Summary We investigated the interaction between indole-3-acetic acid (IAA) and ethylene in the regulation of the seasonal periodicity of tracheid production in 1-year-old balsam fir (Abies balsamea (L.) Mill.) cuttings collected at different times during the dormant period. The cuttings were left with their buds intact or were debudded and treated either apically with IAA or 2-chloroethylphosphonic acid (Ethrel) in lanolin, later-ally with IAA or Ethrel in lanolin, or baslater-ally with Ethrel, Co2+ or Ag+ in deionized water. The treated cuttings were then cultured for up to 5 weeks under controlled environment con-ditions favorable for cambial growth. No change in ethylene evolution was detected during the rest--quiescence transition, when IAA-induced tracheid production increased. The induc-tion of cambial reactivainduc-tion by IAA was associated with a rise in ethylene evolution, but there was no consistent relationship among IAA concentration, tracheid number and ethylene emis-sion. Neither Ethrel, Co2+ nor Ag+ affected tracheid production when applied basally, except for 10 and 100 µM Ethrel and 100 µM Co2+, which were inhibitory. In contrast, ethylene evolution was promoted by Ethrel and inhibited by Co2+, whereas Ag+ had no effect. Similarly, applying Ethrel apically or laterally increased ethylene evolution, but did not promote tracheid production except in the treatment in which 1 mg Ethrel g−1 lanolin was applied laterally to cuttings treated apically with 0.1 mg IAA g−1 lanolin, and in the treatment in which 10 mg Ethrel g−1 lanolin was applied laterally to budded cuttings. We conclude that (1) ethylene evolution is not specifically associ-ated with IAA-induced tracheid production, (2) ethylene does not mimic the promoting effect of IAA on tracheid production, and (3) ethylene can promote tracheid production, but only when its application results in a localized unphysiologically high concentration in the cambial region, which, in turn, in-duces an accumulation of IAA.
Keywords: cambium, 2-chloroethylphosphonic acid, cobalt, dormancy, Ethrel, IAA, silver.
Introduction
Considerable evidence indicates that indole-3-acetic acid (IAA) is involved in the mechanism regulating xylem produc-tion in tree stems. For example, it has been shown that in
1-year-old shoots of Abies balsamea and Pinus sylvestris, debudding concomitantly decreases the production of tra-cheids and the concentration of IAA, whereas both are in-creased by applying IAA either apically to debudded shoots or laterally to budded shoots (Little and Savidge 1987, Sundberg and Little 1990, Little and Sundberg 1991). Other research has demonstrated that IAA transport is basipetally polar in the cambial region, and that stem girdling both increases tracheid production and the IAA concentration above the girdle while reducing both below (Little and Savidge 1987). However, the rate and duration of tracheid production are not clearly related to the concentration of IAA in the cambial region (Sundberg et al. 1991, 1993). Moreover, the cambium’s ability to produce tracheids in response to IAA decreases with increasing age (Little and Savidge 1987, Little and Sundberg 1991) and varies with the time of IAA application. Studies with Abies balsamea and Pinus sylvestris have shown that exogenous IAA promotes tracheid production under environmental conditions favorable for growth if applied during the winter when the cambium is in the quiescence stage of dormancy imposed externally by low temperature, but not if applied during the autumn when the cambium is in the resting stage of dormancy maintained by internal agents or conditions (Little and Savidge 1987, Sund-berg et al. 1987, Little et al. 1990, Mellerowicz et al. 1992). The reduced response to IAA by the resting cambium cannot be attributed to either the accumulation of abscisic acid (Little and Wareing 1981) or the inhibition of IAA transport (Little 1981), nor is it altered by exogenous gibberellic acid or kinetin (Little and Bonga 1974). It is not known whether ethylene affects the seasonal variation in cambial responsiveness to IAA, or if it acts as an intermediate in IAA-induced tracheid production.
