Precambrian Research 105 (2001) 57 – 71
Marine evaporites from an oceanic island in the
Neoproterozoic Adamastor ocean
H.E. Frimmel
a,*, S.-Y. Jiang
baDepartment of Geological Sciences,Uni6ersity of Cape Town,Pri6ate Bag,Rondebosch7701,South Africa bState Key Laboratory of Mineral Deposit Research,Department of Earth Sciences,Nanjing Uni6ersity,
Nanjing210093,People’s Republic of China
Received 28 October 1999; accepted 17 July 2000
Abstract
We report a hitherto unknown occurrence of ancient Neoproterozoic evaporite deposits from an allochthonous terrane in the Pan-African Gariep belt in Namibia. Low contents of Rb, Cs, Ba, Zr, Hf, Th, and U, flat chondrite-normalised rare earth element (REE) patterns,87Sr/86Sr ratios as low as 0.7075, Na – Cl – Br systematics of
fluid inclusion leachates, and highd11B values for stratiform tourmalinites, together with geologic evidence, such as
association with oceanic basalt, gabbro, and stromatolitic dolomite, point to a marine evaporitic origin. An atoll environment on an oceanic island is envisaged as a likely depositional setting. In the associated mafic sequence we found a diamictite with metre-sized ice rafted detritus, suggesting the presence of sea ice cover at relatively low latitude around the time of evaporite deposition. Based on chemostratigraphic (87Sr/86Sr, d13C) comparison with
passive continental margin sediments in the para-autochthonous external part of the Gariep belt, a correlation of the mafic diamicitite with the global Varangian (590 – 560 Ma) glaciation is proposed. © 2001 Elsevier Science B.V. All rights reserved.
Keywords:Gariep belt; Marmora terrane; Neoproterozoic; Evaporite; Boron isotopes; Varangian glaciation
www.elsevier.com/locate/precamres
1. Introduction
The record of Precambrian evaporite deposits is sparse which is largely due to the fact that pri-mary evaporite minerals do not survive even low-grade metamorphism easily and post-depositional metasomatism in these rocks often obliterates
pri-mary geochemical signatures. Yet, knowledge of the distribution of evaporite deposits is pivotal for the reconstruction of stratigraphic correlation, pa-leogeography and paleoclimate. The presence of Archean evaporite deposits in the Barberton greenstone belt, South Africa, has been inferred from d11B values for tourmaline (Byerly and Palmer, 1991). Only few Proterozoic examples of former evaporite occurrences exist. Most of them, such as the 2.1 Ga borate deposits in the Liaohe group of eastern Liaoning, China (Jiang et al., * Corresponding author. Tel.:+24-21-6502901; fax:+
24-21-6503783.
E-mail address:[email protected] (H.E. Frimmel).
1996) and the ]1.7 Ga Thackaringa group (Willyama supergroup) in New South Wales, Aus-tralia (Stevens et al., 1988; Slack et al., 1989), or the Neoproterozoic Duruchaus formation (Nosib group) in the Damara belt, Namibia (Behr et al., 1983), are believed to have been deposited in non-marine, playa lake environments in rift grabens.
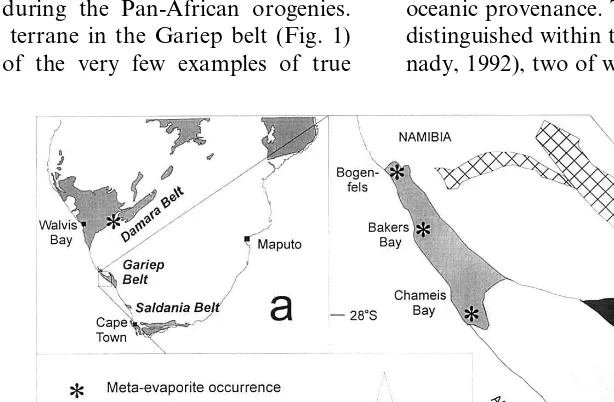
Within the Gariep belt, which forms a coast-parallel branch of the larger network of Pan-African orogenic belts in southwestern Africa (Fig. 1), we found a dolomite-dominated metased-imentary succession that bears many similarities to the inferred former playa deposits in the Dam-ara belt. The succession occurs, however, in a very different geologic setting compared with other Proterozoic former evaporite deposits — it is not associated with rift sediments but with mafic and ultramafic rocks which have been previously de-scribed as representing either an oceanic island or an aseismic ridge (Frimmel et al., 1996a).
One of the enigmas in the reconstruction of Neoproterozoic paleogeography in southwestern Africa is the width, or existence at all, of oceanic basins between the various crustal fragments that amalgamated during the Pan-African orogenies. The Marmora terrane in the Gariep belt (Fig. 1) provides one of the very few examples of true
oceanic crust in the Pan-African belts of south-western Africa. It remains unclear whether this oceanic crust formed in a wide, open ocean basin or in a narrow sea. Thus, the question arises whether inferred former evaporite deposits there are of marine or non-marine origin. To address this question we studied the whole rock geochem-istry and Sr, O and C isotopic compositions of various dolomite beds, determined the mineral chemistry and boron isotopic composition of tourmaline from stratiform tourmalinite, and analysed the chemistry of fluid inclusions in tour-maline, early quartz and calcite veins.
2. Geologic setting and lithology
The Gariep belt evolved from an ocean basin, the so-called Adamastor ocean, that separated the Kalahari craton of southern Africa from the Rio de la Plata craton in South America. The belt is subdivided into an eastern para-autochthonous zone, consisting of rift graben and passive conti-nental margin deposits, and a western, al-lochthonous terrane (Marmora terrane) of oceanic provenance. Three sub-terranes have been distinguished within the latter (Frimmel and Hart-nady, 1992), two of which are dominated by mafic
H.E.Frimmel,S.-Y.Jiang/Precambrian Research105 (2001) 57 – 71 59
Fig. 2. Stratigraphic subdivision of the Chameis group in the Chameis sub-terrane.
on the geology of this unit and a detailed strati-graphic description is being provided elsewhere (Frimmel, 2000). A thick basal sequence of meta-basalt and tuff, metamorphosed to thinly lami-nated greenschist (Dernburg formation) containing bodies of metagabbro and serpentinite (Bakers Bay suite) is capped by carbonate rocks and pelitic to psammitic metasedimentary rocks of the Bogenfels formation (Fig. 2). Geochemi-cally, most of the mafic rocks bear all the hall-marks of oceanic within-plate basalts, whereas, some are better compared with mid-ocean ridge basalt (Frimmel et al., 1996a). Submarine extru-sion of the mafic volcanics is indicated by the evidence of pillow lavas, whereby relatively shal-low water depths are implied by the occurrence of hyaloclastite and mafic breccias. The Dernburg formation is overlain by laminated to massive limestone and dolomite (Dreimaster member, lower Bogenfels formation), in which high Sr contents indicate the original presence of arago-nite (Frimmel, 2000). A relatively deep water anoxic or restricted lagoonal setting is inferred for these carbonate rocks from their high Sr, but also conspicuously high H2S contents. The predomi-nantly siliciclastic upper Bogenfels formation is interpreted to represent flysch sediments that were laid down during the closure of the Adamastor ocean.
