Cortisol in Regular Gamblers
Gerhard Meyer, Berthold P. Hauffa, Manfred Schedlowski, Cornelius Pawlak,
Michael A. Stadler, and Michael S. Exton
Background: Although the effects of gambling on
car-diovascular parameters have been documented, no data exists describing the effect of gambling on stress hor-mone secretion. Our study investigated the effect of gambling on heart rate and salivary cortisol in a casino environment.
Methods: Ten male gamblers participated in both an
experimental and control session. In the experimental session, gamblers played a game of blackjack using their own money. Gamblers played cards in the same setting during the control condition; however, the game was played for accumulation of points rather than money. Heart rate and endocrine parameters were recorded at baseline, 30 min, and 60 min following commencement of each session, and again at completion of the game.
Results: Heart rate increased significantly from baseline
to 30 min in the experimental session and remained elevated for the remainder of the recording period. Sali-vary cortisol was raised at 30 min and further elevated at 60 min during gambling, then returned to control levels following completion of the game.
Conclusions: These data indicate that gambling in a “real
life” situation produces increases in salivary cortisol levels that accompany increased cardiovascular activity. Such effects may contribute to the development of gam-bling addiction. Biol Psychiatry 2000;48:948 –953 © 2000 Society of Biological Psychiatry
Key Words: Gambling, arousal, heart rate, salivary cor-tisol, addiction, field study
Introduction
S
elf-reports of gamblers, as well as observations of gamblers attending casinos, indicate that games of chance can directly affect physiologic, emotional, andmental states (Meyer and Bachmann 2000). The monetary stakes, recognition of risk, and the hope of real winnings produce stimulation, arousal, well-being, and euphoria, as well as feelings of power and success (DeCaria et al 1996; Legg England and Go¨testam 1991).
To date, limited studies have investigated experimen-tally the effect of gambling on physiologic emotional states. A small number of studies have examined heart rate as a psychophysiologic response to gambling behavior, however. These studies generally have demonstrated that heart rate is increased by gambling at blackjack (Anderson and Brown 1984), poker machines (Coulombe et al 1992; Dickerson et al 1992; Leary and Dickerson 1985), or “fruit machines” (Carroll and Huxley 1994; Griffiths 1993), as well as betting on horse racing (Coventry and Norman 1997). Increases in heart rate correlate positively with the magnitude of the stakes (Anderson and Brown 1984), which probably explains why heart rate alterations are smaller in laboratory simulations as opposed to real situations, as well as why smaller activation is seen in the heart rates of slot machine players. Although some data suggest that heart rate responses are greater in high-frequency gamblers as opposed to low-high-frequency gam-blers (Leary and Dickerson 1985), a number of studies have failed to confirm this assumption (Coulombe et al 1992; Coventry and Norman 1997; Dickerson et al 1992; Griffiths 1993). Furthermore, gamblers who are addicted to slot machines show no differences in heart rate reactiv-ity compared with casual gamblers (Carroll and Huxley 1994). Nonetheless, although problem gamblers who are asked to recall and describe winning situations demon-strate a significant increase in heart rate that does not differ from that of regular or casual gamblers, they display greater reactivity in other parameters of physiologic arousal, such as muscular activity and skin conductance (Sharpe et al 1995).
The consistent data showing stimulation of cardiovas-cular activity during gambling are potentially indicative of an acute stress state. Acute psychologic stress produces increased release of catecholamines and pituitary-adrenal hormones (Benschop et al 1996; Oberbeck et al 1998;
From the Institute of Psychology and Cognition Research, University of Bremen, Bremen (GM, MAS); the Department of Hematology/Oncology and Endocri-nology (BPH) and the Institute of Medical Psychology, University of Essen (MS, MSE), Essen; and the Division of Medical Psychology, Hannover Medical School, Hannover (CP), Germany.
Address reprint requests to Gerhard Meyer, Institute of Psychology and Cognition Research, P.O. Box 330440, Bremen 28334, Germany.