There is evidence that ethylene plays a role in the regulation of xylem production in tree stems. First, the application of the ethylene-releasing compound 2-chloroethylphosphonic acid (Ethrel) increases tracheid production in Pinus species. (Barker 1979, Telewski and Jaffe 1986, Yamamoto and Ko-zlowski 1987a, 1987b), as well as xylem increment in several woody angiosperms (Robitaille and Leopold 1974, Phelps et al. 1980, Yamamoto and Kozlowski 1987c, Yamamoto et al. 1987). Ethrel also alters the cell wall composition of Picea abies hypocotyls, increasing the content of cellulose and
de-Interaction between indole-3-acetic acid and ethylene in the control of
tracheid production in detached shoots of
Abies balsamea
LEIF EKLUND
1and C. H. ANTHONY LITTLE
21 Department of Engineering and Natural Sciences, Växjö University, S-351 95 Växjö, Sweden
2 Natural Resources Canada, Canadian Forest Service, P.O. Box 4000, Fredericton, New Brunswick E3B 5P7, Canada
Received December 16, 1993
creasing that of noncellulosic polysaccharides (Ingemarsson et al. 1991a, Eklund 1991a), and promotes xylem differentiation in Lactuca pith explants (Miller et al. 1984, 1985). Reinders-Gouwentak (1965) reported that cambial rest in Fraxinus shoots was overcome by ethylene chlorohydrin treatment. In addition, Co2+, which decreases ethylene production (Yu and Yang 1979, Ingemarsson et al. 1991a), inhibited cell wall deposition, whereas applying Ag+, an inhibitor of ethylene action (Veen and Van de Geijn 1978), increased tracheid size but did not alter cell wall composition in Picea abies (Inge-marsson et al. 1991a, Eklund 1991a). Second, in several tree species, ethylene evolution from the stem is higher during the period of xylem production than when the cambium is dormant (Yamanaka 1985, Eklund 1990, 1991b, 1993b, Ingemarsson et al. 1991b, Eklund et al. 1992). Moreover, mechanical pertur-bation increases both ethylene evolution and tracheid produc-tion in Pinus taeda (Telewski 1990), and there is evidence that cambial region cells of Abies balsamea can convert an ethylene precursor, 1-aminocyclopropane-1-carboxylic acid (ACC), to ethylene (Savidge 1988).
This study was undertaken to determine if ethylene interacts with IAA in the regulation of the annual cycle of cambial activity and dormancy in balsam fir stems, as measured by seasonal changes in tracheid production.
Materials and methods
Plant material, application of growth regulators and culture conditions
Current-year shoots were collected from the upper crown of balsam fir (Abies balsamea (L.) Mill.) trees approximately 25 years old and 4 to 7 m tall, located in a spaced stand at the University of New Brunswick forest in Fredericton, New Brunswick, Canada. In one experiment, cuttings were col-lected from the same trees on September 9, October 21 and December 5, 1992, corresponding approximately to the cam-bial dormancy stages of rest, partial quiescence and full quies-cence, respectively (Little and Bonga 1974, Sundberg et al. 1987). In all other experiments, the cuttings were collected in January to March 1993, during quiescence. On each collection date, enough cuttings were obtained to ensure that every tree was represented in all treatments per experiment. The cuttings were trimmed under tap water at the proximal end to a length of 13 cm below the apical whorl of buds, and subsequently were either left with their buds intact or debudded (final length of debudded cuttings = 11 cm). They then were treated (1) apically, either with 0, 0.1 or 1 mg IAA g−1 lanolin, or with 1 or 10 mg Ethrel g−1 lanolin, (2) laterally, either with 0, 1 or 10 mg Ethrel g−1 lanolin (neutralized Ethrel solution mixed with lanolin), or with 1 mg IAA g−1 lanolin, or (3) basally, either with 0, 0.1, 1, 10, 100, 1000 or 10,000 µM Ethrel, or with 1, 10 or 100 µM cobalt chloride, or with 1, 10 or 50 µM silver thiosulfate in deionized water. Apical treatment involved com-plete debudding, the removal of the needles borne on the uppermost 1 cm of the shoot, and the application of about 0.8 g of lanolin mixture to the apical cut surface. Lateral treatment involved the careful removal of the needles and periderm from
around the shoot circumference at a point located 8--9 cm (budded cuttings) or 7--8 cm (debudded cuttings) below the apex, the application of about 0.5 g of lanolin mixture spread evenly over the surface of the application site, and the covering of this site with aluminum foil. The cuttings were placed upright in beakers with their bases immersed in deionized water (containing Ethrel, Co2+ or Ag+ when these were applied basally) and cultured for up to 5 weeks in a controlled environ-ment chamber providing a photon flux density of 90 µmol m−2 s−1 from combined fluorescent and incandescent lamps, a 16-h photoperiod and a day/night temperature of 23/13 °C. At weekly intervals, basal solutions and IAA-lanolin mixtures were replaced after removing a 1-mm slice from the basal or apical cut surface.