Within the upper Dernburg formation, we found at numerous localities exotic dropstones and lonestones, reaching up to 1.5 m in length, embedded in a greenschist (metatuff) matrix (Fig. 3). The most plausible explanation for the pres-ence of these exotic clasts is that they represent very coarse-grained ice-rafted detritus. Conse-quently, the mafic matrix of this diamictite (Chameis Gate member) is indicative of volcanic activity during times of glaciation.
The rocks of interest to this study occur below the diamictite but within the mafic Dernburg for-mation. They were found at several localities throughout the Chameis sub-terrane (Fig. 1) and comprise a mixed sequence of thinly laminated, highly magnesian calcpelite with stratiform layers and boudins of up to 1.5 m thick tourmalinite, thin chert bands, massive light to medium grey dolomicrite, very coarse-grained, sugary textured, Fig. 3. Diamicite of the Chameis gate member with exotic
granite gneiss dropstones in greenschist matrix; above, metre-size clast, 500 m east of Bogenfels; below, 20 cm long clast pierced into underlying mafic tuff layer (younging direction is towards the fore, i.e. bottom of photo).
white dolosparite, breccia with irregularly shaped clasts of dolomite of the latter type set in an equally coarse-grained, dark grey to pink dolosparite matrix, and massive albitite. This se-quence (Sholtzberg member, Fig. 2) is laterally not continuous but occurs over a strike length of only a few hundred metres. Locally, massive stro-matolitic, Fe-rich dolomite, intercalated within the metabasalt sequence, is developed in the vicin-ity of the mixed sequence. A similar association of oceanic metabasalt, hyaloclastite and, in places, stromatolitic and oolitic dolomite is also known from the Schakalsberge sub-terrane (Fig. 1). That tectono-stratigraphic unit is made up of a thick sequence of greenschist (Grootderm formation) which is capped by dolomite of the Gais member (Frimmel et al., 1996a) — a likely correlative of the Sholtzberg member in the Chameis sub-terrane.
No estimates can be made on the total thick-ness of the various stratigraphic units in the Chameis sub-terrane because of intense folding and thrusting. Although most contacts are tec-tonic, a few examples exist of gabbro having intruded the Sholtzberg member causing decime-ter-thick contact metamorphic aureoles in calc-pelite. In most cases, however, it appears as if the metagabbro bodies were tectonically emplaced with preferential movement along these dolomite-rich strata, which gave rise to a previous interpre-tation of the whole Chameis sub-terrane representing a tectonic melange zone (Frimmel and Hartnady, 1992).
The dominant minerals in the mixed calcareous succession of the Sholtzberg member are dolomite, albite, and quartz. In addition, magne-sioriebeckite, talc, clinochlore, phlogopite, tour-maline, and hematite (replacing either magnetite or pyrite) occur in effectively all rock types but in highly variable proportions. Tourmaline-bearing mineral assemblages found include dolomite – talc – quartz – albite – tourmaline, dolomite – tour-maline – magnesioriebeckite, and dolomite – talc – chlorite – tourmaline. Magnesioriebeckite and al-bite are ubiquitous phases also in many of the mafic rocks in the Chameis sub-terrane and have been previously ascribed to extensive Na-metaso-matism (Frimmel and Hartnady, 1992). The
tim-ing of this metasomatism must have been prior to or during the main phase of orogenic deformation as the sodic amphibole has grown syn-tectonically with respect to the major phase of folding (Frim-mel, 1995). Sodium salts in a sediment, dominated by Mg-carbonate and enriched in B, provide a likely source for the Na-metasomatism, and by analogy with dolomitic rocks containing similar mineral associations in the Duruchaus formation of the Damara belt (Behr et al., 1983), the Sholtzberg member is interpreted as representing a low grade metamorphosed former evaporite de-posit.
3. Dolomite geochemistry
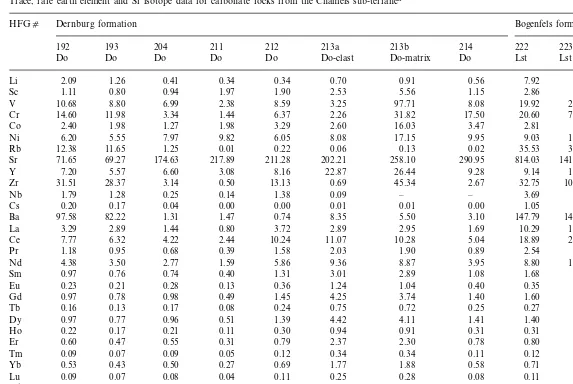
Eleven samples of the various dolomite types in the suspected meta-evaporite sequence were analysed for their trace and rare earth element (REE) contents using an ELAN 6000 ICP-MS (for analytical procedures see Frimmel, 2000) and for their Sr isotopic composition (Table 1) using conventional ion-exchange techniques and a VG Sector 7-collector mass spectrometer (see Frimmel et al., 1996a) at the Department of Geological Sciences, University of Cape Town (UCT). Dolomite samples representing the inferred mer evaporite sequence within the Dernburg for-mation from an area 5 km north of Bakers Bay, at co-ordinates 27°40.11%S, 15°32.35%E (samples HFG211-214) are compared with dolomite and limestone that cap that formation (HFG192-204,) and that lack any relationship to evaporite de-posits, but represent diagenetically modified shal-low marine carbonate deposits from 27°35.59%S, 15°32.20%E. All the dolomite samples from the inferred evaporite sequence display a generally flat chondrite-normalised REE pattern (La/Yb=1.6 – 6.7), and no Ce and Eu anomalies (Fig. 4). A succession of limestone and dolomite, represent-ing the lower Bogenfels formation 7 km to the south (27°41.58%S, 15°32.48%E), differs by display-ing a light REE enrichment (La/Yb=11.7 – 14.5 and 43.6, respectively).