Received December 6, 1999; revised March 15, 2000; accepted March 20, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
Richter et al 1996). To date, only limited data exist reporting the neuroendocrine response to gambling (Schmitt et al 1998). Few studies have examined basal neuroendocrine functions in pathological gamblers com-pared with healthy control subjects (Ramirez et al 1988; Roy et al 1988, 1989). Nonetheless, it is unknown whether these changes are induced by gambling or behaviors comorbid to gambling, or whether they are endocrine precursors for addictive behavior. No research to date has investigated the acute effect of gambling on neuroendo-crine function in a field setting. Therefore, our study aimed to examine the effect of gambling on cardiovascular and neuroendocrine activity in a real gambling situation. Specifically, it examined heart rate and salivary cortisol in casino blackjack players before, during, and after play.
Methods and Materials
Participants
Nineteen blackjack players were approached in a casino and, following an explanation of the study, were asked whether they would like to participate. Of these, 10 individuals signed an informed consent form and were paid 50 deutsche marks for their participation. Aside from those who were unwilling to participate (n55), two of the 19 participants approached were discounted because of alcohol consumption, one had a viral infection, and one was taking blood pressure medication. Thus, a final group of 10 male gamblers (44.562.5 years of age) participated in the current study, all of whom participated in games of chance at least once a week. The protocol of the current study was approved by the local ethics committee for investigations using human subjects.
Heart Rate Measurement
Heart rate was measured continually using a portable heart rate monitor (Accurex Plus, Polar, Bu¨ttelborn, Germany). The ECG was transmitted from a chest patch to a receiver that was worn around the wrist in the mode of a watch. Heart rate was recorded and saved by the receiver in 5-sec intervals. Data were trans-ferred from the receiver to computer using “Polar Interface Plus” software.
Salivary Cortisol Measurement
Unstimulated saliva samples were collected for the detection of cortisol using standard techniques. Briefly, participants were asked to place a cotton wool swab into the mouth for 2 min. The swab was subsequently placed into a prepared tube (Salivette, Sarstedt, Germany), which was stored on ice until collection of all four samples. Tubes were then centrifuged for 10 min and stored at220°C.
Saliva samples were inspected for blood contamination before further analysis, with all samples yielding a clear saliva of low viscosity. Saliva flow was determined by weighing the tubes
before and after saliva sampling. Cortisol concentration in saliva was measured using a commercially available, coated-tube ra-dioimmunoassay (Coat-a-Count, Diagnostic Products, obtained through H. Biermann GmbH, Bad Nauheim, Germany) validated for that matrix (Tunn et al 1992). Measurements were performed in duplicate. Intra- and interassay variability (CV %), as calcu-lated by the Rodbard method (Rodbard 1974), were 5.34 and 12.24 at a physiologic concentration range (0.28 – 0.52mg/dL), 2.09 and 3.67 at a high concentration range (0.798 –1.30mg/dL), and 1.64 and 2.92 at very high concentrations (2.06 –3.36
mg/dL).
Behavioral Measurement
The severity of pathological gambling was assessed using a 20-item questionnaire (Petry 1996; Petry and Baulig 1998). The statements were scored on four-point Likert scales (0 –3), pro-ducing a maximum score of 60. The items assessed preoccupa-tion with gambling, loss of control, escapism, financial indebt-edness, “chasing one’s losses,” illegal acts, feelings of guilt, and suicidal thoughts. Instrumental (Cronbach’sa 5.79) and retest (r5 .80) reliability of the scale are satisfactory. Further, the questionnaire has been demonstrated to discriminate gamblers in treatment (score $ 16) from comparative samples of casual gamblers and bridge players (score , 16), and to diagnose pathological gamblers (according to DSM-III-R criteria).
Procedure
Specific testing dates were arranged with each participant. Each subject participated in an experimental and a control session, with both sessions occurring in the same casino environment. In the experimental session, the heart rate recorder was fitted once the participant arrived at the casino. The device was tested by recording each participant’s heart rate during both a resting position and following movement (seated position and standing up and walking). The participant was then asked to provide the first saliva sample. The participant was subsequently seated at the blackjack table and participated in the game using his own money. Heart rate recording commenced just before the begin-ning of play. The second and third saliva samples were collected from the participant following 30 and 60 min of play respec-tively. The fourth sample was collected following the end of play, with the participants subsequently completing the question-naire. The control session was conducted similarly to the experimental session, with the single difference that participants played a game of cards without any monetary stakes. Both experimental and control sessions occurred between 7:00PMand 11:00PM, with the length of play lasting 2 hours.
