Significant advances in the genetic dissection of
brassinosteroid biosynthesis and signaling have been made during the past few years. Genetic and biochemical data have helped to elucidate the pathways of biosynthesis of
brassinolide, the most active brassinosteroid. In addition, several models have been put forward for the perception of brassinolide by its putative receptor, BRI1, a ubiquitously expressed plasma membrane localized protein kinase. These studies provide the basic framework for future analysis of brassinosteroid signaling.
Addresses
Howard Hughes Medical Institute and Plant Biology Laboratory, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, California 92037 USA
*e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:79–84
1369-5266/00/$ — see front matter © 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations
BR brassinosteroid
CLV1 CLAVATA1
KAPP kinase-associated protein phosphatase
LRR leucine-rich repeat
MVA mevalonic acid
Introduction
In 1979, Grove et al. [1] showed that the unique growth-pro-moting activity of Brassica pollen extracts was conferred by brassinolide, a C-28 steroid with an unusual lactone B-ring structure. Since then, brassinolide and more then 40 analogs have been isolated from a wide variety of plant species and the biological activity of this class of molecules — known as brassinosteroids — has been studied in great detail [2–4]. Their exogenous application has been shown to lead to a spectrum of growth responses, such as stem elongation, inhibition of root growth, leaf epinasty, pollen-tube growth and xylem differentiation, brought about by changes in enzyme activity and gene expression [2–4,5•].
Although the existence and biological activity of these plant steroids had been described in a huge body of litera-ture, they only found their way into the mainstream of plant hormone biology a few years ago, when the available biochemical and physiological data were complemented by the analysis of brassinosteroid (BR)-deficient mutants from Arabidopsis [6,7], pea [8] and tomato [9]. The analysis of these mutants has not only established BRs as essential endogenous growth regulators but has also initiated the search for mutants with defects in BR signal transduction. Surprisingly, screens for BR-insensitive mutants have so far identified only a single signal transduction component [10,11]. Excellent reviews have been published recently [5•,12] and here we focus on the most recent advances in
the analysis of BR biosynthesis and on emerging mecha-nisms of BR modification. In addition, we discuss possible modes of action for BRI1, the putative BR-receptor and describe strategies to identify additional players.
Brassinosteroid biosynthetic mutants
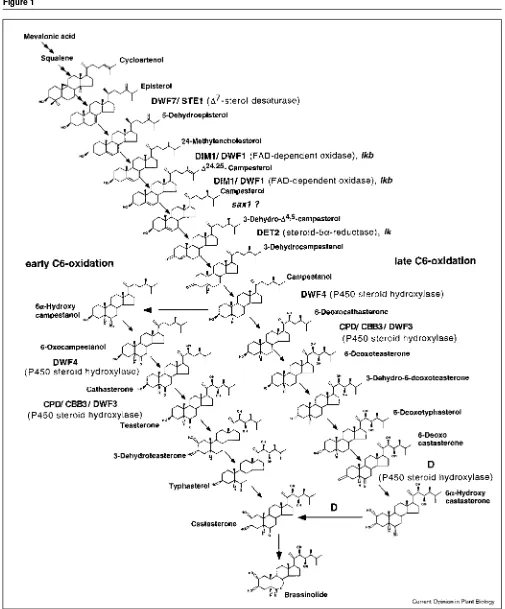
Mutations in at least eight loci of Arabidopsis [6,7,10,13,14,15•,16•] and several additional loci in tomato [9] and pea [8] lead to plants showing phenotypes charac-teristic of BR-deficiency. When grown in the light, these plants are dwarfs with dark-green, curled leaves that show defects in xylem differentiation, and have reduced fertility and apical dominance. In the dark, most of them resemble light-grown seedlings with short hypocotyls, open cotyle-dons and developing leaves. Exogenous application of brassinolide leads to a normalization of their phenotypes. A biosynthetic pathway that had been proposed solely on biochemical analysis [17] provided an excellent framework for the characterization of these mutants and was in turn confirmed and refined by their analysis (Figure 1).