Measurement of tracheid production
Tracheid production was determined in transverse, hand-cut sections obtained from the midpoint of the cutting’s length, unless indicated otherwise. The sections were stained in an aqueous solution of phloroglucinol in 20% hydrochloric acid, which reacts with lignin, and mounted in glycerol. Tracheid production was measured as the number of new cells per radial file on the xylem side of the cambium, recorded at eight equidistant points around the circumference and starting from the last-formed tracheids in the previous year’s latewood. The measurement included only those cells reacting with the stain, i.e., in which at least some lignification had occurred.
Measurement of ethylene evolution
1 ppm ethylene standard (Valley Oxygen, Fredericton, Can-ada) after measuring and subtracting the volume of the needles and stem from the vial volume, and determining the fresh weight of the needles and stem.
Statistical analysis
Analysis of variance was performed on each data set to deter-mine the significance of the effects of concentration, tree and time or position, depending on the experiment. These effects were fixed in the model, except for tree, which was treated as random replication. Where appropriate, the significance (P≤ 0.05) of the difference in effect induced by individual concen-trations at a particular time or position was determined by Fisher’s Least Significant Difference test or a paired t-test.
Results
Ethylene evolution during IAA-induced cambial reactivation in quiescent cuttings
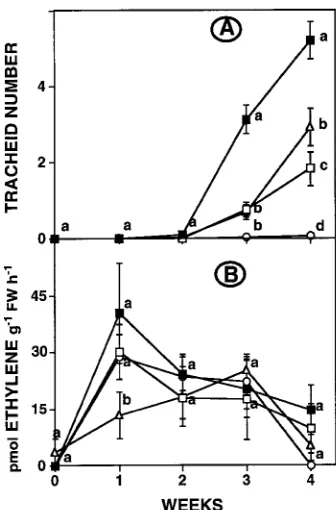
Bud burst and cambial reactivation occurred in the budded control cuttings, and tracheids were present by Week 2 (Fig-ure 1A). As expected, tracheid production was inhibited by debudding and stimulated in debudded cuttings by applying IAA apically, and the extent of the stimulation was positively related to the exogenous IAA concentration. During the first three weeks, the rate of tracheid production in the budded cuttings was similar to that in the debudded cuttings treated with 0.1 mg IAA g−1 lanolin, but subsequently the rate was
higher in the budded cuttings.
The evolution of ethylene from the budded control cuttings increased gradually through Week 3, then declined (Fig-ure 1B). In contrast, in all debudded cuttings regardless of IAA concentration, ethylene evolution increased during the first week and subsequently decreased. The only between-treat-ment difference was evident after one week, when debudded cuttings evolved more ethylene than budded cuttings.
Effect of basal application of Ethrel, Ag2+ and Co2+ on IAA-induced tracheid production and ethylene evolution during the rest--quiescence transition
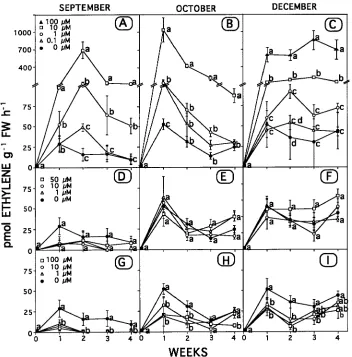
The production of tracheids induced by 1 mg IAA g−1 lanolin in cuttings treated basally with only water increased during the autumn, most noticeably between September 9 and October 21 (Figure 2), reflecting the transition between the cambial dor-mancy states of rest and quiescence. Tracheid production was not affected by Ethrel, Co2+ or Ag+ on September 9 and October
Figure 1. Tracheid number (A) in, and ethylene evolution (B) from, cuttings that were either left intact (budded control, n) or debudded
and treated apically with 0 (s), 0.1 (h) or 1 (j) mg IAA g−1 lanolin.
Mean ± SE, n = 9; within each week, means accompanied by the same letter are not significantly different.
21, but 10--100 µM Ethrel and 100 µM Co2+ were inhibitory on December 5.