Statistical Analysis
Following statistical confirmation of normal distribution, data were analyzed using two-factor repeated measures (condition3
Results
Gambling Characteristics of the Sample
The magnitude of detrimental consequences of gambling behavior for each subject is shown in Table 1. The scores of five participants demonstrated normal gambling behav-ior (indicated by a score between 1 and 15), three had scores that indicated the emergence of a gambling addic-tion (scores between 16 and 25), one displayed a score that indicated medium gambling addiction (a score between 26 and 45), and one participant had a score indicating severe gambling addiction (a score between 46 and 60).
Heart Rate
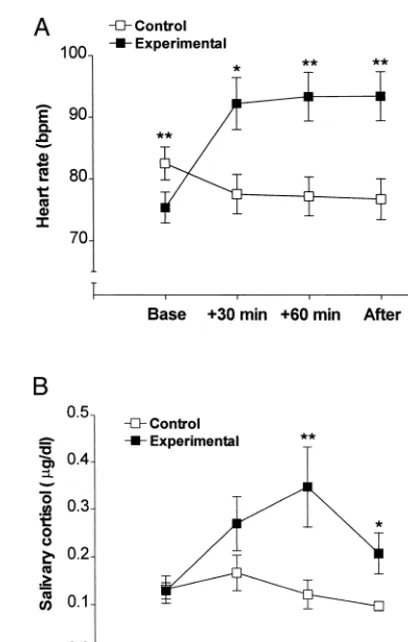
Gambling produced a significant increase in heart rate responses across the testing session compared with the control condition [F(3,27) 5 18.331, p 5 .001; Figure 1A]. Single comparisons revealed higher heart rate at baseline in the control condition; however, control heart rate lowered across the testing session, whereas heart rate increased significantly from baseline to 30 min during gambling by an average of 18 beats per minute. Single subjects revealed heart rate elevations of up to 80 beats per minute. Heart rate remained significantly higher than control levels across the whole gambling session.
Salivary Cortisol
Salivary cortisol was elevated by gambling compared with control conditions [F(3,27) 54.14, p 5.021; Figure 1B]. Single comparisons revealed that the peak cortisol increase at 60 min was significantly higher than control levels. Although salivary cortisol levels dropped following completion of the gambling session, cortisol remained significantly elevated compared with control levels at the end of play.
Relationships among Variables
Correlational analysis was used to examine the effect of age and severity of pathological gambling on heart rate and cortisol responses. Age was not associated with the reactivity of heart rate or salivary cortisol at any time (p. .05). In contrast, pathological gambling severity was negatively correlated with heart rate over the four times of testing in the control condition (baseline, r5 2.661, p5 .038; 130 min, r 5 2.815, p 5 .004; 160 min, r 5 2.830, p 5 .003; baseline, r 5 2.859, p 5 .001). Pathological gambling severity was unrelated to control cortisol levels and to the reactivity of both heart rate and cortisol to gambling, however (p ..05).
Additionally, reactivity of heart rate and cortisol to gambling showed inconsistent positive relationships. Specifically, heart rate responses at each time point during and after gambling were associated with increased cortisol at 130 min and after gambling, but not at 160 min (all
ps,.05).
Figure 1. Heart rate (A) and salivary cortisol (B) levels before gambling, during gambling at 30 min and 60 min, and after gambling. Data are presented as means6SEs. *p,.05; **p, .02.
Table 1. Participant Age and Severity of Pathologic Gambling
Subject Age
Pathologic gambling severity
1 47 17
2 45 31
3 44 7
4 45 5
5 48 19
6 34 5
7 29 3
8 49 5
9 45 24
10 59 46
n510 Mean544.5 Mean516.2
Discussion
Our study demonstrated that alterations in stress hormones accompany the known activation of cardiovascular activ-ity during gambling. Specifically, gambling produced increased levels of salivary cortisol that accompanied an elevated heart rate.