H
Trace, rare earth element and Sr isotope data for carbonate rocks from the Chameis sub-terranea
HFGc Dernburg formation Bogenfels formation
204 193
192 211 212 213a 213b 214 222 223 224
Do Lst
Do-matrix Lst Do
Do-clast
Do Do Do Do Do
0.70 0.91 0.56 7.92 9.73 0.75
Li 2.09 1.26 0.41 0.34 0.34
2.53 5.56 1.15 2.86 3.28
1.90 0.22
0.94 1.97
Sc 1.11 0.80
8.59
10.68 8.80 6.99 2.38 3.25 97.71 8.08 19.92 23.63 1.52
V
6.37
14.60 11.98 3.34 1.44 2.26 31.82 17.50 20.60 76.61 2.33
Cr
2.60 16.03 3.47 2.81 4.49
3.29 1.42
Co 2.40 1.98 1.27 1.98
Ni 6.20 5.55 7.97 9.82 6.05 8.08 17.15 9.95 9.03 13.12 5.72
0.06 0.13 0.02 35.53 39.55
0.22 0.17
12.38
Rb 11.65 1.25 0.01
211.28
71.65 69.27 174.63 217.89 202.21 258.10 290.95 814.03 1412.26 182.96
Sr
8.16
7.20 5.57 6.60 3.08 22.87 26.44 9.28 9.14 14.27 1.00
Y
0.69 45.34 2.67 32.75 101.71
13.13 1.34
Zr 31.51 28.37 3.14 0.50
1.38
1.79 1.28 0.25 0.14 0.09 – – 3.69 6.00 0.20
Nb
0.01 0.01 0.00 1.05 1.52
0.00 0.01
0.00
Cs 0.20 0.17 0.04
8.35 5.50 3.10 147.79 147.69
Ba 97.58 82.22 1.31 1.47 0.74 1.65
2.89 2.95 1.69 10.29 14.29
3.72 2.18
La 3.29 2.89 1.44 0.80
11.07
Ce 7.77 6.32 4.22 2.44 10.24 10.28 5.04 18.89 26.60 3.57
2.03 1.90 0.89 2.54 3.66
1.58 0.41
0.68 0.39
Pr 1.18 0.95
5.86
4.38 3.50 2.77 1.59 9.36 8.87 3.95 8.80 12.61 1.32
Nd
1.31
0.97 0.76 0.74 0.40 3.01 2.89 1.08 1.68 2.49 0.20
Sm
1.24 1.04 0.40 0.35 0.54
0.36 0.08
Eu 0.23 0.21 0.28 0.13
4.25 3.74 1.40 1.60 2.25
Gd 0.97 0.78 0.98 0.49 1.45 0.18
0.75 0.72 0.25 0.27 0.39
0.24 0.03
0.17 0.08
Tb 0.16 0.13
1.39
0.97 0.77 0.96 0.51 4.42 4.11 1.41 1.40 2.14 0.14
Dy
0.30
0.22 0.17 0.21 0.11 0.94 0.91 0.31 0.31 0.49 0.03
Ho
2.37 2.30 0.78 0.80 1.31
0.79 0.07
Er 0.60 0.47 0.55 0.31
0.05 0.12 0.34 0.34 0.11 0.12 0.21 0.01
Tm 0.09 0.07 0.09
1.77 1.88 0.58 0.71 1.22
0.69 0.05
0.53
Yb 0.43 0.50 0.27
0.11
0.09 0.07 0.08 0.04 0.25 0.28 0.08 0.11 0.20 0.01
Lu
0.37
0.79 0.71 0.04 0.02 0.03 1.15 0.06 0.87 2.53 0.03
Hf
0.53 0.99 0.68 1.12 1.27
0.93 0.45
Pb 3.48 2.20 2.16 0.46
0.01 0.73 0.03 0.24 0.11 3.79 5.75 0.21
Th 1.27 0.92 0.11
0.02 0.45 0.43 1.53 1.52
0.27 0.15
0.03
U 0.41 0.50 0.22
87Sr/86Sr 0.71805 0.71798 0.71308 0.70879 0.71026 0.70780 0.70751 0.71104 n.d. n.d. 0.71053
Fig. 4. Chondrite-normalised rare earth element distribution in carbonate rocks from the Chameis sub-terrane.
rock. All of the meta-evaporite samples show a strong depletion in Rb, Cs, and Ba, associated with a slight enrichment in Sr, and some of them are also depleted in Zr, Hf, Th and U. The Dreimaster member dolomite from Bakers Bay follows a similar pattern as the meta-evaporite, whereas an associated limestone is characterised by a marked enrichment in Sr.
The 87Sr/86Sr ratios determined for most of the carbonate samples are influenced by their Rb contents and the addition of radiogenic 87Sr (0.71053 – 0.71805), and they are therefore of no further interest here. However, three meta-evapor-itic dolomite samples (HFG211, 213a, 213b) have extremely low Rb/Sr ratios of B0.0005. They yielded 87Sr/86Sr ratios of 0.7087990.00009, 0.707890.0001 and 0.707590.0001, respectively. In particular the results obtained on the latter two samples, which come from a massive dolomite breccia with HFG213a representing a dolomite clast and HFG213b coarsely recrystallised dolomite matrix, are considered to be least influ-enced by any external radiogenic Sr and are there-fore interpreted as approximating the initial ratio. reference for the trace element distribution in the
inferred meta-evaporite (Fig. 5). That unit lacks mineralogical or textural evidence of a former evaporite but has the appearance and composition of an ordinary dolomitised marine carbonate
H.E.Frimmel,S.-Y.Jiang/Precambrian Research105 (2001) 57 – 71 63
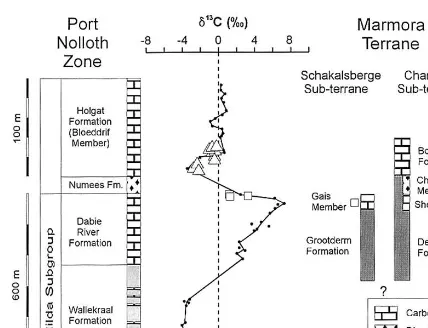
Fig. 6. Stratigraphic correlation between units of the Marmora terrane and the Port Nolloth zone. Referenced13C curve for the latter from Fo¨lling et al. (1998).