Ethylene evolution at the time of collection (Week 0) was undetectable on every collection date (Figure 3). However, after one week of culture, the evolution of ethylene from the cuttings treated basally with only water was about 25 pmol gFW−1 h−1 for the material collected on September 9, and about twice that for the material collected on October 21 and Decem-ber 5. Subsequently, ethylene evolution from these cuttings declined, as observed also in the previous experiment (Fig-ure 1). Applying Ethrel basally increased ethylene evolution to about 1000 pmol gFW−1 h−1, and the extent of the increase was positively related to the Ethrel concentration (Figures 3A--C). Ethylene evolution from all Ethrel-treated cuttings collected on September 9 and October 21 attained a maximum on Weeks 2 and 1, respectively, and subsequently declined. In contrast, ethylene evolution from the Ethrel-treated cuttings collected on December 5 remained high throughout. Applying Ag+ basally did not affect ethylene evolution from the cuttings collected on any date (Figures 3D--F). In contrast, basal appli-cation of Co2+ inhibited ethylene evolution on all collection dates, but the degree of inhibition did not vary with the Co2+ concentration (Figures 3G--I).
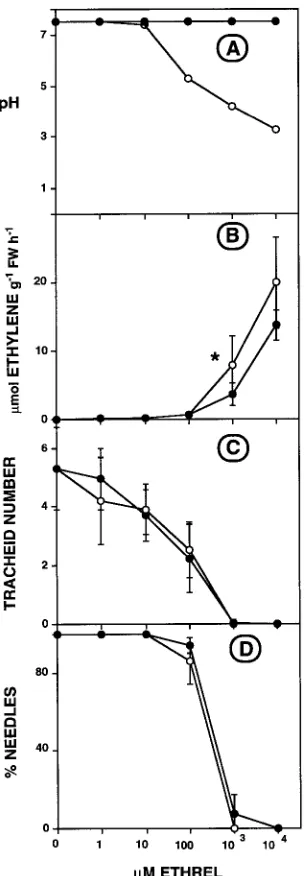
We investigated if the decrease in tracheid production and
stimulation of ethylene evolution induced by the 10 and 100 µM Ethrel (Figures 2A and 3A--C) were attributable to the high concentrations of Ethrel decreasing the pH of the basal solu-tion (Figure 4A). Adjustment to pH 7 reduced ethylene evolu-tion, but only at the very high concentrations of 1 and 10 mM Ethrel, which also caused massive defoliation and inhibited tracheid production (Figures 4B--D). However, neutralization did not alter the effect that Ethrel at any concentration had on tracheid production or defoliation.
We also determined if ethylene emanated from the wounds caused by excision and debudding, and if ethylene evolution was greater from the stem or the needles. At the normal low rate of ethylene evolution, as well as at an Ethrel-induced elevated rate, the evolution of ethylene was not affected by sealing all cut surfaces with a non-ethylene-releasing silicone grease, and the stem evolved about five times more ethylene than the needles (Table I).
Effect of applying IAA and Ethrel laterally or apically on tracheid production and ethylene evolution in quiescent cuttings
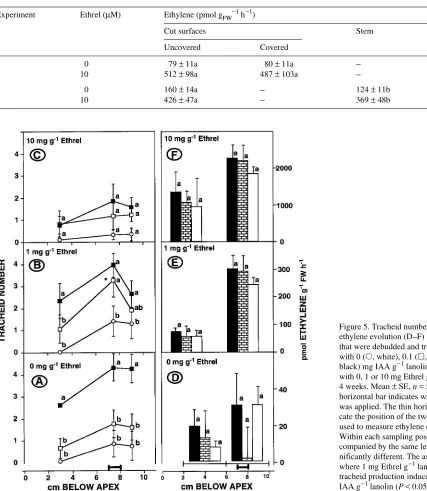
In the absence of Ethrel, tracheid production increased with increasing concentration of apically applied IAA (Figure 5A), whereas ethylene evolution did not (Figure 5D). Lateral
cation of 1 mg Ethrel g−1 lanolin increased the number of tracheids induced by 0.1 mg IAA g−1 lanolin, but only at the Ethrel application site (Figures 5A and 5B). Ethrel at 10 mg g−1 lanolin inhibited IAA-induced tracheid production throughout the cutting (Figures 5A and 5C). Regardless of IAA concentration, 1 and 10 mg Ethrel g−1 lanolin increased ethylene evolution by about 1 and 2 orders of magnitude, respectively (Figures 5D--F). The Ethrel-induced increases were greater in the treated segment than in the control segment. In stems of cuttings whose buds, the major source of endo-genous IAA, were not removed, tracheid production was
pro-moted by lateral application of 0.1 mg and 1 mg IAA g−1 lanolin at, and by 1 mg IAA g−1 lanolin below, the application site (Figure 6A). Lateral application of Ethrel at 10 mg g−1 lanolin inhibited tracheid production above and below the application site. Ethylene evolution was not altered by 0.1 mg IAA g−1 lanolin, whereas 1 mg IAA g−1 lanolin promoted ethylene evolution in the treated segment by about an order of magnitude (Figure 6B). Treatment with 1 mg Ethrel g−1 lanolin had the same effect, whereas 10 mg Ethrel g−1 lanolin stimu-lated the evolution of ethylene from the treated segment by a second order of magnitude and also promoted ethylene evolu-tion from the control segment.