The magnitude of increased heart rate during gambling is average when compared with other studies demonstrat-ing this effect (Anderson and Brown 1984; Carroll and Huxley 1994; Coulombe et al 1992; Coventry and Norman 1997; Dickerson et al 1992; Griffiths 1993; Leary and Dickerson 1985; Sharpe et al 1995). The increase in heart rate is of lower magnitude compared with the acute psychologic stress of parachute jumping (Benschop et al 1996; Richter et al 1996) but of comparable size to the stress of public speech and mental arithmetic (Benschop et al 1994; Burleson et al 1998; Pawlak et al 1999). Never-theless, such models tend to implement brief periods (5–20 min) of stress, with heart rate returning rapidly to baseline following stressor termination (Benschop et al 1994; Benschop et al 1996; Pawlak et al 1999; Richter et al 1996). In contrast, the current data demonstrate that gambling produces a moderate yet sustained heart rate elevation, which persists following termination of the game. Although severity of pathological gambling was unrelated to the reactivity of heart rate during gambling, pathological gambling behavior was associated with lower heart rate across the entire control period. This suggests that persons with less severe gambling behavior may have found the control condition challenging, whereas this condition provoked no arousal in pathological gamblers.
Our study provides the first demonstration that the arousal of gambling induces a secretion of salivary corti-sol. Akin to heart rate elevations, the gambling-induced cortisol secretion is of a lower magnitude than salivary cortisol responses to very brief, intense stressors (Kirsch-baum et al 1996a, 1997, 1999; Kudielka et al 1998); however, the cortisol response to gambling remains ele-vated for a longer period compared with acute stressors, whereby salivary cortisol levels normalize rapidly follow-ing the stressor (Kirschbaum et al 1996b, 1997, 1999; Kudielka et al 1998). The cortisol profile observed cur-rently is similar to that observed in acute stress situations that are of a 1- to 2-hour duration (Kugler et al 1996). Our results therefore suggest that gambling has the potential to produce acute, stresslike endocrine responses over the duration of a gambling episode, which can occur for a number of hours (Meyer 1989).
Physiologic consequences of gambling may contribute to the reinforcement of this behavior; however, the rele-vance of increased cortisol for the maintenance of gam-bling behavior is unclear. It has been posited that
physio-logic responses to gambling enhance mood and that winning has the ability to produce a “euphoric” state (Legg England and Go¨testam 1991). Cortisol may contrib-ute to such mood alterations because accontrib-ute cortisol admin-istration has been shown to enhance positive feelings (Philal et al 1996). Such a position is equivocal because a more sustained increase in cortisol produces depressed mood (Barden et al 1995; Halbreich 1997; Reus et al 1997). Nonetheless, it is possible that cortisol secretion contributes to decreases in tiredness experienced while gambling (Legg England and Go¨testam 1991) because glucocorticoid therapy improves symptoms of clinical fatigue disorders (McKenzie et al 1998; Scott and Dinan 1999).
Some evidence exists to suggest that gambling-induced autonomic arousal acts as a reinforcer of gambling (Sharpe and Tarrier 1993; Sharpe et al 1995). Additionally, it is also possible that the cortisol response to gambling may contribute to the reinforcing properties of this behavior. In support of this proposition, animal models have demon-strated that glucocorticoid activation enhances the acqui-sition and maintenance of drug self-administration, possi-bly via increasing the sensitivity of mesencephalic dopaminergic neurons (Caggiula et al 1998; Goeders 1997; Piazza et al 1991; Piazza and Le Moal, 1998). Whether this model of pharmacologic addiction in animals can be applied to behavioral addiction in humans remains to be investigated.
The increase in cortisol may have implications for the gambling performance of the individual. Because acute increases in glucocorticoids have been demonstrated to have detrimental effects on human memory (Kirschbaum et al 1996b; Lupien et al 1997; Newcomer et al 1999), the cortisol response to gambling may hinder optimal judge-ment within the game. In contrast, neural and behavioral evidence from animal models suggests that although high levels of glucocorticoids impair information processing, mild elevations enhance memory (de Kloet et al 1999). Thus, previous studies suggest that physiologic responses that encourage participation in gambling may also influ-ence the ability to perform the task optimally. The exact nature of this relationship remains equivocal and warrants further investigation.
period of gambling or whether chronic gambling produces alterations in the circadian rhythm of cortisol release.
In addition, our study focused on a small number of male gamblers and one type of gambling. Further inves-tigation is required to establish the generalizability of these effects by examining the physiologic responses of female gamblers, as well as of gamblers playing other games. Additionally, investigations in larger sample sizes are required to examine further the relationship between severity of pathological gambling and physiologic re-sponses. Moreover, because of casino regulations, our study was unable to record whether the subjects were winning or losing money on each game played. Thus, it is difficult to interpret whether the physiologic changes are the consequence of generalized emotional arousal or whether the elation or distress associated with winning and losing money respectively produce contrasting physio-logic responses. Future investigations should incorporate measurements of “winning or losing,” the monetary stakes gambled, and the individual’s mood state to more accu-rately define the psychologic profile that produces in-creased heart rate and salivary cortisol in gamblers.