4. Chemical and boron isotopic composition of tourmaline
Tourmaline occurs in the form of massive, up to 1 m thick, stratiform tourmalinite layers, lenses and boudins within a calcpelite horizon or at the interface with overlying dolomite. In addition, tourmaline is found in bedding-parallel and cross-cutting quartz veins. Tourmaline is a most useful petrogenetic indicator mineral whose chemical composition and boron isotopic composition have been used successfully in the past to discriminate between different environments of tourmaline for-mation and possible boron sources (Henry and Guidotti, 1985; Jiang, 1998). We therefore, analysed the mineral chemistry of tourmaline, us-ing conventional electron microprobe techniques at the Department of Geological Sciences, UCT (for further analytical details see Frimmel et al., 1995), and the boron isotopic composition by negative ion thermal ionisation mass spectrometry at the Max-Planck-Institut fu¨r Chemie in Mainz. For the latter type of analysis, tourmalinite pow-ders were decomposed in tightly-capped Teflon vials with a mixture of HF+HNO3 at tempera-tures of 100°C for about 1 week until they were completely digested. Mannitol was added to the samples before decomposition in order to sup-press boron volatilisation. The boron fractions in the samples were extracted and purified by using a cation-exchange resin (AG 50WX12, 200 – 400 mesh) and a boron-specific resin (Amberlite 743, 40 – 80 mesh; Aggarwall and Palmer, 1995). Dur-ing the period of this study, 25 analyses of the NIST boric acid SRM 951 standard yielded an average 11B/10B ratio of 4.008790.7 (2s). The external 2s precision of the measurements for all samples is estimated to be better than 91.0‰ based on duplicate analyses. The boron isotope data are reported in conventional per mil d nota-tion as d11
Tourmaline has an ideal formula of (XY3Z6BO3)3Si6O18(OH, F)4, where X=Na, Ca, K, or vacancy; Y=Mg, Fe, Mn, Al, and Li; and
Z=Al, Fe, Cr, and V. Formulae were calculated
on the assumptions that B and (OH+F) are present in stoichiometric quantities, possible Si-In addition,d18O and
d13C values for the dolomite samples were used, in conjunction with a larger database for all the carbonate sequences in the Gariep and other Pan-African belts in southwest-ern Africa, for stratigraphic correlation of these units (Frimmel, 2000). The stromatolitic dolomite within the Dernburg formation differs with d13C values of up to 2.82‰ from the carbonate rocks of the Dreimaster member whose d13
C values range from −2.37 to −0.18‰. Using a chemostratigraphic profile through the passive continental margin sequence in the external Gariep belt (Port Nolloth zone) as reference (Fo¨lling et al., 1998), the d13
C of the marine carbonates in the Marmora terrane compare best with those immediately underlying and overlying the glaciogenic Numees formation diamictite (Fig. 6). Thus, a correlation between the stromatolitic Gais and Sholtzberg members in the Marmora terrane with the upper Dabie River formation, which contains similar stromatolites resembling
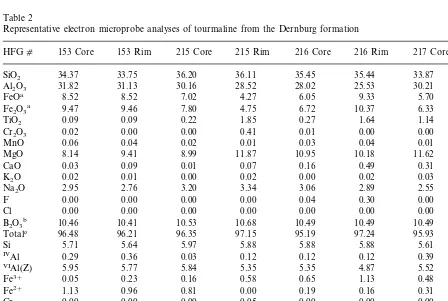
deficiency in the tetrahedral site is balanced by Al, with the remaining Al occupying the Z-site. Fur-thermore, it was assumed that no significant amounts of Li are present as there are no granite or pegmatite to which the tourmaline could be related, and the associated dolomite-rich rocks display very low Li concentrations (B1 ppm). The analysis of some 80 tourmaline grains re-vealed that all of these grains are of dravitic composition (Table 2) with Na/(Na+Ca) of 0.81 – 1.00. No relation exists between composi-tion and mode of occurrence or mineral assem-blage. All analyses show an Al-deficiency in the Z-site and this site was then filled with Fe3+
and Cr. If any Fe was left, it was assigned as Fe2+ to
the Y-site, resulting in Fe2+/(Fe2++Mg) ratios
of up to 0.43, but also in an over-occupancy of
the Y site which suggests that some of that Fe is also present as Fe3+. The real Fe2+/(Fe2+
+Mg)
is more likely close to zero, in which case the bulk of the analyses would plot into the field for meta-carbonate rocks as defined by Henry and Guidotti (1985) Fig. 7). Thus, the tourmaline composition is compatible with an evaporitic origin.
To distinguish between a marine and a non-marine precursor, B isotope ratios can be particu-larly useful. Marine evaporites are characterised by distinctly higher d11
B values (+18.2 to +31.7‰) than non-marine evaporites (Swihart et al., 1986) whose d11
B values (−30.1 to +7.0‰) span the range typical of most other rock types (Jiang, 1998). Four samples of stratiform tourma-linite layers and nodules from the inferred meta-evaporite sequence near Bakers Bay gave very
Table 2
Representative electron microprobe analyses of tourmaline from the Dernburg formation
215 Rim 216 Core
HFGc 153 Core 153 Rim 215 Core 216 Rim 217 Core 217 Rim
33.87
Al2O3 31.82 31.13 30.16 28.52 28.02 25.53 30.21 26.68
FeOa 8.52 8.52 7.02 4.27 6.05 9.33 5.70 9.59
0.27 1.64 1.14 1.58
TiO2 0.09 0.09 0.22 1.85
MgO 8.14 9.41 8.99 11.87 10.95 10.18 11.62 10.72
0.03 0.09 0.01 0.07
CaO 0.16 0.49 0.31 0.47
K2O 0.02 0.01 0.00 0.02 0.00 0.02 0.03 0.03
Na2O 2.95 2.76 3.20 3.34 3.06 2.89 2.55 2.47
0.00 0.00 0.00 0.04
F 0.00 0.30 0.00 0.00
96.48 96.21 96.35 97.15 95.19 95.93 96.42
Totalc
5.95 5.77 5.84 5.35 5.35 4.87 5.52 5.11
VIAl(Z)
0.05 0.23 0.16 0.58 0.65 0.48
Fe3+ 1.13 0.89
0.00 0.19 0.16 0.31 0.46
0.81 0.96
1.13 Fe2+
0.00 0.00 0.00 0.05 0.00 0.00 0.00 0.00
Cr
0.20
0.01 0.01 0.03 0.23 0.03 0.20 0.14
Ti
0.01
0.01 0.01 0.00 0.00 0.00 0.00 0.00
Mn
2.51
2.02 2.34 2.21 2.88 2.71 2.87 2.69
Mg
0.00 0.00 0.01 0.01
K 0.00 0.00 0.00 0.00
Na 0.95 0.89 1.02 1.05 0.98 0.93 0.82 0.81
aCalculated Fe3+/Fe2+minimum ratio as required to completely fill Z-site. bCalculated assuming 3 B per formula unit.
