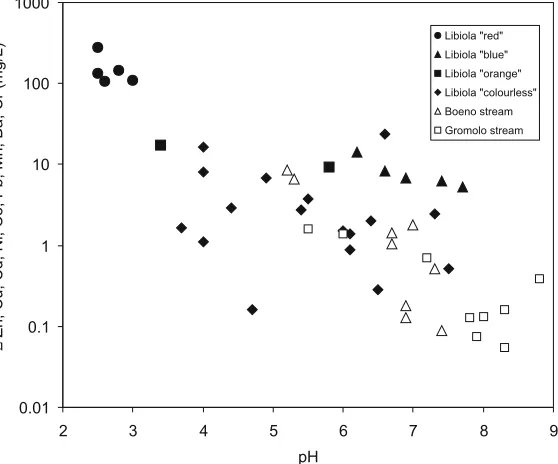
5.8; May 1998) to blue (pH 6.9; October 1998) was accompanied by only a slightly decrease in dissolved Fe, Cu and Al, this being ascribable to the small variation of pH. In the same site, a marked decrease of dissolved Fe, Cu and Al was observed by Dinelli et al. (2001) for a variation of pH from 3.8 (orange water) to 6.2 (blue water). A slogan may be that orange and orange exist, one being more bluish than another; and (3) in the Rio Boeno, whitish colloidal deposits (Cu-Al sulphate hydrate?) were observed in water at site 15 close to the confluence with the Gromolo River in December 1997 and May 1998 at pH of 6.9±0.2, but not in October 1998 at pH 5.3± 0.1. This disappearing precipitate represents an example of ephemeral geochemical barrier controlled by the flow rate (i.e. the rainfall) of water, that is added to the permanent geochemical barriers activat-ed by mixing of acid mine discharge with slightly basic or nearly neutral water along the Gromolo River itself. The relation between total trace element concentration and pH is shown in Fig.2. As expected the trend is negative for both mine waters and stream waters.
At Vigonzano, the chemical characteristics of waters are similar to those at Libiola, with a composition of Ca-SO4 type for the mine water
(colourless and blue) and Ca-HCO3 type for the
unpolluted creek (June 1997; no mine water reaching the creek). When polluted (November 1997), the creek water is of Ca-SO4type. The blue coloured sediments
in June 1997 refer to highly evaporated water, which also shows higher Cu and Al concentration values. However, trace metals are low or below the detection limits, also in the high sulphate waters.
4.2 Mineral-solution Equilibrium
The activity plots for the Fe2O3/FeO–SO3–Al2O3–
CaO–H2O and CuO/Cu2O–SO3–Al2O3–CaO–H2O
systems for red waters and Fe2O3/FeO–SO3–CaO–
H2O and CuO/Cu2O–SO3–CaO–H2O systems for
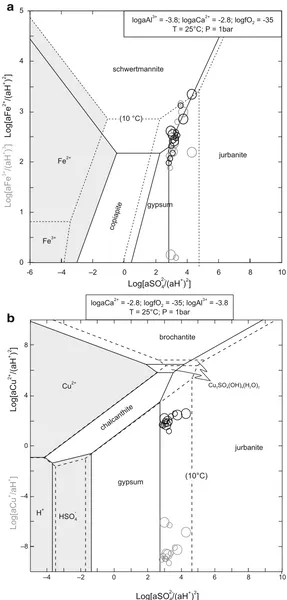
Fig. 3 Activity plots for the Fe2O3–FeO–SO3–
Al2O3–CaO–H2O (a) and
CuO–Cu2O–SO3–Al2O3– CaO–H2O (b) systems at 10° and 25°C for red waters at Libiola. Large and small circles refer respectively to this work and literature data, the bold rim denoting the Fe (III) and Cu (II) systems and the light rim the Fe(II) and Cu (I) systems. Ambient chemi-cal parameters in the plots are mean values calculated from the analytical data
a
a
b
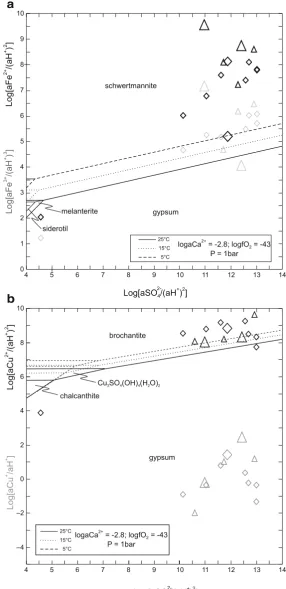
Fig. 4 Activity plots for theFe2O3–FeO–SO3–CaO–H2O
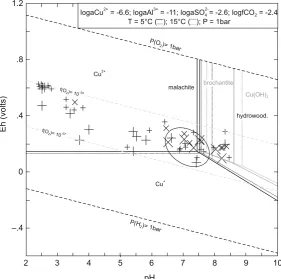