Sterols are the precursors of BR-biosynthesis and feeding studies have indicated that the defect in the dw7 mutant [16•] lies before the production of 24-methylencholesterol in the sterol biosynthetic pathway. Detailed studies using 13C-labeled mevalonic acid (MVA) and compactin, an inhibitor of MVA biosynthesis, pinpointed the defect to the ∆7 sterol C-5 desaturation step, which is catalyzed by the previously identified STE1 gene product [18]. Sequence analysis confirmed that the dwf7 mutants are indeed allelic to the ste1mutant [16•,19]. Mutations in the DIMINUTO(DIM)/DWARF1(DWF1) locus [20,21], which encodes an integral endoplasmic reticulum membrane pro-tein [15•] with a domain conserved in flavin adenine dinucleotide (FAD)-dependent oxidases, have recently also been shown to affect sterol synthesis. Feeding with deuterium-labeled 24-methylencholesterol [15•] and analysis of endogenous intermediates [15•,22•] show that this mutant is defective in the conversion of 24-methyl-enecholesterol to campesterol. The same reaction has been shown to be defective in the lkbmutant of pea [23].
The finding that the DET2 gene encodes a steroid 5α-reductase [6] limited the step it could possibly catalyze to the conversion of campesterol to campestanol — the first reaction specific to the BR-pathway. The fact that it only converts 3-oxo-∆4,5-steroids [24], however, led to the assumption that the conversion from campesterol to campestanol had to involve additional steps [25]. The exis-tence of the suggested pathway from campesterol via (24R)-24-methylcholest-4-en-3-one to (24R)-24methyl-5α-cholestan-3-one to campestanol with DET2 catalyzing the second reaction has recently been confirmed by the detection of the proposed intermediates [26].
Brassinosteroid signal transduction: still casting the actors
Figure 1
The brassinolide biosynthetic pathway. Steps from squalene to campesterol, one of the major phytosterols found in membranes, represent a sterol-specific pathway and steps leading from
campesterol to brassinolide represent the BR-specific pathway. In the early C6-oxidation pathway, hydroxylation of the side chain occurs after
C6 oxidation, whereas in the late C6-oxidation pathway the
The analysis of the defect in the recently described sax1 mutant [27•] has been limited by the fact that the early intermediates of the brassinosteroid pathway do not show biological activity in feeding experiments. Using C22-hydroxylated analogs of (24R)-24-methylcholest-4-en-3-one and (24R)-24methyl-5α-cholestan-3-one, the authors show that the det2 mutant is rescued by the 22-OH-3-one whereas the sax1mutant is not, indicating that SAX1 acts at an earlier step [27•]. Interestingly, the sax1 mutant, when grown in the dark, does not show the de-etiolated phenotype [28•] that was used to identify mutants like det2 [6] and cpd [7]. It will therefore be important to determine whether or not the proposed C22a-hydroxy subpathway exists and whether it is active in a light-specific fashion.
After the synthesis of campestanol the biosynthetic path-way branches into the early and late C6 oxidation pathpath-ways ([17]; Figure 1). Both pathways coexist in Arabidopsisbut in tomato the late C-6 oxidation pathway seems to pre-dominate [29••]. The BR-responsive tomato dwarf (d) mutant was found to be caused by the inactivation of a cytochrome P450 classified as CYP85 [9]. Quantitative analysis of endogenous BRs and the functional expression of Dwarfin yeast have established that it catalyzes the con-version of 6-deoxocastasterone to castasterone — the last step before the conversion to brassinolide [29••].