In summary, the current study demonstrates that in addition to increasing cardiovascular activity, gambling in a real-life situation produces increases in salivary cortisol concentrations. Further investigation is required to reveal the exact characteristics of this response, as well as the implications for gambling behavior.
References
Anderson G, Brown RI (1984): Real and laboratory gambling, sensation seeking and arousal. Br J Psychol 75:401– 410. Barden N, Reul JM, Holsboer F (1995): Do antidepressants
stabilize mood through actions on the hypothalamic-pituitary-adrenocortical system? Trends Neurosci 18:6 –11.
Benschop RJ, Jacobs R, Sommer B, Schu¨rmeyer TH, Raab H-R, Schmidt RE, Schedlowski M (1996): Modulation of the immunologic response to acute stress in humans byb -block-ade or benzodiazepines. FASEB J 10:517–524.
Benschop RJ, Nieuwenhuis EES, Tromp EAM, Godaert GLR, Ballieux RE, van Doornen LJP (1994): Effects ofb -adrener-gic blockade on immunolo-adrener-gic and cardiovascular changes induced by mental stress. Circulation 89:762–769.
Burleson MH, Malarkey WB, Cacioppo JT, Poehlmann KM, Kiecolt-Glaser JK, Berntson GG, et al (1998): Postmeno-pausal hormone replacement: effects on autonomic, neuroen-docrine, and immune reactivity to brief psychological stres-sors. Psychosom Med 60:17–25.
Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C, et al (1998): The role of corticosteroids in nicotine’s physiological and behavioral effects.
Psychoneuroendocri-nology 23:143–159.
Carroll D, Huxley JAA (1994): Cognitive, dispositional, and psychophysiological correlates of dependent slot machine
gambling in young people. J Appl Sociol Psychol 24:1070 – 1083.
Coulombe A, Ladouceur R, Desharnais R, Jobin J (1992): Erroneous perceptions and arousal among regular and occa-sional video poker players. J Gambling Stud 8:235–244. Coventry KR, Norman AC (1997): Arousal, sensation seeking
and frequency of gambling in off-course horse racing bettors.
Br J Psychol 88:671– 681.
DeCaria CM, Hollander E, Grossman R, Wong CM, Mosovich SA, Cherkasky S (1996): Diagnosis, neurobiology, and treat-ment of pathological gambling. J Clin Psychiatry 57:80 – 84. de Kloet ER, Oitzl MS, Joe¨ls M (1999): Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci 22:422– 426.
Dickerson M, Hinchy J, England SL, Fabre J, Cunningham R (1992): On the determinants of persistent gambling behavior. I. High-frequency poker machine players. Br J Psychol 83:237–248.
Goeders NE (1997): A neuroendocrine role in cocaine reinforce-ment. Psychoneuroendocrinology 22:237–259.
Griffiths M (1993): Tolerance in gambling: An objective mea-sure using the psychophysiological analysis of male fruit machine gamblers. Addict Behav 18:365–372.
Halbreich U (1997): Hormonal interventions with psychophar-macological potential: an overview. Psychopharmacol Bull 33:281–286.
Kirschbaum C, Bono EG, Rohleder N, Gessner C, Pirke KM, Salvador A, et al (1997): Effects of fasting and glucose load on free cortisol responses to stress and nicotine. J Clin
Endocrinol Metab 82:1101–1105.
Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellham-mer DH (1999): Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154 –162. Kirschbaum C, Schommer N, Federenko I, Gaab J, Neumann O,
Oellers M, et al (1996a): Short-term estradiol treatment enhances pituitary-adrenal axis and sympathetic responses to psychosocial stress in healthy young men. J Clin Endocrinol
Metab 81:3639 –3643.
Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH (1996b): Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci 58:1475–1483.
Kudielka BM, Hellhammer J, Hellhammer DH, Wolf OT, Pirke K-M, Varadi E, et al (1998): Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandro-sterone treatment. J Clin Endocrinol Metab 83:1756 –1761. Kugler J, Reintjes F, Tewes U, Schedlowski M (1996):
Compe-tition stress in soccer coaches increases salivary immunoglob-ulin A and salivary cortisol concentrations. J Sports Med Phys
Fitness 36:117–120.