Marini et al. 2003 for Libiola). Other potential sulphate minerals are melanterite and siderotil in blue waters (Fig.4a) and copiatite in red waters. As shown in the Eh vs pH diagram of Fig.5, the blue/greenish-blue colour of precipitates from mine waters at Libiola and Vigonzano can be related to deposition of secondary Cu-minerals like malachite, brochantite and hydrowoodwardite. Thermodynamic affinities to equilibrium with respect to relevant minerals for both red and blue waters are reported in Table 6. The list of the many secondary minerals observed at Libiola (Bertolani 1952; Antofilli et al. 1983; Carbone et al. 2002, 2007) includes all the above minerals, except jurbanite. The presence of jurbanite was hypothesized by Derron et al. (2002) and Marini et al. (2003), but it was not identified, most probably because it forms in acid mine spoils as an amorphous or poorly crystalline precipitate.
4.3 Hydrogen and Oxygen Isotope Composition of Waters
The isotopic analyses at Libiola were carried out on water samples collected in December 1997 from rills
and discharges in the mining area, the Rio Boeno and the Gromolo River (Tables 2 and 3). The results are fairly uniform and in keeping with a meteoric origin of the waters, i.e. the mean δ18O of−5.6‰ and δ2H
of−32.7‰values are quite consistent with the yearly
weighted means of precipitation collected in the period 1991–1997 at the Sestri Levante station
(δ18O =−5.5‰ and δ2H =−34.6‰; Longinelli and
Selmo 2003). However, the individual data do not fit perfectly the meteoric line at Sestri Levante (Fig. 6), as extrapolated from the Longinelli and Selmo’s (2003) data, as well as that given by Dubuis et al. (1998) from the regression of mean monthly values between 1988 and 1991. The disagreement involves, along with mine waters and streams of the Gromolo valley, also streams, springs and wells of the nearby Petronio valley (see Dubuis et al.
1998). Probably, the waters under question do not correspond to yearly means, but to an incomplete set of precipitation events, these being influenced by seasonal factors as the amount of precipitation, atmospheric temperature and origin of disturbances. Likely, the waters in the study area are related to a shallow hydrologic circulation. Samples appreciably
Table 6 Resume of the thermodynamic affinities to equilibrium (saturation indexes) with respect to relevant alteration minerals, gas fugacity and labile parameters of the studied red and blue mine waters
Number of samples Libiola Blu Waters Libiola Red Waters Vigonzano
7 14 9
Median Mean ± SD Median Mean ± SD Median Mean ± SD
pH 7.16 7.06 ± 0.48 2.53 2.60 ± 0.14 7.30 7.08 ± 1.47
T (°C) 15.50 15.38 ± 1.08 14.40 14.27 ± 1.85 8.6 10.37 ± 5.15
Eh (mV) 217 213 ± 101 608 582 ± 58 204 240 ± 107
Logf(O2) −42.5 −42.8 ± 5.9 −33.8 −35.3 ± 3.8 −42.7 −42.9 ± 1.9
Logf(CO2) −2.57 −2.48 ± 0.65 – – – −2.78 −2.36 ± 1.30
Brochantite 0.13 1.03 ± 2.01 −16.2 −15.8 ± 1.4 1.32 −1.72 ± 4.99
Chalcanthite −5.26 −5.20 ± 0.74 −2.48 −2.53 ± 0.24 −5.78 −6.41 ± 2.28
Ferrihydrite −2.06 −2.66 ± 1.06 −6.49 −7.00 ± 1.45 −6.43 −6.26 ± 3.39
Gypsum −0.41 −0.40 ± 0.18 −0.17 −0.17 ± 0.15 −1.36 −1.46 ± 0.41