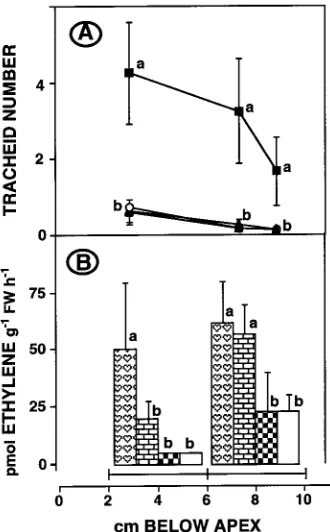
We also investigated the effect on tracheid production and ethylene evolution of separately applying IAA and Ethrel in lanolin to the apex of debudded cuttings. Treatment with 1 mg IAA g−1 lanolin promoted tracheid production along the cut-ting length, but only increased ethylene evolution from the basal segment (Figure 7). Neither 1 mg nor 10 mg Ethrel g−1 lanolin induced tracheid production, although the higher con-centration stimulated the evolution of ethylene from the basal segment to the same extent as did 1 mg IAA g−1 lanolin and, in addition, increased ethylene evolution from the apical seg-ment.
Discussion
About 20% of the ethylene evolved from debudded cuttings emanated from the needles, and undetectable amounts were produced by, and transmitted through, the wounds created by excising the buds and the cutting itself (Table 1). Thus, the bulk of the ethylene evolved from the defoliated stem, but the relative amounts emanated from the needle scars and the un-wounded stem parts is not known. The major source of ethyl-ene in the stem is the combined phloem and cambial tissue (Yamanaka 1985, Telewski 1990). Hence, we assume that the bulk of the ethylene evolved from a cutting in the absence of Ethrel reflects production by the cambial region. We also assume that the evolution of ethylene induced by applying Ethrel, IAA, Co2+ and Ag+ reflects the level of ethylene in the cambial region.
Our results provide evidence for and against the hypothesis that ethylene interacts with IAA in regulating tracheid produc-tion. The hypothesis is supported by the finding that lateral application of 1 mg Ethrel g−1 lanolin to debudded cuttings treated apically with 0.1 mg IAA g−1 lanolin (Figure 5) and lateral application of 10 mg Ethrel g−1 lanolin to budded cuttings (Figure 6) increased ethylene evolution and tracheid production at the application site. Lateral Ethrel application has also been observed to promote tracheid production locally in other conifers (Barker 1979, Telewski and Jaffe 1986, Yamamoto and Kozlowski 1987a, 1987b). However, four other observations indicate that IAA-induced tracheid production does not involve ethylene. First, there was no consistent rela-tionship between ethylene evolution and exogenous IAA con-centration or tracheid production along the length of either debudded cuttings treated apically with IAA (Figures 1, 5 and 7) or budded cuttings treated laterally with IAA (Figure 6). Figure 4. Ethrel solution pH (A), ethylene evolution (B), tracheid
Second, varying ethylene evolution by basal application of Ethrel and Co2+ to cuttings in which the cambium was in different stages along the rest--quiescence transition had no corresponding effect on IAA-induced tracheid production. Ethrel always increased ethylene evolution, as expected, but never tracheid production (Figures 2A, 3A--C and 4). Applica-tion of Co2+ caused the expected decrease in ethylene evolu-tion, but tracheid production was not inhibited except by 100 µM Co2+ in cuttings collected in December, which we specu-late was a toxic concentration at that time (Figures 2C and
3G--I). Third, Ag+, which blocks ethylene action, never inhib-ited ethylene evolution or IAA-induced tracheid production (Figures 2B and 3D--F). Finally, applying Ethrel apically to debudded cuttings stimulated ethylene evolution, but not tra-cheid production (Figure 7). It follows from the above obser-vations that Ethrel promoted tracheid production only when applied both laterally and below an IAA source, and that this promotion was restricted to the Ethrel application point.
