Summary Both short- and long-term effects of Mg defi-ciency on carbohydrate metabolism were investigated in 6-year-old clonal Norway spruce (Picea abies (L.) Karst.) trees cultivated in sand culture with an optimal supply of nutrients, except for Mg which was supplied at 0.203, 0.041 and 0.005 mM to provide optimal, moderately deficient and se-verely deficient Mg supply, respectively. Annual changes in carbohydrate concentrations (starch, sucrose, glucose and fruc-tose) were analyzed and diurnal changes were investigated on a single day during the summer.
Older needles of trees in the moderate Mg-deficiency treat-ment developed tip-yellowing symptoms, whereas current-year needles remained green. The severe Mg-deficiency treatment led to pronounced yellowing symptoms in needles of all ages. Increased carbohydrate concentrations were observed before needle yellowing occurred. Diurnal and annual changes in carbohydrates were similar in all treatments; however, car-bohydrate concentrations were influenced by Mg supply. In both Mg-deficiency treatments, starch concentrations in-creased in needles, especially during summer and autumn. Starch accumulation was more pronounced at the beginning of the Mg-deficiency treatments than at the end of the treatments. Sucrose, and to a minor extent, glucose and fructose concen-trations tended to increase in response to Mg deficiency. The consequences of Mg deficiency on carbohydrate metabolism are discussed with respect to reduced plant growth and de-creased transport rates of carbohydrates to sink organs. Keywords: carbohydrate metabolism, fructose, glucose, growth, starch accumulation, sucrose.
Introduction
There is evidence that, in Norway spruce (Picea abies (L.) Karst.) needles from stands showing forest decline symptoms, changes in pool sizes of carbohydrates are caused by Mg deficiency. Accumulations of starch in yellowing needles of declining Norway spruce trees have been observed by Fink (1983), Oren et al. (1988) and Forschner et al. (1989); however, Hampp and colleagues did not find any changes in starch concentrations, perhaps as a result of the interactive effects of other stress factors, although they did observe a longer reten-tion of starch in yellowing needles than in green needles during
late autumn (Hampp et al. 1987, Einig and Hampp 1990). Because interactions with environmental factors, such as drought, acid deposition and air pollutants (e.g., Vogels et al. 1986, Landoldt and Krause-Lüthy 1989, Hampp 1992), may alter the carbohydrate pools, the effects of Mg deficiency on carbohydrate metabolism need to be studied under controlled conditions.
A deficiency in Mg nutrition can affect carbon metabolism in many ways causing reductions as well as accumulations of specific carbohydrates. Because Mg is a constituent of the chlorophyll molecule and is also a cofactor of a series of enzymes involved in carbon fixation, it is essential for photo-synthesis (Laing and Christeller 1976, Lorimer et al. 1976). Magnesium is also required for ATP metabolism and is, there-fore, essential for many metabolic processes, including carbo-hydrate metabolism and the synthesis of proteins, fats and nucleic acids. It is also a key element in membrane transport (Marschner 1986).
The main objective of this study was to test the hypothesis that Mg deficiency results in the accumulation of carbohy-drates in Norway spruce needles. We investigated short-term and long-term effects of Mg deficiency on starch, sucrose, glucose and fructose concentrations in needles, and we also evaluated diurnal and annual changes in carbohydrates with respect to Mg nutrition and needle age.
Materials and methods
Plant material and study design
Two experiments, one long term (Experiment 1) and one short term (Experiment 2), were conducted during the growing sea-sons of 1990 and 1991, respectively. In Experiment 1, 100 six-year-old Norway spruce trees of Clone 1382/113 (prove-nance Rothenkirchen 84011, Frankenwald, Germany) were planted in an outdoor facility in spring 1988. In Experiment 2, 100 five-year-old Norway spruce trees of Clone 1213/113 of the same provenance were planted in October 1990. In both experiments, the nonmycorrhizal trees were cultivated in sand culture in 70-liter pots and individually supplied with nutrients by means of a continuously circulating solution, which was replaced once a week. The nutrient solutions were adjusted to achieve optimum growth according to the principles set out by
The influence of magnesium deficiency on carbohydrate
concentrations in Norway spruce (
Picea abies
) needles
BEATE MEHNE-JAKOBS
Institut für Forstbotanik und Baumphysiologie, Albert-Ludwigs-Universität, Bertoldstrasse 17, D-79085 Freiburg, Germany
Received April 5, 1994
Ingestad (1959, 1979). One liter of nutrient solution contained: 50 mg KNO3, 68 mg KH2PO4, 65 mg Ca(NO3)2⋅4H2O, 11.5 mg (NH4)2HPO4, 90 mg NH4NO3, 50 mg MgSO4⋅7H2O, 5 mg Fe-EDTA, 800 µg MnSO4⋅4H2O, 80 µg CuSO4⋅5H2O, 200 µg ZnSO4⋅7H2O, 600 µg H3BO3, 17.5 µg Na2MoO4⋅1H2O plus magnesium as specified. Magnesium was added to the nutrient solutions to provide concentrations of 0.203 (optimal, Treat-ment 1), 0.041 (moderately deficient, TreatTreat-ment 2) and 0.005 mM (severely deficient, Treatment 3). Sulfate in the Mg-defi-ciency treatments was supplied as Na2SO4⋅10H2O in concen-trations corresponding to the deficiency treatments.