Goethite 5.32 4.82 ± 1.27 1.32 1.17 ± 0.94 4.30 3.87 ± 1.54
Hematite 11.6 10.6 ± 2.53 3.58 3.30 ± 1.87 9.53 8.67 ± 3.09
Hydrowoodwardite 2.20 2.20 ± 0.68 −3.82 −3.79 ± 0.42 2.01 1.31 ± 1.74
Jurbanite −4.35 −4.35 ± 1.40 −0.42 −0.52 ± 0.52 −3.48 −3.80 ± 2.21
Malachite −0.05 0.34 ± 0.99 – – 0.65 −0.51 ± 2.48
Schwertmannite 8.89 5.31 ± 10.73 −5.39 −6.77 ± 7.50 −1.00 −3.43 ± 7.10
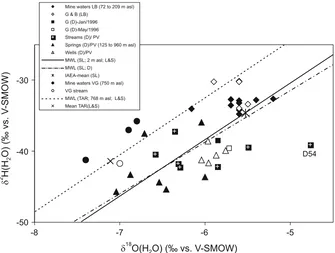
Fig. 6 Hydrogen and oxygen isotope composition of water occurrences in the Gromolo River valley (mine and stream water samples) and the contiguous Petronio River valley (stream, spring and well water samples). Data are from this work and Dubuis et al. (1998), respectively. Meteoric water lines are from Longinelli and Selmo (2003) and Dubuis et al. (1998) at Sestri Levante, and from Longinelli and Selmo (2003)
deviating on the right of the meteoric line as observed in the Petronio valley are likely affected by evaporation rather than by oxygen isotopic exchange with mine sulphate as interpreted by Dubuis et al. (1998) for a stream water sample (D54). In fact, even at pH=1 the sulphate–water oxygen isotope exchange at 25°C should reach half-equilibration in 25 to 100 yr (van Stempvoort and Krouse1994and references therein).
At Vigonzano, the analyses refer to water samples along a very small rill plodding through the waste dump, and the Vigonzano stream, all collected in November 1997. Theδ2H andδ18O values fit closely
the meteoric line reported by Longinelli and Selmo (2003) for the Tarsogno station at 768 m a.s.l. in the Apennines, located about 25 km SE from Vigonzano, and about 30 km inland from the Sestri Levante station. As at Libiola, the deviations from the meteoric line may be due to seasonal isotopic effects or a higher intercept value of the meteoric line at Vigonzano compared to Tarsogno, or both.
No appreciable isotopic differences are observed at Libiola and Vigonzano between mine waters (coloured or colourless) and stream waters, thus supporting no significant isotopic effects due to alteration of rocks and formation of secondary silicate minerals (enriched in 18O and depleted in 2H; e.g. Savin 1980 and references therein), as expected for processes occurring under high water to mineral ratio conditions. Also significant isotopic effects on the initial (meteoric) water due to O-isotope exchange with sulphates and Fe-oxyhydroxides produced by pyrite and chalcopyrite oxidation should be excluded (Fig.6), as they should shift the mine waters to the right of the meteoric line (Spangenberg et al.2007).
4.4 Sulphur Isotope Composition of Ore Sulphide and Aqueous Sulphate
Some pyrite-chalcopyrite ore specimens (massive, disseminated and stockwork) from the Libiola and Vigonzano deposits and dissolved sulphate in mine waters were analysed for theirδ34S values.