The identification of the corresponding mutant in Arabidopsis would be of particular interest as it has been shown that the early C6-oxidation is predominant in this plant in the dark while the late C6-oxidation pathway pre-vails in the light [14,25]. A mutant specific to one of the two pathways could help to reveal if the level of active BRs are regulated by differential biosynthetic pathways. Levels of active hormones are in many cases regulated by devel-opmental and environmental cues and some well-known mechanisms seem to operate in the regulation of active BR levels. Feedback inhibition of biosynthetic genes by end products is a classic mechanism in hormone homeostasis [30] and the CPD gene has been shown to be negatively regulated by brassinolide in a protein-synthesis dependent manner [31]. The finding that the two BR-insensitive mutants bri1from Arabidopsis and lkafrom pea accumu-late brassinolide and some of its precursors [23,32] indicates that the feedback inhibition is mediated by BRI1-dependent signal transduction.
Metabolic inactivation through modification is another important mechanism in the control of the steady-state level of active hormones. Sulfotransferases have been shown to modulate the activity of steroid hormones [33] and it has recently been show that a sulfotransferase from Brassica catalyzes the O-sulfonation at position 22 of 24-epicathasterone in vitroand abolishes its biological activity
Figure 2
Current Opinion in Plant Biology
(a) (b) (c)
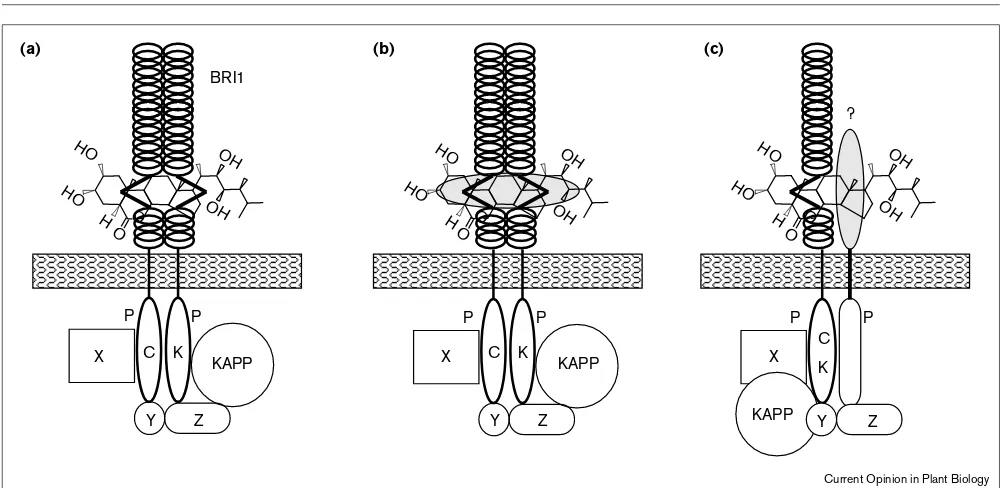
Models for BRI1 in BR signaling. (a)BRI1 is the bona fide receptor for brassinolide, which binds directly to either the LRRs (loops) or the 70-amino-acid island domain of the extracellular domain. BRI1 could either be present as a homodimer and binding of the ligand would induce activity of the intracellular kinase-domain (CK) or, alternatively, binding of the ligand might induce homodimerization, which in turn leads to kinase activity. (b)BRI1 is the receptor for brassinolide complexed to a
[34]. It will be of great importance to see if this happens in vivo and if one of the other identified plant sulfotrans-ferases acts on brassinolide as a substrate. Interestingly, expression of these genes is induced by salicylic acid indi-cating that crosstalk between BR and pathogen signaling might occur at the level of hormone inactivation. Hydroxylation is another important modification leading to inactivation of a number of hormones [35,36]. A mutant phenotype indistinguishable from a BR-deficient mutant has been shown to be caused by the overexpression of a P450 steroid hydroxylase. Feeding experiments using labeled brassinolide have shown that overexpression of the P450 steroid hydroxylase gene leads to increased levels of 26-hydroxybrassinolide whereas brassinolide and some of its precursors are reduced [37].