Sampling conditions
In Experiment 1, current-year to 2-year-old needles were taken from the fourth whorl between 0800 and 0930 h MET (Mid-European Time) in May, June, August and October 1990, and in March 1991 for carbohydrate analysis. In Experiment 2, needles were taken from current-year and 1-year-old twigs of the third whorl in July, August and September 1991 for carbo-hydrate analysis. Samples were collected between 1100 and 1230 h MET at high irradiance (> 1200 µmol m−2 s−1) after a period of at least 2 days with bright sunshine. In both experi-ments, samples were taken from six trees per Mg treatment, and all samples were taken from the sun-exposed, upper side of the twigs. For the study of diurnal changes in carbohydrates, needles from trees in the optimal-Mg treatment and the severe Mg-deficiency treatment were sampled from current-year and 1-year-old shoots every 2 and 4 h, respectively, between 0400 and 2200 h MET on August 22, 1991, a day following a period of high temperatures and high irradiance.
For the determination of Mg, needle samples were taken from current-year to 2-year-old southerly exposed twigs of the fourth whorl in October 1990 and 1991. Five samples of each treatment and age class were combined to form one mixed sample. Magnesium was assayed in two and five replicates of the mixed samples in 1990 and 1991, respectively.
Temperature, relative humidity and solar irradiance, meas-ured with a quantum sensor (Li-Cor Inc., Lincoln, NE), were recorded at each sampling time.
The needle samples were immediately frozen in liquid nitro-gen and stored at −80 °C. Frozen needle tissue was homoge-nized with a microdismembrator (Braun, Melsungen, Germany), and the resulting powder was freeze-dried at −25 °C and stored under vacuum at −20 °C.
Measurements
Concentrations of sucrose, glucose and fructose in the needles were determined according to Einig and Hampp (1990). Two mg of lyophilized needle homogenate was extracted in 1 ml of 65% (v/v) aqueous ethanol at 70 °C for 1 h. Activated charcoal (2%, w/v) was used in order to reduce the blank reading. The mixture was then centrifuged at 10,000 g for 5 min, and 20 to 200 µl aliquots of the supernatant were assayed according to Jones and Outlaw (1981).
To determine needle starch concentrations, 2 to 6 mg aliquots of needle homogenate were extracted with 0.5 ml of 0.5 M NaOH in Eppendorff vials at ambient temperature for
1 h (Dekker and Richards 1971). To remove free glucose, the extract was incubated at 95 °C for 3 min, adjusted to pH 4.6 with 0.5 M acetic acid, and then centrifuged at 10,000 g for 5 min (Einig and Hampp 1990). Aliquots of 20 to 60 µl of the supernatant were used for the enzymatic determination of starch according to Outlaw and Manchester (1979).
Chlorophyll concentrations were determined after extrac-tion in 80% acetone (Ziegler and Egle 1965), and calculated according to Lichtenthaler (1987). Magnesium concentrations of needles were analyzed by the AAS--AES flame technique (Perkin Elmer 4000, Ueberlingen, Germany) after dry ashing and digestion with HNO3-HCl.
Statistical analysis
Means and standard errors (SE) were calculated separately for each sampling time and each needle age class. Differences among the treatments within each needle age class were evalu-ated by analysis of variance and Tukey’s test performed with the SPSS-PC+ software package.