4.4.1 Ore Sulphides
All but one massive sulphides at Libiola show uniform
δ34S values between 5.0 and 6.7‰(mean 6.1‰; n=
4). A specimen has a quite differentδ34S of 1.5‰, but
no peculiar mineral paragenesis is observed. Dissem-inated sulphides are enriched in the heavy isotope by about 3‰, with δ34S values between 8.2 and 10.1‰
(mean 9.4‰; n=4). The only one vein sulphide
displays an intermediate δ34S of 7.2‰. Additional
massive sulphide ore, not checked for mineralogy, yieldedδ34S values of−0.5, 4.5 and 5.6‰. It may be
concluded that the δ34S values of massive ore range
from−0.5 to 6.7‰, with a clustering between 4.5 and
6.7‰. The ore can have been partially remobilized by
Alpine metamorphic fluids, thus providing H2S for
the precipitation of secondary vein sulphides (L4-A;
δ34S=7.2‰), these being enriched in34S with respect
to their source (on average 5.8‰) because of the
positive sulphur isotope fractionation between mineral and aqueous sulphide (e.g. Ohmoto and Rye 1979). Disseminated pyrite in basalt is considerably enriched in34S with respect to both massive and vein sulphide, with δ34S values as high as 10.1‰. Stockwork ore
enriched in 34S relative to massive ore is reported by Carvalho et al. (1999) from the Iberian Pyrite Belt. The δ34S data from Vigonzano mine refer to
dissem-inated and stockwork sulphide, with values of 7.3 and 6.9‰.
As in other volcanic-hosted massive sulphide (VMS) deposits, the δ34S values of the sulphides at
Libiola and Vigonzano do no allow in discriminating between genetic models as chemical reduction of marine sulphate or leaching of sulphur from basalts. It can be only observed that they are within the range of
−2 to 9‰ found for most VMS deposits, including
ophiolite-hosted Cyprus-type deposits (Cook and
Fig. 7 δ34S SO2
4
vs SO24
Hoefs1997; Hannington et al.1998; Huston1999), as well as in reasonable agreement with those of 0 to 5‰
reported for modern base metal sulphide deposits on the oceanic floor in various geodynamic settings (Bortnikov and Vikent’ev2005).
4.4.2 Mine Water Sulphate
At Libiola, the δ34S values of 5.6 to 8.4‰ (mean 6.5‰; n= 8) measured in the aqueous sulfate
throughout the mine area are well within the range of ore sulphides, as expected due to the negligible fractionation involved in pyrite (and presumably chalcopyrite) oxidation to sulphate in acid mine drainage (Taylor et al. 1984; see also Ohmoto and Rye1979).
The δ34
S values appear to be independent of pH and chemical composition, including the SO4
con-centration. Colourless waters (pH = 4 to 6.6; 400 to 550 mg/L SO4) show quite uniform δ34S(SO4)
values of 6.2 to 6.5‰, and identical δ34S values of
5.6 and 5.8‰are shown by sulphate from red (pH =
2.5; 5100 mg/L SO4) and blue (pH 6.6; 1650 mg/L
SO4) waters. However, sulphate in the orange water
from mine site 16 (pH = 3.4; 1350 mg/L SO4) has a
distinctly higher δ34S of 8.4‰. The34S-enrichment
can be attributed to an inhomogeneous isotopic composition of metal sulphides through the deposit. Isotopic effects related to the precipitation of secondary sulphates during the evolutionary path of red water to orange to blue water should be excluded, as negligible to very small isotopic fractionation of sulphur are involved in precipitating Al-hydroxysulphates (e.g. basaluminite; Prietzel and Mayer 2005).
At Vigonzano, theδ34S values of aqueous sulphate
in the mine rill are between 0.6 and 1.5‰, slightly higher than in the bordering stream (−0.1‰). When
coupled with the available δ34
S values of 6.9–7.3‰
measured in sulphide veinlets in basalt and serpentin-ite, values in the range−0.1 to 7.3‰may be assigned
to the ore. This range of values for mineral sulphides is nearly equal to that found at Libiola, and coherently the same origin should be concluded for the miner-alization at Vigonzano.
All the available samples do not show any δ34S
(SO4) vs (SO4) correlation (Fig. 7), in keeping with
the low to null sulphur isotope fractionation involved in the sulphide to sulphate oxidation process.
5 Conclusions
The geochemical characteristics of rills draining tailings impoundments and of bordering streams were investigated at the abandoned Fe–Cu (Zn)
ophiolite-hosted Libiola and Vigonzano mines in northern Apennines.