cCorrected for F
H.E.Frimmel,S.-Y.Jiang/Precambrian Research105 (2001) 57 – 71 65
Fig. 7. Cation variation diagrams for tourmalines from the Dernburg formation, Marmora terrane; data fields after Henry and Guidotti (1985), 1, Li-rich felsic intrusives; 2, Li-poor felsic intrusives; 3, hydrothermally altered granites; 4 and 5, Al-saturated and Al-undersaturated, respectively, metapelities and metapsammites, 6, Fe3+-rich quartz-tourmaline rocks,
calc-silicates, and metapelites; 7, low-Ca ultramafic rocks; 8, metacarbonate rocks and metapyroxenites.
low d11B of
+4.0‰ (Fig. 8). All in all, thed11B values for tourmaline grains from the Chameis sub-terrane, though spanning over a relatively large interval, are, in general, very high and provide strong evidence of a marine evaporitic origin, although a continental setting with highly evolved seawater-derived brines cannot be excluded.
5. Fluid inclusion chemistry
Eleven samples of quartz-chlorite-tourmaline veins, coarse-grained sparry, pink calcite veins within dolomite, a quartz vein within a black chert at the base of the evaporite-derived dolomite, and stratiform tourmalinite all of which, based on microthermometric and textural observations, appear to be dominated by only a single fluid inclusion generation were selected for crush-leach analysis at the Department of Geolog-ical Sciences, UCT, to determine the relative abundance of dissolved ionic species. The fluid inclusions are undersaturated to saturated two or three-phase (vapour, liquid, with or without halite daughter crystal) aqueous and CO2-rich. They are 1 – 5 mm in size and display highly variable liquid, vapour ratios which is most likely due to necking of these fluid inclusions after they have reached the liquid – vapour curve on their retrograde P–T
path. About 500 mg of cleaned mineral separate were crushed under doubly distilled de-ionised high d11B values of +10.7 to
+27.5‰ (Fig. 8). One tourmaline sample from a tourmaline-rich dolomite from a similar succession at Chameis Bay also yielded a highd11
B value of+20.1‰. In contrast, one tourmaline sample from a very coarse-grained tourmaline aggregate from a mag-nesian calcpelite about 1 km to the east of the sample locality near Bakers Bay has a relatively
Table 3
Sample average concentrations (in mg/l) of solutes in fluid inclusions from the Sholtzberg member
Na
Sample typea NH
4 K Mg Mn Ca Cl Br SO4
1 29.18 0.00 7.54 4.87 0.00 29.02 72.58 0.13 1.90
0.00 7.06 4.40 0.00
28.25 26.95
1 63.23 0.21 2.19
2 3.00 0.00 0.92 1.39 0.00 8.61 4.91 0.03 2.63
0.21 2.02 0.31 0.54
16.30 27.90
3 28.45 0.10 17.49
0.27 2.43 0.92 0.85
3 21.83 63.96 39.08 0.15 113.47
0.08 3.71 5.17 0.00
3.34 68.79
4 3.95 0.00 81.26
0.06 2.85 4.43 0.00 30.74 4.03 0.00 113.54
4 2.40
0.09 2.44 3.79 0.00
2.15 23.18
4 4.28 0.00 315.29
2.26
5 0.14 0.69 1.93 0.00 2.38 5.45 0.00 2.00
0.60
5 0.10 0.45 1.87 0.00 2.71 4.20 0.00 2.28
0.07 0.43 1.58 0.00
0.00 1.77
5 3.20 0.00 0.56
a1, Sparry calcite vein; 2, quartz vein in chert; 3, quartz–chlorite–tourmaline vein in meta-evaporite; 4, tourmaline nodule; 5, stratiform tourmalinite layer.
water and the resultant leachate was analysed using high performance ion chromatography (HPIC) for Na+, K+, NH
4
+, Ca2+, Mg2+, Mn2+,
Cl−, F−, NO
3
−, SO
4
2− (reflecting total dissolved
sulphur) and Br−. The choice of eluent (Na
2CO3 and NaHCO3) for the analysis of anion concentra-tions precluded the determination of carbonic spe-cies in the leachates. Precision is estimated to be better than 92% for most ions except for K+
, for which it is 94%. The lower limits of detection are on the order of 0.001 mg/l (for further details on the technique see Frimmel et al., 1999). Each sample was analysed at least twice and the means of the concentrations obtained are reported (Table 3). As the exact volume of fluid inclusions taken up into the leachate during crushing is not known, the absolute concentrations are of little significance but molar element ratios (Fig. 9) are more informa-tive. No F− and NO3− were detected and NH4+
was found only in small amounts. Similarly, none of the samples analysed contained measurable Mn2+, except for the quartz vein in the chert.
Using HPIC, Br− was below the detection limit
and was then analysed for using ICP-MS, which is by an order of magnitude more sensitive for this element. The Br− contents were found to be very
low and in the tourmalinite-hosted fluid inclusions not detectable. The dominant cation in all samples is Ca2+, followed by Na+, Mg2+, and K+ (Fig.
9a). Among the anions, Cl− dominates except for
some tourmaline-hosted inclusions in which SO42−
is present in higher concentrations (Fig. 9b). Charge balance calculation suggests the presence of a major additional anion, most likely CO32−
, which could not be analysed for directly.