Results
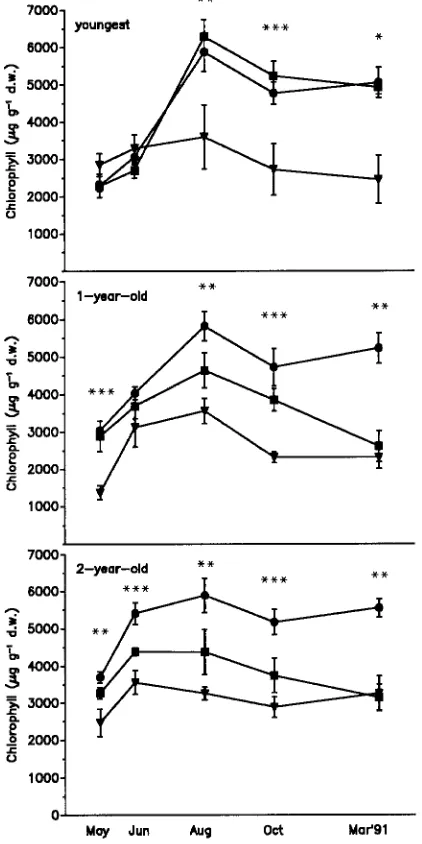
After 3 years of adaptation to Mg deficiency, Mg concentra-tions in needles of Norway spruce trees closely paralleled the nutrient regimes, with lowest values in the severe Mg-defi-ciency treatment (Table 1). Trees in the long-term moderate Mg-deficiency treatment showed typical symptoms of Mg deficiency, including tip-yellowing of the 1- and 2-year-old needles. The current-year needles, which developed in May, remained green throughout the growing season, and their chlo-rophyll concentrations did not differ from those of the controls (Figure 1). In contrast, in the long-term, severe Mg-deficiency treatment, needles of all age classes displayed pronounced yellowing (Figure 1). The short-term Mg-deficiency treat-ments resulted in lower needle Mg concentrations than the long-term Mg-deficiency treatments (Table 1). In the short-term Mg-deficiency treatments, the first deficiency symptoms became visible in July. One-year-old needles of trees in the short-term, moderate Mg-deficiency treatment exhibited tip-yellowing, whereas the current-year needles remained green and had chlorophyll concentrations comparable to those of
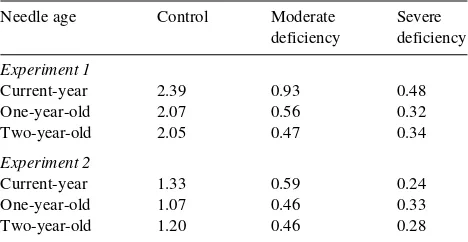
Table 1. Magnesium concentrations (mg gDW−1) in current-year, and 1- and 2-year-old needles of Norway spruce trees subjected to long-term (Experiment 1, n = 2) or short-term (Experiment 2, n = 5) Mg-deficiency treatments.
control needles (Table 2). In the short-term, severe Mg-defi-ciency treatment, yellowing symptoms were most pronounced among the current-year needles. Throughout the growing sea-son, chlorophyll concentrations of needles in the short-term severe Mg-deficiency treatment were significantly lower than those of control needles (Table 2).