At Libiola, the newly acquired chemical results corroborate those from previous investigations, thus providing additional support to existing geochemical models in terms of metal distribution, solid phases precipitation, water–rock interaction and mixing reaction paths, and environmental problems. At Vigonzano, the chemical characteristics of waters are similar to those at Libiola. Low crystalline hydro-woodwardite, brochantite and malachite are responsi-ble of the blue-green colour in the near neutral waters. In both localities, the hydrogen and oxygen isotope composition of waters agrees with their meteoric origin. Acid to neutral mine waters do not show any significant isotope shift with respect to the initial water, in spite of the oxidation of even large amounts of pyrite/chalcopyrite ore.
The sulphur isotope composition of aqueous sulphate in mine rills matches that of sulphide ore, in keeping with the almost null isotopic effects expected in the oxidation of sulphide to sulphate in surface environments. The isotopic signature of mas-sive ore specimens is consistent with those reported for most volcanic-hosted massive sulphide deposits, in-cluding other Cyprus-type deposits, as well as with that of modern base metal sulphide deposits on the oceanic floor in various geodynamic settings.
References
Accornero, M., Marini, L., Ottonello, G., & Vetuschi Zuccolini, M. (2005). The fate of major constituents and chromium and other trace elements when acid waters from the derelict Libiola mine (Italy) are mixed with stream waters. Applied Geochemistry,20, 1368–1390.
Alpers, C. N., & Blowes, D. W. (Eds.) (1994).Environmental Geochemistry of Sulfide Oxidation. American Chemical Society, Symposium Series, vol. 550, 681 pp.
Antofilli, M., Borgo, E., & Palenzona, A. (1983). I nostri minerali: geologia e mineralogia in Liguria. Sagep Editrice, Genova, 295 pp.
Bethke, C. M. (2002). The Geochemist’s Workbench. A User’s Guide to Rxn, Act2, Tact, React, and Gtplot Release 4.0. Urbana, IL: University of Illinois Hydrogeology Program, 236 pp.
Bonatti, E., Zerbi, M., Kay, R., & Rydell, H. (1976). Metalliferous deposits from the Apennine ophiolites: Mesozoic equivalents of modern deposits from oceanic spreading centers.Geological Society American Bulletin, 87, 83–94.
Bortnikov, N. S., & Vikent’ev, I. V. (2005). Modern base metal sulphide mineral formation in the world ocean.Geology of Ore Deposits,47, 13–44.
Boschetti, T., & Toscani, L. (2008). Springs and streams of the Taro-Ceno catchments (Northern Apennine, Italy): reac-tion path modeling of waters interacting with serpenti-nized-ultramafic rocks.Chemical Geology(submitted). Bravo-Suárez, J. J., Páez-Mozo, E. A., & Oyama, S. T. (2004).
Models for the estimation of thermodynamic properties of layered double hydroxides: application to the study of their anion exchange characteristics.Química Nova, 27, 574–581.
Bruni, J., Canepa, M., Cipolli, F., Marini, L., Ottonello, G., Vetuschi Zuccolini, M., et al. (2002). Irreversible water-rock mass transfer accompanying the generation of the neutral, Mg-HCO3and high-pH, Ca-OH spring waters of
the Genova province, Italy. Applied Geochemistry, 17, 455–474.
Carbone, C., Marescotti, P., Cabella, R., & Lucchetti, G. (2002). Sulphide alteration and related secondary minerals from the Libiola mine (Eastern Liguria, Italy). In:PLINIUS, 28, 88–89 (abstracts 82° Convegno SIMP, Cosenza, 18–20 September 2001). Litografia Felici, Pisa.
Carbone, C., Marescotti, P., Cabella, R., Lucchetti, G., & Martinelli, A. (2007). Mineralogical features of varicol-ored stream sediments from acid sulfate waters in Libiola mine (Italy). InEpitome, vol. 2, 431–432. Geoitalia 2007, 6th Italian Forum of Earth Sciences, September 12–14, 2007, Rimini. FIST, Federazione Italiana di Scienze della Terra.