Brassinosteroid signal transduction
In contrast to the analysis of BR-biosynthesis, attempts to genetically identify components of BR signal transduction pathways have been less fruitful. Screens based on the inhibition of root growth by BRs [10] and the assumption that a signal transduction mutant should look like a biosyn-thetic mutant but should be BR-insensitive [11] have identified multiple mutations but the vast majority of them turned out to be alleles of a single locus, BRI1. This gene encodes a leucine-rich repeat (LRR) receptor kinase with a putative extracellular amino-terminal domain con-sisting of a leucine zipper indicative of the formation of homodimers or heterodimers. This is followed by 25 LRRs with a domain of 70 amino acids buried between LRRs 21 and 22, a transmembrane domain and a cytoplasmic ser-ine/threonine kinase domain. Analysis of bri1 alleles ([11,32]; D Friederichsen, J Chory, unpublished results) has highlighted the importance of the kinase domain — the activity of which has been shown in E. coliand in mam-malian cells (J Li, J Chory, unpublished data) — as well as the 70-amino-acid island domain for BRI1’s function. Given its molecular structure and the fact that a BRI1 fusion with green fluorescent protein (GFP) localizes to the plasma membrane (D Friederichsen, J Chory, unpub-lished results), it seems most likely that BRI1 functions as a cell surface receptor that transduces the BR signal into the cytoplasm through protein phosphorylation. Such a model is quite attractive because BRI1 would be the first example of a membrane-bound steroid receptor. The pres-ence of such receptors has been assumed in animal cells, where they are thought to mediate the nongenomic effects of steroids [38], but the corresponding molecules have not yet been identified. However, LRRs are known to mediate protein–protein interactions [39] and have so far not been shown to interact with small molecules such as steroids. This shifts the attention to the unique 70-amino-acid island domain, which has been found to be mutated in a number of bri1alleles whereas only a single mutant allele affecting the LRRs has been identified ([32]; Figure 2a). Currently, there are no reports of direct binding of brassi-nolide to BRI1 and it remains open if this simplest model holds true.
The identification of Arabidopsissequences encoding pro-teins with homology to mammalian steroid-binding proteins lends support to a second model in which brassi-nolide is presented to BRI1 as a complex with a steroid-binding protein (Figure 2b). In both models, BRI1 could either pre-exist as a homodimer or homodimeriza-tion could be induced by ligand-binding, which in turn would lead to phosphorylation of the kinase domain as has been shown for the transforming growth factor β(TGFβ) receptors [40]. Alternatively, BRI1 might form het-erodimers either with other known LRR-kinases or with yet unknown proteins (Figure 2c). BRI1 seems to be expressed ubiquitously and brassinolide has been found in many tissues raising the question of how specificity is achieved in this signaling system. Models involving inter-action partners for BRI1 address this issue, as they could be the limiting partners expressed in a temporally or spa-tially restricted manner. Transgenic approaches to test the different models for BRI1 action have been hampered by frequent co-suppression effects (J Li, Z Wang, J Chory, unpublished results) and the biochemical analysis of BRI1 will therefore be of pivotal importance.
A recent study of CLAVATA1 (CLV1) [41••], another LRR kinase similar to BRI1, provides an excellent example of the biochemical analysis of a LRR-receptor protein complex. The authors found that CLV1 is present in two complexes of 185 kDa and 450 kDa (see also review by Fletcher and Meyerowitz, pp 23–30). The size and stability of the small-er complex suggests the formation of a disulfide-linked heterodimer. The formation of the bigger complex is depen-dent on the presence of CLV3, which has been idepen-dentified as a potential ligand of CLV1 [42]. In addition, they identify the kinase-associated protein phosphatase (KAPP) [43], which had previously been shown to be a negative regulator of CLV1 signaling [44], and a RhoGTPase-related protein as parts of the 450 kDa complex. KAPP has been shown to interact in vitro with a number of LRR-kinases [43,45] including BRI1 (J Li, B Williams, E Meyerowitz, J Chory, unpublished results), but the analysis of transgenic lines overexpressing or underexpressing KAPP has so far not pro-vided in vivoevidence for this interaction. The fact that a RhoGTPase was identified in this complex is exciting and it will be very interesting to see if the possible connection to a mitogen-actived protein kinase (MAPK) pathway turns out to be true not only for CLV1 but also for other LRR-kinase signaling pathways.