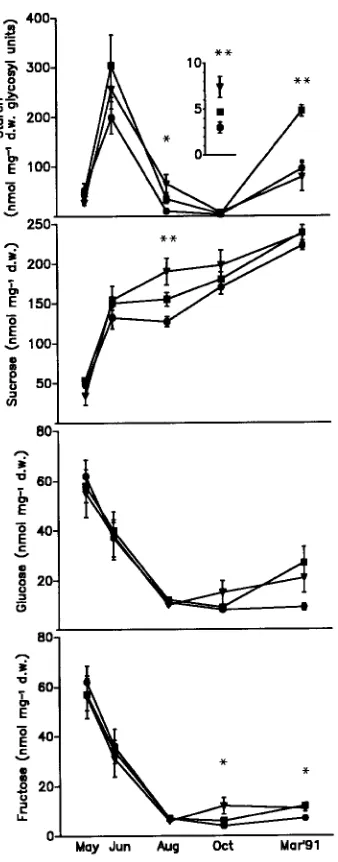
As a consequence of long-term Mg deficiency, starch con-centrations increased in the current-year needles after their maturation from August onward (Figure 2). In addition, the Mg-deficiency treatments resulted in higher sucrose and fruc-tose concentrations in the current-year needles in August 1990 and March 1991, respectively, than in the corresponding
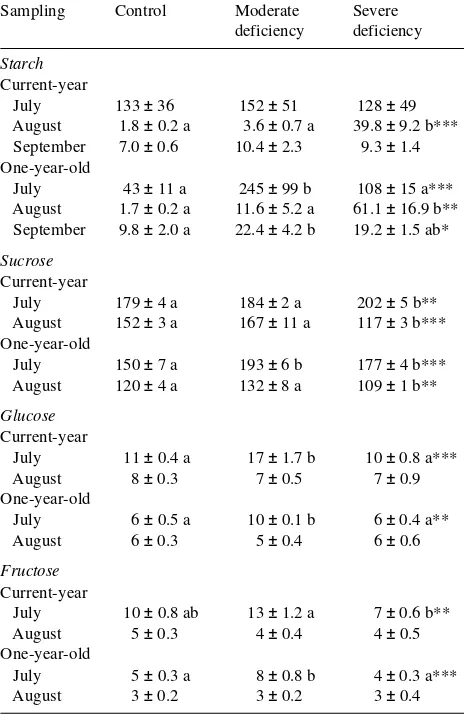
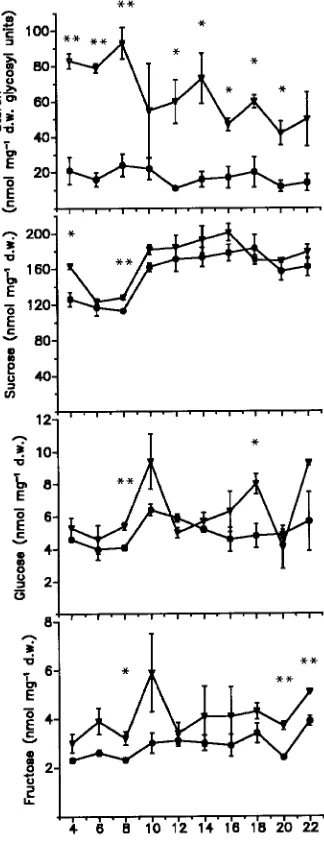
con-trol needles. One-year-old Mg-deficient needles exhibited a decrease in starch accumulation in May, when the develop-ment of new shoots started (Figure 3), and this was accompa-nied by increased concentrations of glucose and fructose in May and June. Significant increases in sucrose concentrations occurred in 1-year-old needles in the moderate- and severe Mg-deficiency treatments in May and October, respectively. As observed in current-year needles, the Mg-deficiency treat-ments caused increases in starch concentrations of 2-year-old needles in August and October and increases in sucrose con-centrations throughout the measuring period (Figure 4). The well-known annual changes in starch, glucose and fructose concentrations, which generally reflect growth and develop-mental processes in the trees, were observed in all treatments. The accumulation of starch in the short-term Mg-deficiency treatment was even more pronounced than in the long-term experiment, especially in 1-year-old needles (Table 3). The needles in the moderate Mg-deficiency treatment exhibited peak concentrations of glucose and fructose in July. A signifi-cant increase in sucrose concentration also occurred in July in both current-year and 1-year-old needles of the severe Mg-de-ficiency treatment and in 1-year-old needles of the moderate Mg-deficiency treatment. However, in August, sucrose con-centrations decreased in both needle age classes of the severe Mg-deficiency treatment in parallel with large increases in starch concentrations.
In August 1991, diurnal changes in carbohydrate concentra-tions were followed in samples from trees in the control and severe Mg-deficiency treatments after a period of high peratures and intensive irradiance. During daytime, the tem-perature reached 35 °C, and relative humidity declined to about 20% (Figure 5). As a consequence of the hazy sky, photosynthetically active radiation (PAR) was saturating for photosynthesis for only a few hours around noon. Starch con-centrations of the current-year control needles showed only minor alterations during daytime, whereas starch concentra-tions in the severe Mg-deficiency treatment decreased throughout the day (Figure 6). Although chlorophyll concen-trations were reduced in current-year needles of the severe Figure 1. Chlorophyll concentrations in current-year, and 1- and
2-year-old needles of Norway spruce trees adapted to Mg deficiency (Experiment 1) (d controls, j moderate Mg deficiency, and . severe
Mg deficiency). Means of nine samples ± SE are presented for each month (significant differences between treatments: * = P < 0.05, ** = P < 0.01, and *** = P < 0.001).