Carvalho, D., Barriga, F. J. A. S., & Munhà, J. (1999). Bimodal siliciclastic systems—the case of the Iberian Pyrite Belt. In: C. T. Barrie & M. D. Hannington, (Eds.) Volcanic Associated Massive Sulfide Deposits: Processes and Examples in Modern and Ancient Settings. Review in Economic Geology 8, 375–408. Society of Economic Geologists, Inc., Littleton, Colorado.
Chou, I. M., Seal II., R. R., & Hemingway, B. S. (2002). Determination of melanterite–rozenite and chalcanthite– bonattite equilibria by humidity measurements at 0.1 MPa. American Mineralogist,87, 108–114.
Christov, C. (2003). Thermodynamic study of the co-crystallization of ammonium, sodium and potassium alums and chromium alums. Computer Coupling of Phase Diagrams and Thermochemistry,27, 153–160. Cook, N. J., & Hoefs, J. (1997). Sulphur isotope characteristics
of metamorphosed Cu (Zn) volcanogenic massive sulphide deposits in the Norwegian Caledonides.Chemical Geolo-gy,135, 307–324.
Cortecci, G., Dinelli, E., Lucchini, F., & Vaselli, O. (2001). Hydrogeochemical and isotopic investigations in the
abandoned Fe–Cu mine of Libiola (northern Italy). In R. Cidu (Ed.) Proceedings of the Tenth International Symposium on Water–Rock Interaction WRI-10 (pp. 1197–1200). Lisse: A.A. Balkema Publ. (Villasimius, Italy, 10–15 June 2001).
Danielsson, L. G., Magnusson, B., & Westerlund, S. (1978). An improved metal extraction procedure for the determi-nation of trace metals in seawater by atomic absorption spectrotometry with electrothermal atomization.Analytica Chimica Acta,98, 47–57.
Derron, M. H. (1999). Interaction eau–roche de basse tempér-ature: géochimie des métaux dans l’altèration météorique des roches mafiques alpine. Thése de doctorat. Faculté des Sciences de l’Université de Lausanne.
Derron, M. H., Hunziker, J., & Pfeifer, H. R. (2002). Géochimie des eaux acides de l’ancienne mine de cuivre de Libiola (Ligurie, Italie).Bulletin de la Société Vaudoise des Sciences Naturelles,88, 175–194.
Dinelli, E., Cortecci, G., & Lucchini, F. (1996). Geochemical characterization of sulphide waste rock piles from mining workings in Northern Apennines, Emilia province, Italy. Mineralogica et Petrographica Acta,39, 109–123. Dinelli, E., & Lombini, L. (1996). Metal distribution in plants
growing on copper mine spoils in Northern Apennies, Italy: the evaluation of seasonal variations. Applied Geochemistry,11, 375–385.
Dinelli, E., Lucchini, F., Fabbri, M., & Cortecci, G. (2001). Metal distribution and environmental problems related to sulfide oxidation in the Libiola copper mine area (Ligurian Apennines, Italy). Journal of Geochemical Exploration, 74, 141–152.
Dinelli, E., Morandi, N., & Tateo, F. (1998). Fine-grained weathering products in waste disposal from two sulphide mines in the northern Apennines, Italy.Clay Minerals,33, 423–433.
Dinelli, E., & Tateo, F. (2001a). Factors controlling heavy-metal dispersion in mining areas: the case of Vigonzano (northern Italy), a Fe–Cu sulfide deposit associated with ophiolitic rocks. Environmental Geology, 40, 1138– 1150.
Dinelli, E., & Tateo, F. (2001b). Sheet silicates as effective carriers of heavy metals in the ophiolitic mine area of Vigonzano (northern Italy).Mineralogical Magazine, 65, 121–132.
Dinelli, E., & Tateo, F. (2002). Different types of fine-grained sediments associated with acid mine drainage in the Libiola Fe–Cu mine area (Ligurian Apennines, Italy). Applied Geochemistry,17, 1081–1092.
Dubuis, R., Moulin, C., Pfanzelter, A., & Roch, K. (1998). Etude geochimique et isotopique des eaux des bassins versants Petronio & Gromolo. Faculté des Sciences de l’Universitè de Lausanne.