Conclusions
could be a result of mutations in these loci either causing lethality or being undetectable because of redundancy. In both cases, a second generation of more sophisticated mutant screens designed to avoid these problems will be necessary. Suppressor screens in different genetic back-grounds — either performed as loss-of-function screens using traditional mutagens or as gain-of-function screens using activation tagging — are well on their way. Together with the available molecular tools for the identification of interacting proteins, casting the roles next to the diva BRI1 should be completed in the not too distant future.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Grove MD, Spencer GF, Rohwedder WK: Brassinolide, a plant growth-promoting steroid isolated from Brassica napuspollen.
Nature1979, 281:216-217.
2. Mandava NB: Plant growth-promoting brassinosteroids.Annu Rev Plant Physiol Plant Mol Biol1988, 39:23-52.
3. Sakurai A, Fujioka S: The current status of physiology and biochemistry of brassinosteroids.Plant Growth Regulation1993,
13:147-159.
4. Yokota T: The structure, biosynthesis and function of brassinosteroids.Trends Plant Sci1997, 2:137-147.
5. Clouse S, Sasse J: Brassinosteroids: essential regulators of plant
• growth and development.Annu Rev Plant Physiol Plant Mol Biol 1998, 49:427-451.
This review is the most recent comprehensive overview of reseach biosyn-thesis of brassinosteroids, physiology of brassinosteroid action and signal transduction.
6. Li J, Nagpal P, Vitart V, McMorris TC, Chory J: A role for
brassinosteroids in light-dependent development of Arabidopsis. Science1996, 272:398-401.
7. Szekeres M, Németh K, Koncz-Kálmán Z, Mathur J, Kauschmann A, Altmann T, Rédei GP, Nagy F, Schell J, Koncz C: Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis.Cell1996,
85:171-182.
8. Nomura T, Nakayama M, Reid JB, Takeuchi Y, Yokota T: Blockage of brassinosteroid biosynthesis and sensitivity causes dwarfism in garden pea.Plant Physiol1997, 113:31-37.
9. Bishop GJ, Harrison K, Jones JD: The tomato Dwarf gene isolated by heterologous transposon tagging encodes the first member of a new cytochrome P450 family.Plant Cell1996, 8:959-969.
10. Clouse S, Langford M, McMorris TC: A brassinosteroid-insensitive mutant in Arabidopsis thalianaexhibits multiple defects in growth and development.Plant Physiol1996, 111:671-678.
11. Li J, Chory J: A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction.Cell1997, 90:929-938.
12. Altmann T: A tale of dwarfs and drugs: brassinosteroids to the rescue.Trends Genet1998, 14:490-495.
13. Kauschmann A, Jessop A, Koncz C, Szekeres M, Willmitzer L, Altmann T:
Genetic evidence for an essential role of brassinosteroids in plant development.Plant J1996, 9:701-713.
14. Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA:
The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22a-hydroxylation steps in brassinosteroid biosynthesis.Plant Cell1998, 10:231-243.
15. Klahre U, Noguchi T, Fujioka S, Takatsuto S, Yokota T, Nomura T,
• Yoshida S, Chua NH: The Arabidopsis DIMINUTO/DWARF1 gene encodes a protein involved in steroid synthesis.Plant Cell1998,
10:1677-1690.
Feeding experiments described in this paper show that DIM/DWF1 acts in the sterol synthesis pathway in the conversion of 24-methylencholesterol to
campesterol. The localization of a GFP-fusion as well as biochemical analy-sis suggest that DIM/DWF1 is an integral membrane protein probably asso-ciated with the endoplasmic reticulum.