Table 2. Chlorophyll concentrations (µg gFW−1) in current-year and 1-year-old needles of Norway spruce trees subjected to short-term Mg-deficiency treatments (Experiment 2, means ± SE, n = 6). Signifi-cant differences between the treatments: ** = P < 0.01, *** = P < 0.001. Values followed by different letters differ significantly among the treatments (Tukey’s test, P < 0.05).
Sampling Control Moderate Severe deficiency deficiency
Current-year needles
July 1730 ± 48 a 1731 ± 56 a 1343 ± 117 b** August 2013 ± 108 a 1777 ± 75 ab 1347 ± 179 b** September 2020 ± 71 a 1981 ± 52 a 1431 ± 140 b***
One-year-old needles
Mg-deficiency treatment, starch accumulation was five times greater than that of the control needles. Sucrose concentrations rose in the morning and then remained constant throughout the day. Treatment-related increases in sucrose concentration were only observed in the morning. In the evening, glucose and fructose concentrations increased in the Mg-deficiency treat-ment. Short-term Mg deficiency also resulted in increased starch concentrations in 1-year-old needles, but values varied greatly (Figure 7).
Discussion
Symptoms of Mg deficiency in Norway spruce typically occur as tip-yellowing in older needles, whereas the current-year needles remain green (Baule and Fricker 1967, Zöttl 1985, Bergmann 1988). A similar pattern of symptoms was observed in trees in the short-term, moderate Mg-deficiency treatment; however, even current-year needles developed severe chlorosis in the short-term, severe Mg-deficiency treatment. As observed in the field (Mies and Zöttl 1985, Lange et al. 1989), the yellowing symptoms were more intense on the upper, sun-ex-Figure 2. Starch, sucrose, glucose and fructose concentrations in
current-year needles of Norway spruce trees adapted to Mg deficiency (Experiment 1) (d controls, j moderate Mg deficiency, and . severe Mg deficiency). Means of six samples ± SE are presented for each month (significant differences between treatments: * = P < 0.05, and ** = P < 0.01). Note the additional scale for the October data.
posed sides of the needles than on the lower, shaded sides. This pattern of deficiency symptoms continued throughout the 3-year study and was not accompanied by tree mortality. Chlo-rophyll concentrations were reduced when the Mg concentration of the needles was less than 0.5 mg gDW−1. This value is between the threshold values of 0.7 mg gDW−1 reported for Norway spruce seedlings (Ingestad 1962) and 0.3 mg gDW−1 reported for older trees (Reemtsma 1986, Köstner 1989, Schulze 1989). The differences in the threshold values might be associated with tree age or with differences in nitrogen nutrition (Ingestad 1979).
The observed diurnal and annual dynamics of carbohydrate concentrations in the needles confirms earlier studies (Senser et al. 1975, Ericsson 1979, Fink 1983, Forschner et al. 1989, Figure 4. Starch, sucrose, glucose and fructose concentrations in
2-year-old needles of Norway spruce trees adapted to Mg deficiency (Experiment 1) (d controls, j moderate Mg deficiency, and . severe
Mg deficiency). Means of six samples ± SE are presented for each month (significant differences between treatments: * = P < 0.05, ** = P < 0.01, and *** = P < 0.001). Note the additional scale for the October data.
Table 3. Starch (nmol glycosyl units mgDW−1), sucrose, glucose and fructose concentrations (nmol mgDW−1) in current-year and 1-year-old needles of Norway spruce subjected to short-term Mg-deficiency treatments (Experiment 2, means ± SE, n = 6; for further explanations, see Table 2).