Ferrario, A. (1973). I giacimenti cupriferi nelle pillow lavas della Liguria orientale. Rendiconti Società Italiana di Mineralogia e Petrolologia,29, 485–495.
Ferrario, A., & Garuti, G. (1980). Copper deposits in the basal breccia and volcano-sedimentary sequences of the eastern Ligurian ophiolites (Italy).Mineralium Deposita,15, 291– 303.
studies and research topics. Reviews in Economic Geology, vol. 6. Society of Economic Geologists, Inc. Littleton, Colorado.
Hannington, M. D., Galley, A. G., Herzig, P. M., & Petersen, S. (1998). Comparison of the TAG mound and stockwork complex with Cyprus-type massive sulphide deposits. In P. M Herzig, S.E. Humphris, D.J. Miller & R.A. Zierenberg (Eds.) Proceed. Ocean Drilling Program, Scientific Results, 158, 389–415. College Station, TX (Ocean Drilling Program).
Hemingway, B. S., Seal, R. R. II., & Chou, I. M. (2002). Thermodynamic data for modeling acid mine drainage problems: compilation and estimation of data for selected soluble iron-sulfate minerals. U.S. Geological Survey Open File Report 02-161, 13 pp. Reston, VA. Retrieved January 11, 2008, fromhttp://pubs.usgs.gov/of/2002/of02-161. Holt, B. D., & Engelkemeir, A. G. (1970). Thermal
decompo-sition of barium sulphate to sulphur dioxide for mass spectrometric analysis. Analytical Chemistry, 42, 1451– 1453.
Huston, D. L. (1999). Stable isotopes and their significance for understanding the genesis of volcanic-hosted massive sulphide deposits: a review. In: C. T. Barrie & M. D. Hannington, (Eds.) Volcanic Associated Massive Sulfide Deposits: Processes and Examples in Modern and Ancient Settings. Reviews in Economic Geology, vol. 8, 157–179. Society of Economic Geologists. Inc., Littleton, Colorado. Jambor, J. L., Blowes, D. W., & Ritchie, A. I. M. (Eds) (2003). Environmental Aspects of Mine Wastes. Mineralogical Association of Canada, Short-Course Series, vol. 31, 430 pp. Longinelli, A., & Selmo, E. (2003). Isotopic composition of precipitation in Italy: a first overall map. Journal of Hydrology,270, 75–88.
Lottermoser, B. G. (2003). Mine wastes: Characterization, treatment, and environmental impacts p. 277. Berlin: Springer-Verlag.
Ludwig, B., Beese, F., & Michel, K. (2005). Modelling cation transport and pH buffering during unsaturated flow through intact subsoils.European Journal of Soil Science, 56, 635–645.
Majzlan, J., Navrotsky, A., McCleskey, R. B., & Alpers, C. N. (2006). Thermodynamic properties and crystal structure refinement of ferricopiapite, coquimbite, rhomboclase, and Fe2(SO4)3(H2O)5. European Journal of Mineralogy, 18,
175–186.
Marini, L., Saldi, G., Cipolli, F., Ottonello, G., & Vetuschi Zuccolini, M. (2003). Geochemistry of water discharges from the Libiola mine, Italy. Geochemical Journal, 37, 199–216.
Ohmoto, H., & Rye, R. O. (1979). Isotopes of sulfur and carbon. In H. L. Barnes (Ed.)Geochemistry of Hydrother-mal Ore Deposits, 2nd ed (pp. 509–567). New York: Wiley & Sons Inc.
Parkhurst, D. L., & Appelo, C. A. J. (1999).User’s guide to PHREEQC (version 2)—a computer program for specia-tion, batch-reacspecia-tion, one-dimensional transport and in-verse geochemical calculation. Washington DC: US Department of the Interior, US Geological Survey. Peltier, E., Allada, R., Navrotsky, A., & Sparks, D. L. (2006).
Nickel solubility and precipitation in soils: a thermody-namic study.Clays and Clay Minerals,54, 153–164.
Plumlee, G. S., & Logsdon, M. J. (Eds) (1999). The environmental geochemistry of mineral deposits; partA: processes, techniques, and health issues. Reviews in Economic Geology, vol. 6A, 371 pp. Society of Economic Geologists, Inc. Littleton, Colorado.