The different effects of basally and laterally applied Ethrel on tracheid production may reflect the way in which the two Table 1. Ethylene evolution from cuttings that were debudded and treated apically with 1 mg IAA g−1 lanolin and basally with water containing 0 or 10 µM Ethrel for 4 weeks. Ethylene evolution was measured before and after covering all cut surfaces with silicone grease (Experiment 1), and before and after separating the cutting into stem and needles (Experiment 2). Mean ± SE, n = 6; within each row, means accompanied by the same letter are not significantly different.
Experiment Ethrel (µM) Ethylene (pmol gFW−1 h−1)
Cut surfaces Stem Needles
Uncovered Covered
1 0 79 ± 11a 80 ± 11a --
--10 512 ± 98a 487 ± 103a --
--2 0 160 ± 14a -- 124 ± 11b 59 ± 10c
10 426 ± 47a -- 369 ± 48b 62 ± 14c
Figure 5. Tracheid number (A--C) in, and ethylene evolution (D--F) from, cuttings that were debudded and treated apically with 0 (s, white), 0.1 (h, brick) or 1 (j,
methods of application supplied ethylene to the cambial re-gion. All solutions applied basally (Figure 4) and used to make the lanolin mixtures (data not shown) had a pH > 4, thus Ethrel decomposed to ethylene nonbiologically (Biddle et al. 1976) before and after being taken up by the cutting. After uptake, Ethrel and ethylene in the solution applied basally moved upwards in the transpiration stream (Jackson and Campbell 1975, Foster et al. 1992, Eklund 1993a) and passed into the cambial region along the entire transpiration pathway. In con-trast, laterally applied Ethrel and derived ethylene reached the cambial region after localized passage through the cortex and phloem. We speculate that applying Ethrel laterally, but not basally, raised the ethylene concentration in the cambial region to an unphysiologically high level sufficient to induce a local accumulation of IAA, which, in turn, caused the increase in tracheid production (Sundberg and Little 1990, Little and Sundberg 1991). The cause of the presumptive increase in IAA concentration is not known, because the IAA concentration in a particular tissue is determined by the relative rates of biosyn-thesis, conjugation, catabolism and transport, and ethylene is known to affect each of these processes (Abeles et al. 1992).
However, the possibility that Ethrel affects tracheid production in a way other than, or in addition to, an ethylene--IAA inter-action, e.g., directly or through a metabolite other than ethyl-ene, cannot be excluded.
The poor relationship between ethylene evolution and tra-cheid production (Figures 1, 5--7) suggests that the higher rate of ethylene evolution observed when the cambium is active than when it is dormant (Figures 1--3; Yamanaka 1985, Eklund 1990, 1991b, 1993b, Ingemarsson et al. 1991b, Eklund et al. 1992) is not associated specifically with tracheid production. This is supported by the finding that ethylene evolution from the control cuttings collected in September, in which no tra-cheids were produced, also increased during the experimental period (Figures 2 and 3). Furthermore, the magnitude of the increase in ethylene evolution from IAA-treated cuttings, which formed many tracheids, was similar to that from cut-tings treated with lanolin only, in which tracheid production did not occur (Figure 1).
Acknowledgment
Supported by a grant from the Swedish Council for Forestry and Agriculture Research to L.E. We thank Dr. R.A. Savidge for the use of a gas chromatograph, Ms. S.A. Martin for statistical advice, and Rhône-Poulenc Canada Ltd. for a gift of Ethrel.