In order to assess the extent to which water derived from the evaporation of seawater is
H.E.Frimmel,S.-Y.Jiang/Precambrian Research105 (2001) 57 – 71 67
Fig. 10. Na – Cl – Br systematics of fluid inclusion leachates from various vein types in the meta-evaporites sequence of the Dernburg formation (for legend see Fig. 9). Analytical uncer-tainties correspond to size of symbols.
6. Discussion
Extensive alkali-metasomatism in the whole Chameis sub-terrane is indicated by the wide-spread occurrence of magnesioriebeckite and al-bite in proportions which cannot be explained by any common igneous or sedimentary bulk rock composition (Frimmel and Hartnady, 1992). The source of the alkalis is most readily found in the dolomitic rocks that enclose, in most places tec-tonically, large blocks of mafic and ultramafic rocks. A Na-rich sedimentary precursor of the dolomite is postulated because of the widespread presence of massive albitite and albite-rich dolomite, and the ubiquitous occurrence of mag-nesioriebeckite and phlogopite in the dolomite. From the lack of calcite in the presence of dolomite+talc+quartz, a very Mg-rich pre-metamorphic carbonate precursor rock, rich in magnesite, is inferred. The presence of massive tourmalinite implies a major B source which, apart from granitic/pegmatitic melts, is most read-ily provided by a high evaporation rate of seawa-ter. No granitic or pegmatitic bodies are known from the whole of the Chameis sub-terrane and its exposed neighbouring tectonic units. Although there are few isolated occurrences of post-oro-genic carbonatite bodies in the wider region (e.g. Cooper and Reid, 1998), their small size and age cannot be reconciled with the regional (100 km) scale of alkali-metasomatism. The presence of an initially highly magnesian, alkali-rich sediment with mobilisation of the alkalis in particular dur-ing diagenesis and regional, syn-orogenic meta-morphism is therefore considered the most plausible explanation for the mineral distribution observed.
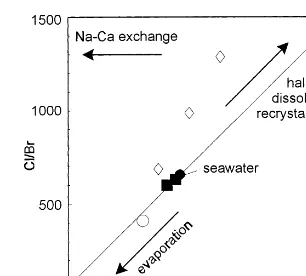
Although reflecting the composition of a post-depositional fluid, the limited fluid inclusion data obtained on the various vein samples, in particu-lar their high overall salinity and their Na:Cl:Br proportions point toward a strong involvement of seawater in the make-up of the post-depositional local pore water. Seawater is characterised by a negative Ce anomaly because of its oxidative re-moval (Elderfield and Greaves, 1982), whereas positive Ce anomalies may be suggestive of either a reducing marine environment (De Baar et al., volved in the make up of a fluid, the Na – Cl – Br
relations are particularly useful (Kesler et al., 1995; Viets et al., 1996). Due to the very small partition coefficient of Br into the major evaporite minerals, a sensitive monitor of the degree of evaporation is given by the Cl/Br ratio of the residual brine. As seawater evaporates, the Cl/Br ratio of the residual brine remains similar to that of seawater (Cl/Br=662) until halite saturation is reached. Further evaporation and precipitation of halite leads to a progressive decrease of the Cl/Br and Na/Br ratios. Residual brines that become physically separated from their evaporite minerals will have Cl/Br ratios that reflect the degree of evaporation. Similarly, dissolution of halite and other evaporite minerals in meteoric water or seawater will cause Cl/Br ratios that are very high in comparison to those of the residual brines.
1988) or an evaporative lacustrine environment (Mo¨ller and Bau, 1993) and are typically associ-ated with negative Eu anomalies. The lack of a positive Ce anomaly and a slightly negative to absent Eu anomaly in the inferred meta-evaporite samples may be viewed, therefore, as pointing against a lacustrine but toward a marine, slightly reducing environment. Such a mildly reducing environment might be indicated also by the lack of NO3− in those fluid inclusion leachate samples
that contain small amounts of NH4+ as the NH4+/
NO3−ratio seems to be a monitor of redox
poten-tial (Frimmel et al., 1999).
A continental influence should be reflected by a strong light REE enrichment. Of all the carbonate rocks analysed, the evaporite-derived dolomite samples display the least light REE enrichment. In fact their REE patterns are markedly flat (Fig. 4) and conform to those of the oceanic island basalts in the Dernburg formation (Frimmel et al., 1996a). Also, the strong depletion of the evap-orite-derived dolomite in Rb, and to a lesser extent in Ba, Zr, Hf, and Th, reflects the lack of detrital components from a continental source. Further evidence of a lack of continental input comes from the Sr isotope data. Although the 87
Sr/86
Sr ratio of seawater fluctuated greatly dur-ing the Neoproterozoic era, the near-primary87Sr/ 86Sr ratios obtained for the evaporite-derived
dolomite are close to the lower end of the poten-tial range of Neoproterozoic seawater composi-tion. Any continental influence should be reflected by an increase in 87Sr/86Sr.
A marine origin of the inferred meta-evaporite is also supported by the B isotopic composition. The possible B sources for the tourmalines are either hydrothermal fluids related to the mafic oceanic volcanism or seawater. Taking the maxi-mumd11
B value measured (+27.5‰) and allow-ing for isotope fractionation between tourmaline and aqueous fluid (Palmer et al., 1992), a tourma-line formation temperature of 200°C would corre-spond to a d11B
fluid value around 40‰ which is typical of seawater. The minimum d11B value obtained for tourmalinite in the meta-evaporite succession (10.7‰) can be explained by either a lower d11Bfluid (24‰) at the same temperature as above or by a lower temperature (50°C) and an
isotopic composition typical of seawater, or by a combination of both possibilities. The theoretical temperatures calculated are in perfect agreement with an expected tourmaline formation during diagenesis. As there is no obvious reason for tourmaline formation to take place over a temper-ature interval of some 150°C, the lower d11B values obtained may be indicative of mixing of seawater with submarine hydrothermal fluids as-sociated with the igneous activity that is manifest in the Dernburg formation. By analogy, the high proportions of SO42− detected in some of the
tourmalinite-hosted fluid inclusions could be re-lated either to evaporitic sulphates or to sulphuric volatiles exhaled by hydrothermal vents on the volcanic edifice.