Sampling Control Moderate Severe deficiency deficiency
Starch Current-year
July 133 ± 36 152 ± 51 128 ± 49 August 1.8 ± 0.2 a 3.6 ± 0.7 a 39.8 ± 9.2 b*** September 7.0 ± 0.6 10.4 ± 2.3 9.3 ± 1.4 One-year-old
July 43 ± 11 a 245 ± 99 b 108 ± 15 a*** August 1.7 ± 0.2 a 11.6 ± 5.2 a 61.1 ± 16.9 b** September 9.8 ± 2.0 a 22.4 ± 4.2 b 19.2 ± 1.5 ab*
Sucrose Current-year
July 179 ± 4 a 184 ± 2 a 202 ± 5 b** August 152 ± 3 a 167 ± 11 a 117 ± 3 b*** One-year-old
July 150 ± 7 a 193 ± 6 b 177 ± 4 b*** August 120 ± 4 a 132 ± 8 a 109 ± 1 b**
Glucose Current-year
July 11 ± 0.4 a 17 ± 1.7 b 10 ± 0.8 a*** August 8 ± 0.3 7 ± 0.5 7 ± 0.9 One-year-old
July 6 ± 0.5 a 10 ± 0.1 b 6 ± 0.4 a** August 6 ± 0.3 5 ± 0.4 6 ± 0.6
Fructose Current-year
July 10 ± 0.8 ab 13 ± 1.2 a 7 ± 0.6 b** August 5 ± 0.3 4 ± 0.4 4 ± 0.5 One-year-old
July 5 ± 0.3 a 8 ± 0.8 b 4 ± 0.3 a*** August 3 ± 0.2 3 ± 0.2 3 ± 0.4
Einig and Hampp 1990, Einig et al. 1990, 1991). Similar diurnal and annual fluctuations were observed in all treat-ments, but carbohydrate concentrations were influenced by the Mg-deficiency treatments. Needles in both Mg-deficiency treatments exhibited an increase in starch concentrations, es-pecially during summer and autumn, and in the short-term Mg-deficiency treatment. In addition, sucrose and, to a minor extent, glucose and fructose concentrations increased in re-sponse to the Mg-deficiency treatments. Similar increases in carbohydrates have been observed in leaves of Mg-deficient herbaceous plants in association with decreases in the accumu-lation of assimilates in sink organs (Beringer and Forster 1981,
Fischer and Bussler 1988, Fischer and Bremer 1993). Fink (1991) observed starch accumulation in the chloroplasts of Norway spruce needles, suffering from experimentally in-duced Mg deficiency.
The accumulation of starch began before the reduction in chlorophyll concentration occurred. Starch accumulation was highest in needles exhibiting slight yellowing symptoms and decreased again when yellowing became severe (Figures 1 and 2, Tables 2 and 3). The accumulation of carbohydrates leads to reduced activities of Calvin-cycle enzymes (Krapp et al. 1991, Stitt et al. 1991). Under high irradiance, a reduction in Calvin-cycle enzyme activities could result in an over-energization Figure 6. Diurnal course of starch, sucrose, glucose and fructose
concentrations in current-year needles of Norway spruce trees under short-term Mg-deficiency treatment (Experiment 2) (d controls, and
. severe Mg deficiency). Means of three samples ± SE are presented
for each month (significant differences between treatments: * = P < 0.05, and ** = P < 0.01).
Figure 7. Diurnal course of starch, sucrose, glucose and fructose concentrations in 1-year-old needles of Norway spruce trees under short-term Mg-deficiency treatment (Experiment 2) (d controls, and
. severe Mg deficiency). Means of three samples ± SE are presented
and over-reduction of thylakoids and thus cause oxidative stress. Evidence for this interpretation is provided by the find-ing that the activity of antioxidative systems in Mg-deficient Norway spruce needles increased before reductions in chloro-phyll concentrations occurred (J. Schittenhelm, S. Westphal, E. Wagner and B. Mehne-Jakobs, unpublished results).
The accumulation of carbohydrates in Mg-deficient needles could arise as a result of either a malfunction of phloem loading, or a reduced demand for carbohydrates in growing tissues that depend on assimilates such as roots, trunks or developing shoots (Rufty and Huber 1983, Schaffer et al. 1986, Sawada et al. 1987, Rufty et al. 1988). In Mg-deficient bush beans (Phaseolus vulgaris L.), reductions in leaf area incre-ment of developing leaves occur before sucrose and starch accumulate in mature leaves (Fischer and Bremer 1993). In trees, the interactions between assimilating leaves and growing tissues are more complex, because spring growth is dependent on stored carbohydrates assimilated during the preceding growing season (Kozlowski et al. 1991). Nevertheless, early growth reductions, especially in the roots, have been observed in Mg-deficient Norway spruce (Ingestad 1959, 1979), indicat-ing that carbohydrate accumulation in assimilatindicat-ing needles is closely associated with reduced growth rates.