Figure 6. Tracheid number (A) in, and ethylene evolution (B) from, budded cuttings treated laterally either with 0 (s, white), 0.1 (h,
brick) or 1 (j, black) mg IAA g−1 lanolin, or with 1 (n, checkered) or 10 (m, hearts) mg Ethrel g−1 lanolin for 4 weeks. Mean ± SE, n = 5. The thick horizontal bar indicates where the lanolin mixtures were applied. The thin horizontal bars indicate the position of the two segments used to measure ethylene evolution. Within each sampling position, means accompanied by the same letter are not significantly different.
References
Abeles, F.B., P.W. Morgan and M.E. Saltveit. 1992. Ethylene in plant biology. 2nd Edn. Academic Press, San Diego, California, 414 p. Barker, J.E. 1979. Growth and wood properties of Pinus radiata in
relation to applied ethylene. N.Z. J. For. Sci. 9:15--19.
Biddle, E., D.G.S. Kerfoot, Y.H. Kho and K.E. Russel. 1976. Kinetic studies of the thermal decomposition of 2-chloroethylphosphonic acid in aqueous solution. Plant Physiol. 58:700--702.
Eklund, L. 1990. Endogenous levels of oxygen, carbon dioxide and ethylene in stems of Norway spruce trees during one growing season. Trees 4:150--154.
Eklund, L. 1991a. Relations between indoleacetic acid, calcium ions and ethylene in the regulation of growth and cell wall composition in Picea abies. J. Exp. Bot. 42:785--789.
Eklund, L. 1991b. Hormone levels in the cambial region of Picea abies during the onset of cambial activity. Physiol. Plant. 82:385--388. Eklund, L. 1993a. Movement and possible metabolism of ethylene in
dormant Picea abies. Plant Growth Regul. 12:37--41.
Eklund, L. 1993b. Seasonal variation of O2, CO2 and ethylene in oak and maple stems. Can. J. For. Res. 23:2608--2610.
Eklund, L., E. Cienciala and J.-E. Hällgren. 1992. No relation between drought stress and ethylene production in Norway spruce. Physiol. Plant. 87:297--300.
Foster, K.R., D.M. Reid and R.P. Pharis. 1992. Ethylene biosynthesis and ethephon metabolism and transport in barley. Crop Sci. 32:1345--1352.
Ingemarsson, B.S.M., L. Eklund and L. Eliasson. 1991a. Ethylene effects on cambial activity and cell wall formation in hypocotyls of Picea abies seedlings. Physiol. Plant. 82:219--224.
Ingemarsson, B.S.M., E. Lundqvist and L. Eliasson. 1991b. Seasonal variation in ethylene concentration in the wood of Pinus sylvestris L. Tree Physiol. 8:273--279.
Jackson, M.B. and D.J. Campbell. 1975. Movement of ethylene from roots to shoots, a factor in the response of tomato plants to water-logged soil conditions. New Phytol. 74:397--406.
Little, C.H.A. 1981. Effect of cambial dormancy state on the transport of [1-14C]indol-3-ylacetic acid in Abies balsamea shoots. Can. J. Bot. 59:342--348.
Little, C.H.A. and J.M. Bonga. 1974. Rest in the cambium of Abies balsamea. Can. J. Bot. 52:1723--1730.
Little, C.H.A. and R.A. Savidge. 1987. The role of plant growth regulators in forest tree cambial growth. Plant Growth Regul. 6:137--169.
Little, C.H.A. and B. Sundberg. 1991. Tracheid production in response to indole-3-acetic acid varies with internode age in Pinus sylvestris stems. Trees 5:101--106.
Little, C.H.A. and P.F. Wareing. 1981. Control of cambial activity and dormancy in Picea sitchensis by indol-3-ylacetic and abscisic acids. Can. J. Bot. 59:1480--1493.
Little, C.H.A., B. Sundberg and A. Ericsson. 1990. Induction of acropetal 14C-photosynthate transport and radial growth by indole-3-acetic acid in Pinus sylvestris shoots. Tree Physiol. 6:177--189. Mellerowicz, E.J., W.K. Coleman, R.T. Riding and C.H.A. Little.
1992. Periodicity of cambial activity in Abies balsamea. I. Effects of temperature and photoperiod on cambial dormancy and frost hardiness. Physiol. Plant. 85:515--525.