In conclusion, all our new data are in line with the presence of marine evaporites within a forma-tion that is otherwise dominated by oceanic mafic rocks. As most of these mafic rocks represent either former oceanic islands or an aseismic ridge (Frimmel et al., 1996a), and because of the associ-ation with stromatolitic dolomite, the most likely depositional environment we envisage for the for-mation of the evaporite is that of an atoll, sur-rounded by stromatolitic reef mounds on top of a guyot.
7. Paleogeographic and climatic implications
H.E.Frimmel,S.-Y.Jiang/Precambrian Research105 (2001) 57 – 71 69
Neoproterozoic glacial deposits have been de-scribed only from rift grabens or continental mar-gins (e.g. Young, 1995). If the depositional environment that is preferred for the meta-eva-porite in the Dernburg formation of the Marmora terrane is correct, a sea ice cover away from the continental margin and reaching to relatively low latitudes has to be inferred.
An unresolved problem is the age of this glacia-tion. No direct age data exist for the volcano-sed-imentary succession in the Chameis sub-terrane but, based on chemostratigraphic and biostrati-graphic (Conophyton-like stromatolites) correla-tion with the para-autochthonous passive continental margin sequence (Port Nolloth group) in the external Gariep belt, some constraints can be set on the age of the Dernburg formation. Two stratigraphically different glaciogenic diamictite horizons are distinguished in the Port Nolloth group, namely the Kaigas and the Numees forma-tion. For the Kaigas formation, a correlation with the global c. 750 Ma Sturtian glaciation is well established by single zircon age data from overly-ing felsic rift volcanics (Frimmel et al., 1996b). The age of the younger Numees formation di-amictite is, however, problematic. The marine car-bonates above the Kaigas and Numees formation diamictites, respectively, can be distinguished by their characteristic geochemical signatures. The d13C of the carbonates above the Kaigas forma-tion diamictite follow, starting from depleted val-ues around −4‰, a pronounced positive excursion, reaching up to +8‰, just to decline again immediately underneath the Numees forma-tion diamictite. The carbonates above the Numees formation diamictite start again with negative d13C ratios of −4‰ but then remain fairly con-stant around 0‰ (Fig. 6). The pre-Numees car-bonates are characterised by low 87
Sr/86
Sr ratios between 0.7071 and 0.7076 whereas those above this younger diamictite have relatively high 87
Sr/ 86Sr ratios of 0.7083 – 0.7088 (Fo¨lling and
Frim-mel, 1999). These chemical data would be in line with a correlation of the Numees formation with the global Varangian glaciation (Asmerom et al., 1991). Such a correlation has found further sup-port by recent Pb – Pb carbonate ages which are interpreted to date early diagenesis (Fo¨lling et al.,
2000). Carbonates overlying the Kaigas formation diamictite yielded an age of 728932 which is indistinguishable from a Pb – Pb single zircon age of 74196 for associated felsic volcanics (Frimmel et al., 1996b). The cap carbonates above the Nu-mees formation diamictite gave an age of 555928 Ma thus strongly supporting a correlation of the Numees glaciation with the global Varangian glacial epoch for which a time span between 590 and 560 Ma has been suggested (Saylor et al., 1998).
The cap carbonates on top of the Dernburg formation mafic diamictite in the Marmora ter-rane have geochemical (high Sr content) and iso-topic (d13C) characteristics very similar to those of the cap carbonates above the Numees formation diamictite (Fig. 6), for which a post-Varangian age has been inferred above. By analogy we as-sume that the diamictite in the Dernburg forma-tion is a reflecforma-tion of Varangian glaciaforma-tion. A direct d13C correlation between the meta-evapor-ites described in this study and open marine car-bonates elsewhere was not attempted because of the restricted nature (and thus potential C iso-topic peculiarities) that is postulated for the depo-sitional environment of the former. However, d13
C data for dolomite of the Gais member, which is considered the best stratigraphic equivalent of the meta-evaporites available within the Marmora terrane, are in good agreement with a strati-graphic position immediately below the glacial deposits (Fig. 6).
Possibly more reliable than d13C data are the near-primary 87Sr/86Sr ratios. For the Sholtzberg member, this ratio was found to be similar to that typical of pre-Varangian carbonate rocks else-where (e.g. Asmerom et al., 1991; Fo¨lling and Frimmel, 1999). The relatively low near-primary 87
Sr/86
Sr ratios determined for the Sholtzberg member not only provide additional support for its marine origin but are probably also the best available evidence for a post-Sturtian/pre-Varangian age.
from South Australia (Schmidt and Williams, 1995). Our findings provide further support, though from a very different area, for the exis-tence of low-latitude glaciation, not only near coast lines but also in the oceanic realm for this time.
Acknowledgements
This work was funded through a South African National Research Foundation grant to HEF and a China National Science Foundation grant (no. 49925306) and an Alexander von Humboldt Foundation grant to SYJ. We thank Namdeb Ltd., in particular R. Burrell, J. Ward and R. Spaggiari for their permission to enter the re-stricted Diamond area and for their financial and logistic support. Thanks are also due to A. Spa¨th who acquired the ICP-MS data and to B. Saylor who provided useful comments on an earlier ver-sion of the manuscript. G.H. Swihart and J. Veizer are thanked for reviewing the manuscript.
References
Aggarwall, J.K., Palmer, M.R., 1995. Boron isotope analysis: a review. Analyst 120, 1301 – 1307.
Asmerom, Y., Jacobsen, S.B., Knoll, A.H., Butterfield, N.J., Swett, K., 1991. Strontium isotopic variations of Neoproterozoic seawater: implications for crustal evolu-tion. Geochim. Cosmochim. Acta 55, 2883 – 2894. Behr, H.J., Ahrendt, H., Porada, H., Ro¨hrs, J., Weber, K.,
1983. Upper proterozoic playa and sabkha deposits in the Damara orogen, SWA/Namibia. In: Miller, R.M.G. (Ed.), Evolution of the Damara Orogen of South West Africa/ Namibia, vol. 11. Geological Society of South Africa, Johannesburg, pp. 1 – 20.
Byerly, G.R., Palmer, M.R., 1991. Tourmaline mineralization in the barberton greenstone belt, South Africa: early Archean metasomatism by evaporite-derived boron. Con-trib. Mineral. Petrol. 107, 387 – 402.