Acknowledgments
This research was supported by the Deutsche Forschungsgemein-schaft (DFG). The technical help of Rainer Baritz, Susanne Röske, Julia Streif and Stefan Türk is gratefully acknowledged.
References
Baule, H. and C. Fricker. 1967. Die Düngung von Waldbäumen. Bayerischer Landwirtschaftsverlag, München, 259 p.
Bergmann, W. 1988. Ernährungsstörungen bei Kulturpflanzen. G. Fischer Verlag, Stuttgart, New York, 762 p.
Beringer, H. and H. Forster. 1981. Einfluss variieter Mg-Ernährung auf Tausendkorngewicht und P-Franktionen des Getreidekorns. Z. Pflanzenern. Bodenkd. 144:8--15.
Dekker, R.F.M. and G.N. Richards. 1971. Determination of starch in plant material. J. Sci. Food Agric. 22:441--444.
Einig, W. and R. Hampp. 1990. Carbon partitioning in Norway spruce: amounts of fructose-2,6-bisphosphate and of intermediates of starch/sucrose synthesis in relation to needle age and degree of needle loss. Trees 4:9--15.
Einig, W., P. Weidmann, B. Egger and R. Hampp. 1990. Diurnale Änderungen im Gehalt an Intermediaten des Energie- und Kohlen-hydrat-Stoffwechsels in Fichtennadeln. Kernforschungszentrum Karlsruhe, KfK-PEF 61:311--321.
Einig, W., P. Weidmann, R. Hampp, Ch. Hagg, U. Rinderle and H.K. Lichtenthaler. 1991. Tagesgänge der Photosyntheseaktivität in Fichtennadeln: Gaswechsel, Energiehaushalt und Regulation des Kohlenhydratstoffwechsels. Kernforschungszentrum Karlsruhe, KfK-PEF 80:71--80.
Ericsson, A. 1979. Effects of fertilization and irrigation on the sea-sonal changes of carbohydrate reserves in different age-classes of needle on 20-year-old Scots pine trees (Pinus sylvestris). Physiol. Plant. 45:270--280.
Fink, S. 1983. Histologische und histochemische Untersuchungen an Nadeln erkrankter Tannen und Fichten im Südschwarzwald. Allg. Forstzeitschr. 38:660--663.
Fink, S. 1991. Structural changes in conifer needles due to Mg and K deficiency. Fert. Res. 27:23--27.
Fischer, E.S. and W. Bussler. 1988. Effects of magnesium deficiency on carbohydrates in Phaseolus vulgaris. Z. Pflanzenernähr. Bod-enkd. 151:295--298.
Fischer, E.S. and E. Bremer. 1993. Influence of magnesium deficiency on rates of leaf expansion, starch and sucrose accumulation, and net assimilation in Phaseolus vulgaris. Physiol. Plant. 89:271--276. Forschner, W., V. Schmitt and A. Wild. 1989. Investigations on the
starch content and ultrastructure of spruce needles relative to the occurrence of novel forest decline. Bot. Acta 102:208--221. Hampp, R. 1992. Comparative evaluation of the effects of gaseous
pollutants, acidic deposition, and mineral deficiencies on the carbo-hydrate metabolism of trees. Agric. Ecosyst. Environ. 42:333--364. Hampp, R., W. Einig and R. Keil. 1987. Profile biochemischer Pa-rameter in pflanzlichen Geweben und Zellen mit unterschiedlich ausgeprägten Schadsymptomen. Kernforschungszentrum Karls-ruhe, KfK-PEF 32:1--48.
Ingestad, T. 1959. Studies on the nutrition of forest tree seedlings. II. Mineral nutrition of spruce. Physiol. Plant. 12:568--593.
Ingestad, T. 1962. Macroelement nutrition of pine, spruce and birch seedlings in nutrient solutions. Medd. Statens Skogsforskn. 51:1--150.
Ingestad, T. 1979. Mineral nutrient requirements of Pinus sylvestris and Picea abies seedlings. Physiol. Plant. 45:373--380.
Jones, M.G.K. and W.H. Outlaw. 1981. Enzymatic assay for sucrose. In Techniques in Carbohydrate Metabolism. Ed. H.L. Kornberg. B 302 Elsevier, Amsterdam, pp 1--8.
Köstner, B.M. 1989. Jahresverlauf der Chloroplastenpigmente von ungeschädigten und chlorotischen Fichten an einem Waldschadens-standort im Fichtelgebirge in Abhängigkeit von Alter und Mineral-stoffgehalt der Nadeln. Ph.D. Thesis. Bayerische Julius-Maximilians-University, Würzburg, 163 p.