Leary K, Dickerson M (1985): Levels of arousal in high- and low-frequency gamblers. Behav Res Ther 23:635– 640. Legg England S, Go¨testam KG (1991): The nature and treatment
of excessive gambling. Acta Psychiatr Scand 84:113–120. Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair
McKenzie R, O’Fallon A, Dale J, Demitrack M, Sharma G, Deloria M, et al (1998): Low-dose hydrocortisone for treat-ment of chronic fatigue syndrome: A randomized controlled trial. JAMA 280:1061–1066.
Meyer G (1989): Glu¨cksspieler in Selbsthilfegruppen: Erste Ergebnisse einer empirischen Untersuchung. Suchtgefahren 35:217–234.
Meyer G, Bachmann M (2000): Spielsucht—Ursachen und
Therapie. Berlin: Springer.
Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, et al (1999): Decreased memory performance in healthy humans induced by stress-level cortisol treatment.
Arch Gen Psychiatry 56:527–533.
Oberbeck R, Schu¨rmeyer T, Jacobs R, Benschop RJ, Sommer B, Schmidt RE, et al (1998): Effects ofb-adrenoceptor blockade on stress-induced adrenocorticotrophin release in humans.
Eur J Appl Physiol 77:523–526.
Pawlak C, Jacobs R, Mikeska E, Ochsmann S, Lombardi MS, Kavelaars A, et al (1999): Patients with systemic lupus erythematosus differ in their immunological response to acute psychological stress. Brain Behav Immun 13:287–302. Petry J (1996): Psychotherapie der Glu¨cksspielsucht.
Heidel-berg, Germany: Beltz.
Petry J, Baulig T (1998): Kurzfragebogen zum
Glu¨cksspielver-halten. Mu¨nchwies, Germany: Psychosomatische Fachklinik
Mu¨nchwies.
Philal W, Krug R, Pietrowsky R, Fehm HL, Born J (1996): Corticosteroid receptor mediated effects on mood in humans.
Psychoneuroendocrinology 21:515–523.
Piazza P, Le Moal M (1998): The role of stress in drug self-administration. Trends Pharmacol Sci 19:67–74. Piazza PV, Maccari S, Deminie`re J-M, LeMoal M, Morme`de P,
Simon H (1991): Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl
Acad Sci U S A 88:2088 –2092.
Ramirez LF, McCormick RA, Lowy MT (1988): Plasma cortisol
and depression in pathological gamblers. Br J Psychiatry 153:684 – 686.
Reus VI, Wolkowitz OM, Frederick S (1997): Antiglucocorti-coid treatments in psychiatry. Psychoneuroendocrinology 22:S121–S124.
Richter SD, Schu¨rmeyer TH, Schedlowski M, Ha¨dicke A, Tewes U, Schmidt RE, et al (1996): Time kinetics of the endocrine response to acute psychological stress. J Clin Endocrinol
Metab 81:1956 –1960.
Rodbard D (1974): Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem 20:1255–1270.
Roy A, Adinoff B, Roehrich L, Lamparski D, Custer R, Lorenz V, et al (1988): Pathological gambling: A psychobiological study. Arch Gen Psychiatry 45:369 –373.
Roy A, DeJong J, Ferraro T, Adinoff B, Gold P, Rubinow D, et al (1989): CSF GABA and neuropeptides in pathological gamblers and normal controls. Psychiatry Res 30:137–144. Schmitt LH, Harrison GA, Spargo RM (1998): Variation in
epinephrine and cortisol excretion rates associated with be-havior in an Australian Aboriginal community. Am J Phys
Anthropol 106:249 –253.
Scott LV, Dinan TG (1999): The neuroendocrinology of chronic fatigue syndrome: Focus on the hypothalamus-pituitary-adre-nal axis. Funct Neurol 14:3–11.
Sharpe L, Tarrier N (1993): Towards a cognitive-behavioural model of problem gambling behaviour. Br J Psychiatry 162:407– 412.
Sharpe L, Tarrier N, Schotte D, Spence SH (1995): The role of autonomic arousal in problem gambling. Addiction 90:1529 – 1540.