Miller, A.R., W.L. Pengelly and L.W. Roberts. 1984. Induction of xylem differentiation in Lactuca by ethylene. Plant Physiol. 75:1165--1166.
Miller, A.R., D.L. Crawford and L.W. Roberts. 1985. Lignification and xylogenesis in Lactuca pith explants cultured in vitro in the presence of auxin and cytokinin: a role for endogenous ethylene. J. Exp. Bot. 36:110--118.
Morgan, P.W., C.-J. He, J.A. De Greef and M.P. De Proft. 1990. Does water deficit stress promote ethylene synthesis by intact plants? Plant Physiol. 94:1616--1624.
Phelps, J.E., E.A. McGinnes, M. Saniewski, J. Pieniazek and M. Smolinski. 1980. Some anatomical observations on the effect of morphactin IT 3456 and ethrel on wood formation in Salix fragilis L. IAWA Bull. 1:76--82.
Reinders-Gouwentak, C.A. 1965. Physiology of the cambium and other secondary meristems of the shoot. In Encyclopedia of Plant Physiology. Vol. 15. Ed. W. Ruhland. Springer-Verlag, New York, pp 1077--1105.
Robitaille, H.A. and A.C. Leopold. 1974. Ethylene and the regulation of apple stem growth under stress. Physiol. Plant. 32:301--304. Savidge, R.A. 1988. Auxin and ethylene regulation of diameter growth
in trees. Tree Physiol. 4:401--414.
Sundberg, B. and C.H.A. Little. 1990. Tracheid production in response to changes in the internal level of indole-3-acetic acid in 1-year-old shoots of Scots pine. Plant Physiol. 94:1721--1727.
Sundberg, B., C.H.A. Little, R.T. Riding and G. Sandberg. 1987. Levels of endogenous indole-3-acetic acid in the vascular cambium region of Abies balsamea trees during the activity--rest--quiescence transition. Physiol. Plant. 71:163--170.
Sundberg, B., C.H.A. Little, K. Cui and G. Sandberg. 1991. Level of endogenous indole-3-acetic acid in the stem of Pinus sylvestris in relation to the seasonal variation of cambial activity. Plant Cell Environ. 14:241--246.
Sundberg, B., A. Ericsson, C.H.A. Little, T. Näsholm and R. Gref. 1993. The relationship between crown size and ring width in Pinus sylvestris L. stems: dependence on indole-3-acetic acid, carbohy-drates and nitrogen in the cambial region. Tree Physiol. 12:347--362.
Telewski, F.W. and M.J. Jaffe. 1986. Thigmomorphogenesis: the role of ethylene in the response of Pinus taeda and Abies fraseri to mechanical perturbation. Physiol. Plant. 66:227--233.
Telewski, F.W. 1990. Growth, wood density, and ethylene production in response to mechanical perturbation in Pinus taeda. Can. J. For. Res. 20:1277--1282.
Veen, H. and S.C. Van de Geijn. 1978. Mobility and ionic form of silver as related to longevity of cut carnations. Planta 140:93--96. Yamamoto, F. and T.T. Kozlowski. 1987a. Effects of flooding, tilting
of stems, and ethrel application on growth, stem anatomy and ethylene production of Pinus densiflora seedlings. J. Exp. Bot. 38:293--310.
Yamamoto, F. and T.T. Kozlowski. 1987b. Effect of ethrel on growth and stem anatomy of Pinus halepensis seedlings. IAWA Bull. 8:11--19.
Yamamoto, F. and T.T. Kozlowski. 1987c. Effects of flooding, tilting of stems, and ethrel application on growth, stem anatomy, and ethylene production of Acer platanoides seedlings. Scand. J. For. Res. 2:141--156.
Yamamoto, F., G. Angeles and T.T. Kozlowski. 1987. Effect of ethrel on stem anatomy of Ulmus americana seedlings. IAWA Bull. 8:3--9.
Yamanaka, K. 1985. Ethylene production in Chamaecyparis obtusa phloem and xylem tissues in response to wounding. Mokuzai Gak-kaishi 31:703--710.
Yamanaka, K. 1986. Wound ethylene production by phloem and cam-bium tissues in conifers. Mokuzai Gakkaishi 32:136--139. Yu, Y.B. and S.F. Yang. 1979. Auxin-induced ethylene production and