Kozlowski, T.T., P.J. Kramer and S.G. Pallardy. 1991. The physiologi-cal ecology of woody plants. Academic Press, San Diego, 657 p. Krapp, A., W.P. Quick and M. Stitt. 1991. Ribulose-1,5-bisphosphate
carboxylase-oxygenase, other Calvin-cycle enzymes, and chloro-phyll decrease when glucose is supplied to mature spinach leaves via the transpiration stream. Planta 186:58--69.
Laing, W.A. and J.T. Christeller. 1976. A model for the kinetics of activation and catalysis of ribulose-1,5-bisphosphate carboxylase. Biochem. J. 159:563--570.
Landoldt, W. and B. Krause-Lüthy. 1989. Nationales Forschungspro-gramm 14+. ‘‘Waldschäden und Luftverschmutzung in der Schweiz’’. Teilprojekt ‘‘Negativbegasung’’, WSL, CH-8903 Bir-mensdorf, pp 1--120.
Lange, O.L., R.M. Weikert, M. Wedler, J. Gebel and U. Heber. 1989. Photosynthese und Nährstoffversorgung von Fichten aus einem Waldschadensgebiet auf basenarmem Untergrund. Allg. Forst-zeitschr. 44:55--65.
Lorimer, G.H., M.R. Badger and T.J. Andres. 1976. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and mag-nesium ions. Equilibra, kinetics, a suggested mechanism, and physiological implications. Biochemistry 15:529--536.
Lichtenthaler, H.K. 1987. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 148:350--382. Marschner, H. 1986. Mineral nutrition of higher plants. Academic
Press, London, 674 p.
Oren, R., E.-D. Schulze, K.S. Werk, J. Meyer, B.U. Schneider and H. Heilmeier. 1988. Performance of two Picea abies (L.) Karst. stands at different stages of decline. I. Carbon relations and stand growth. Oecologia 75:25--37.
Outlaw, W.H. and J. Manchester. 1979. Guard cell starch concentration quantitatively related to stomatal aperture. Plant Physiol. 64:79--82. Reemtsma, J.B. 1986. Der Magnesium-Gehalt von Nadeln nied-ersächischer Fichtenbestände und seine Beurteilung. Allg. Forst-und Jagdztg. 157:196--200.
Rufty, T.W., Jr. and S.C. Huber. 1983. Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1,6-bisphosphatase in response to source--sink alterations. Plant Physiol. 72:474--480.
Rufty, T.W., Jr., S.C. Huber and R.J. Volk. 1988. Alterations in leaf carbohydrate metabolism in response to nitrogen stress. Plant Physiol. 88:725--730.
Sawada, S., H. Kawamura, T. Hayakawa and M. Kasal. 1987. Regula-tion of photosynthetic metabolism by low-temperature treatment of roots of single-rooted soybean plants. Plant Cell Physiol. 28:235--241.
Schaffer, A.A., K.-C. Liu, E.F. Goldschmidt, C.D. Boyer and R. Goren. 1986. Citrus leaf chlorosis induced by sink removal: starch, nitrogen, and chloroplast ultrastructure. J. Plant Physiol. 124:111--121.
Schulze, E.-D. 1989. Air pollution and forest decline in a spruce (Picea abies) forest. Science 244:776--783.
Senser, M., F. Schötz and E. Beck. 1975. Seasonal changes in structure and function of spruce chloroplasts. Planta 126:1--10.
Stitt, M., A. von Schaewen and L. Willmitzer. 1991. ‘Sink’ regulation of photosynthetic metabolism in transgenic tobacco plants express-ing yeast invertase in their cell wall involves a decrease of the Calvin-cycle enzymes and an increase of glycolytic enzymes. Planta 183:40--50.
Vogels, K., R. Guderian and G. Masuch. 1986. Studies on Norway spruce (Picea abies (L.) Karst.) in damaged forest stands and in climatic chamber experiments. In Acidification and Its Policy Im-plications. Ed. T. Schneider. Elsevier, Amsterdam, pp 171--186. Ziegler, R. and K. Egle. 1965. Zur quantitativen Analyse der
Chloro-plastenpigmente. Beitr. Biol. Pflanz. 41:11--37.