Cooper, D.L., Reid, D.L., 1998. Nepheline-so¨vites as parental magmas in carbonatite complexes: evidence from Dicker Willem, southwest Namibia. J. Petrol. 39, 2123 – 2136. De Baar, H.J.W., German, C.R., Elderfield, H., Gaans, P.V.,
1988. Rare earth element distribution in anoxic waters of the Cariaco trench. Geochim. Cosmochim. Acta 52, 1203 – 1219.
Elderfield, H., Greaves, M.J., 1982. The rare earth elements in seawater. Nature 296, 214 – 219.
Fo¨lling, P.G., Frimmel, H.E., 1999. Chemostratigraphical cor-relation between Neoproterozoic sequences in the external Gariep belt and the Kango inlier of the Saldania belt. J. Afr. Earth Sci. 28 (4A), 23 – 24.
Fo¨lling, P., Frimmel, H.E., Zartman, R.E., 1998. Chemostrati-graphic correlation and Pb – Pb dating of metasedimentary sequences in the Gariep belt: evidence for two different diamictites. J. Afr. Earth Sci. 27 (1A), 76 – 77.
Fo¨lling, P., Zartman, R.E., Frimmel, H.E., 2000. A novel approach to double-spike Pb – Pb dating of carbonate rocks: examples from Neoproterozoic sequences in south-ern Africa. Chem. Geol., 171, 83 – 109.
Frimmel, H.E., 1995. Metamorphic evolution of the Gariep belt. South Afr. J. Geol. 98, 176 – 190.
Frimmel, H.E., 2000. The stratigraphy of the Chameis sub-ter-rane in the Gariep belt in southwestern Namibia: In Miller, R.M.G. (Ed.), Henno Martin Commemorative Volume. Communications of the Geological Survey of Namibia 13, in press.
Frimmel, H.E., Hartnady, C.J.H., 1992. Blue amphiboles and their significance for the metamorphic history of the Pan-African Gariep belt, Namibia. J. Metamorphic Geol. 10, 651 – 669.
Frimmel, H.E., Frank, W., 1998. Neoproterozoic tectono-ther-mal evolution of the Gariep belt and its basement, Namibia/South Africa. Precamb. Res. 90, 1 – 28.
Frimmel, H.E., Hoffmann, D., Watkins, R.T., 1995. An Fe analogue of kinoshitalite from the Broken Hill massive sulfide deposit in the Namaqualand metamorphic complex, South Africa. Am. Mineralogist 80, 833 – 840.
Frimmel, H.E., Hartnady, C.J.H., Koller, F., 1996a. Geo-chemistry and tectonic setting of magmatic units in the Pan-African Gariep belt, Namibia. Chem. Geol. 130, 101 – 121.
Frimmel, H.E., Klo¨tzli, U.S., Siegfried, P.R., 1996b. New Pb – Pb single zircon age constraints on the timing of Neoproterozoic glaciation and continental break-up in Namibia. J. Geol. 104, 459 – 469.
Frimmel, H.E., Hallbauer, D.K., Gartz, V.H., 1999. Gold mobilizing fluids in the Witwatersrand basin: composition and possible sources. Mineral. Petrol. 66, 55 – 81. Henry, D.J., Guidotti, C.V., 1985. Tourmaline as a
petroge-netic indicator mineral: an example from the staurolite-grade metapelites of NW Maine. Am. Mineralogist 70, 1 – 15.
Jiang, S.-Y., 1998. Stable and radiogenic isotope studies of tourmaline: an overview. J. Czech Geol. Surv. 43, 75 – 90. Jiang, S.-Y., Palmer, M.R., Peng, Q.-M., Yang, J.-H., 1996. Chemical and stable isotopic compositions of Proterozoic metamorphosed evaporites and associated tourmalines from the Houxianyu borate deposit, eastern Liaoning, China. Chem. Geol. 135, 189 – 211.
H.E.Frimmel,S.-Y.Jiang/Precambrian Research105 (2001) 57 – 71 71
Mo¨ller, P., Bau, M., 1993. Rare-earth patterns with positive cerium anomaly in alkaline waters from Lake Van, Turkey. Earth Planetary Sci. Lett. 117, 671 – 676.
Palmer, M.R., London, D., Morgan, G.B., Babb, H.A., 1992. Experimental determination of fractionation of 11B/10B between tourmaline and aqueous vapor: a temperature-and pressure-dependent isotopic system. Chem. Geol. 101, 123 – 130.
Saylor, B.Z., Kaufman, A.J., Grotzinger, J.P., Urban, F., 1998. A composite reference section for terminal Protero-zoic strata of southern Namibia. J. Sedimentary Res. 68, 1223 – 1235.
Schmidt, P.W., Williams, G.E., 1995. The Neoproterozoic climatic paradox: equatorial paleolatitude for marinoan glaciation near sea level in South Australia. Earth Plane-tary Sci. Lett. 134, 107 – 124.
Slack, J.F., Palmer, M.R., Stevens, B.P.J., 1989. Boron isotope evidence for the involvement of non-marine evaporites in the origin of the Broken Hill ore deposits. Nature 342, 913 – 916.
Stevens, B.P.J., Barnes, R.G., Brown, R.E., Stroud, W.J., Willis, I.L., 1988. The Willyama supergroup in the Broken Hill and Euriowie blocks, New South Wales. Precamb. Res. 40/41, 297 – 327.
Swihart, G.H., Moore, P.B., Callis, E.L., 1986. Boron isotopic composition of marine and non-marine evaporites. Geochim. Cosmochim. Acta 50, 1297 – 1301.
Torsvik, T.H., Lohmann, K.C., Sturt, B.A., 1995. Vendian glaciations and their relation to the dispersal of Rodinia: paleomagnetic constraints. Geology 23, 727 – 730. Viets, J.G., Hofstra, A.H., Emsbo, P., 1996. Solute
composi-tions of fluid inclusions in sphalerite from North American and European Mississippi valley-type ore deposits: ore fluid derived from evaporated seawater. In: Sangster, D.F. (Ed.), Carbonate-Hosted Lead – Zinc Deposits. Society of Economic Geologists, Littleton, pp. 465 – 482.
Young, G.M., 1995. Are Neoproterozoic glacial deposits pre-served on the margins of Laurentia related to the fragmen-tation of two supercontinents? Geology 23, 153 – 156.