16. Choe S, Noguchi T, Fujioka S, Takatsuto S, Tissier CP, Gregory BD,
• Ross AS, Tanaka A, Yoshida S, Tax FE, Feldmann KA: The
Arabidopsis dwf7/ste1 mutant is defective in the delta7 sterol C-5 desaturation step leading to brassinosteroid biosynthesis.Plant Cell1999, 11:207-221.
Detailed feeding studies show that dwf7has a defect in ∆7sterol C-5
desat-uration, a reaction catalyzed by the previously cloned STE1locus. Sequence analysis confirms that the dwf7alleles are indeed strong alleles of STE1. 17. Fujioka S, Sakurai A: Brassinosteroids.Nat Prod Rep1997, 14:1-10.
18. Gachotte D, Meens R, Benveniste P: An Arabidopsis mutant deficient in sterol biosynthesis: heterologous complementation by ERG 3 encoding a delta 7-sterol-C-5-desaturase from yeast.
Plant J1995, 8:407-416.
19. Husselstein T, Schaller H, Gachotte D, Benveniste P: Delta7-sterol-C5-desaturase: molecular characterization and functional expression of wild-type and mutant alleles.Plant Mol Biol1999,
39:891-906.
20. Feldmann KA, Marks MD, Christianson ML, Quatrano RA: A dwarf mutant of Arabidopsisgenerated by T-DNA insertion mutagenesis.
Science1989, 243:1351-1354.
21. Takahashi T, Gasch A, Nishizawa N, Chua N-H: The DIMINUTOgene of Arabidopsisis involved in regulating cell elongation.Genes Dev 1995, 9:97-107.
22. Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, Noguchi T, Fujioka S,
• Takatsuto S, Tanaka A, Yoshida S et al.: The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis.Plant Physiol 1999, 119:897-907.
In addition to the analysis of the defective step, the authors analyzed multi-ple dwf1alleles and the results suggest that the potential FAD-binding domain of DWF1 is important for its enzymatic function.
23. Nomura T, Kitasaka Y, Takatsuto S, Reid JB, Fukami M, Yokota T:
Brassinosteroid/sterol synthesis and plant growth as affected by
lka and lkb mutations of Pea.Plant Physiol1999, 119:1517-1526.
24. Li J, Biswas MG, Chao A, Russell DW, Chory J: Conservation of function between mammalian and plant steroid 5a-reductases.
Proc Natl Acad Sci USA1997, 94:3554-3559.
25. Fujioka S, Li J, Choi YH, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J, Sakurai A: The Arabidopsis
deetiolated2 mutant is blocked early in brassinosteroid biosynthesis.Plant Cell1997, 9:1951-1962.
26. Noguchi T, Fujioka S, Takatsuto S, Sakurai A, Yoshida S, Li J, Chory J: Arabidopsis det2is defective in the conversion of (24R)-24-methylcholest-4-en-3-one to (24R)-24-methyl-5a-cholestan-3-one in brassinosteroid biosynthesis.Plant Physiol1999, 120:833-839.
27. Ephritikhine G, Pagant S, Fujioka S, Takatsuto S, Lapous D, Caboche M,
• Kendrick R, Barbier-Brygoo H: The sax1mutation defines a new locus involved in the brassinosteroid biosynthesis pathway in
Arabidopsis thaliana.Plant J1999, 18:315-320.
The phenotype of the sax1mutant can be rescued by BR application and here the authors describe rescue experiments using various intermediates including C22-hydroxylated analogs of some of the early intermediates. In contrast to their native counterparts, these analogs are active and, although it remains to be shown whether they exist in nature, they will be valuable tools for the dissection of early steps of the biosynthetic pathway. 28. Ephritikhine G, Fellner M, Vannini C, Lapous D, Barbier-Brygoo H: The
• sax1dwarf mutant of Arabidopsis thalianashows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid.Plant J1999, 18:303-314.
The sax1mutant was isolated in a screen for auxin-hypersensitive mutants and further analysis revealed that it shows striking abscisic acid-hypersensi-tivity. This is of particular interest as it shows that brassinosteroid-deficient mutants might be valuable tools to dissect the interaction of brassinos-teroids with other plant hormones.
29. Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S,
•• Takatsuto S, Jones JD, Kamiya Y: The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis.Proc Natl Acad Sci USA1999, 96:1761-1766.
a P450 involved in BR-biosynthesis. Expressing the DWARF protein in yeast, they show that it catalyzes the conversion of 6-deoxocastasterone to castasterone.
30. Brown MS, Goldstein JL: The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor.Cell1997, 89:331-340.
31. Mathur J, Molnar G, Fujioka S, Takatsuto S, Sakurai A, Yokota T, Adam G, Voigt B, Nagy F, Maas C et al.: Transcription of the Arabidopsis
CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids.Plant J1998,
14:593-602.
32. Noguchi T, Fujioka S, Choe S, Tax FE, Takatsuto S, Yoshida S, Feldmann KA: Brassinosteroid-insensitive dwarf mutant bri1of
Arabidopsisaccumulates native brassinosteroids.Plant Physiol 1999, 121:743-752.
33. Strott CA: Steroid sulfotransferases.Endocrinol Rev1996,
17:670-697.
34. Rouleau M, Marsolais F, Richard M, Nicolle L, Voigt B, Adam G, Varin L:
Inactivation of brassinosteroid biological activity by a salicylate-inducible steroid sulfotransferase from Brassica napus.J Biol Chem1999, 274:20925-20930.
35. Kayser H, Winkler T, Spindler-Barth M: 26-hydroxylation of ecdysteroids is catalyzed by a typical cytochrome P-450-dependent oxidase and related to ecdysteroid resistance in an insect cell line.Eur J Biochem1997, 248:707-716.
36. Thomas SG, Phillips AL, Hedden P: Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation.Proc Natl Acad Sci USA1999, 96:4698-4703.
37. Neff M, Nguyen E, Malancharuvil S, Fujioka T, Noguchi H, Seto M, Tsubuki T, Honda S, Takatsuto S, Yoshida S, Chory J: BAS1: A gene
regulating brassinosteroid levels and light responsiveness in
Arabidopsis. Proc Natl Acad Sci USA, 1999, 96:15316-15323.
38. Christ M, Haseroth K, Falkenstein E, Wehling M: Nongenomic steroid actions: fact or fantasy?Vitam Horm1999, 57:325-373.
39. Kobe B, Deisenhofter J: The leucine-rich repeat: a versatile binding motif.Trends Biochem Sci1994, 19:415-421.
40. Massague J: TGF-bsignal transduction.Annu Rev Biochem1998,
67:753-791.
41. Trotochaud AE, Hao T, Wu G, Yang Z, Clark SE: The CLAVATA1
•• receptor-like kinase requires CLAVATA3 for its assembly into a signaling complex that includes KAPP and a Rho-related protein.
Plant Cell1999, 11:393-406.
Similar to BRI1 (the putative BR receptor) CLV1 is a LRR receptor-like kinase and in this paper the authors use straightforward biochemistry to show that CLV1 is present as a disulfide-linked multimer in two different complexes, which potentially reflect the inactive and active states. The big-ger complex is dependent on the presence of CLV3, the potential ligand and contains a Rop GTPase. It will be interesting to see if CLV1 turns out to be a paradigm for other LRR-RLKs.
42. Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM:
Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems.Science1999, 283:1911-1914.
43. Stone JM, Collinge MA, Smith RD, Horn MA, Walker JC: Interaction of a protein phosphatase with an Arabidopsis serine-threonine receptor kinase.Science1994, 266:793-795.
44. Williams RW, Wilson JM, Meyerowitz EM: A possible role for kinase-associated protein phosphatase in the Arabidopsis CLAVATA1 signaling pathway.Proc Natl Acad Sci USA1997, 94:10467-10472.
45. Braun DM, Stone JM, Walker JC: Interaction of the maize and