Pollard, A. M., Thomas, R. G., & Williams, P. A. (1992). The stabilities of antlerite and Cu3SO4(OH)4*2H2O: their
formation and relationships to other copper(II) sulfate minerals.Mineralogical Magazine,56, 359–365. Powell, K. J., Brown, P. L., Byrne, R. H., Gajda, T., Hefter, G.,
Sjöberg, S., et al. (2007). Chemical speciation of environ-mentally significant heavy metals with inorganic ligands. Part 2: The Cu2+–OH−, Cl−, CO2
systems (IUPAC Technical Report).Pure Applied Chem-istry,79, 895–950.
Prietzel, J., & Mayer, B. (2005). Isotopic fractionation of sulfur during formation of basaluminite, alunite and natroalunite. Chemical Geology,215, 525–535.
Reardon, E. J. (1988). Ion interaction parameters for aluminum sulfate and application to the prediction of metal sulfate solubility in binary salt systems. Journal of Physical Chemistry,92, 6426–6431.
Savin, S. M. (1980). Oxygen and hydrogen isotope effects in low-temperature mineral–water interactions. In P. Fritz, & J. Ch. Fontes (Eds.)Handbook of environmental isotope geochemistry 1, the terrestrial environment, A(pp. 283– 327). New York: Elsevier.
Seal, R. R., & Hammarstrom, J. M. (2003). Geoenvironmental models of mineral deposits—Examples from massive sulphide and gold deposits. In: J. L. Jambor, D. W. Blowes & A. I. M. Ritchie (Eds.),Environmental Aspects of Mine Wastes. Mineralogical Association of Canada Short Courses Series, 31, 11–50.
Seal, R. R. II., Hammarstrom, J. M., Foley, N. K., & Alpers, C. N. (2002). Geoenvironmental models for seafloor massive sulfide deposits. In R. R. Seal II, & N. K. Foley (Eds.)Progress on Geoenvironmental Models for selected Mineral Deposit Types. U.S. Geological Survey Open-File Report 02-195, chapter L, pp. 196–212. Retrieved January 11, 2008, fromhttp://pubs.usgs.gov/ of/2002/of02-195/.
Singh, S. S. (1980). Thermodynamic properties of synthetic basic aluminite [Al4(OH)10SO4*5H2O] from solubility
data.Canadian Journal of Soil Science,60, 381–384. Spangenberg, J. E., Dold, B., Vogt, M. L., & Pfeifer, H. R.
(2007). Stable hydrogen and oxygen isotope composition of waters from mine tailings in different climatic environ-ments. Environmental Science & Technology, 41, 1870– 1878.
Spooner, E. T. C., Beckinsale, R. D., Fyfe, W. S., & Smewing, J. D. (1974).18O enriched ophiolitic metabasic rocks from E. Liguria (Italy), Pindos (Greece) and Trodos (Cyprus).Contributions to Mineralogy and Petrology,47, 41–62.
Taylor, B. E., Wheeler, M. C., & Nordstrom, D. K. (1984). Stable isotope geochemistry of acid mine drainage: experimental oxidation of pyrite.Geochimica Cosmochi-mica Acta,48, 2669–2678.
Tumiati, S., Godard, G., Masciocchi, N., Martin, S., & Ponticelli, D. (2008). Environmental factors controlling the precipitation of Cu-bearing hydrotalcite-like com-pounds from mine waters. The case of the "Eve verda" spring (Aosta Valley, Italy).European Journal of Miner-alogy, 20 (in press).
van Stempvoort, D. R., & Krouse, H. R. (1994). Controls ofδ18O
in sulfate. Review of experimental data and application to specific environment. In C. N. Alpers & H. Krouse (Eds.).
Environmental Geochemistry of Sulfide Oxidation. ACS Symposium Series 550, Washington, D.C., 446–480. Verdes, G., Robert, G., & Sylvie, C. (1992). Thermodynamic
properties of the aluminate ion and of bayerite, boehmite, diaspore, and gibbsite.European Journal of Mineralogy, 4, 767–792.