T
he uptake and transport of inorganic nitrogen is essential for plant growth. Following the carrier-mediated uptake of nitrate or ammonium from the soil, or of ammonium derived from symbiotic dinitrogen fixation, inorganic nitrogen is assimilated into amino acids in an energy-requiring process1. Theassimilation of ammonium usually occurs in the roots, whereas nitrate assimilation, depending on the species and environmental conditions, occurs either in the roots or in the leaves, after trans-port via the xylem2. From the leaves, excess amino acids can be
exported via the phloem. Thus, amino acids are present in both xylem and phloem, and can be withdrawn from the vascular sys-tem by cells that depend on an external supply, such as apices, newly developing tissues and reproductive organs. In most plants, the spectra of amino acids found in the phloem and xylem appear to be similar, the major components being amides such as glutamine and asparagine, and acidic amino acids such as glutamate and as-partate. However, the relative concentrations of these amino acids are very different: the xylem contains low concentrations (e.g. 3– 20 mMin Urtica3); and the phloem contains higher concentrations
(e.g. 60–140 mMin sugar beet4
). The phloem, as a living system with cytoplasmic continuity between cells, mediates transport intracellularly, whereas the xylem can be considered to be an extra-cellular compartment. Because of loading of amino acids from the xylem to the phloem (e.g. in the case of seeds that receive their organic nitrogen mainly via the phloem), the composition of the phloem sap can differ along its path5
. Such complexity in compo-sition, and the multiplicity of cell types that are involved in long-distance transport during the life cycle, suggests that multiple, highly regulated transport systems for amino acids exist. There is now physiological and genetic evidence for the activity of multi-ple, carrier-mediated transport systems responsible for the uptake and transfer of amino acids6
. A molecular dissection of amino acid transport and characterization of individual carriers is thus required.
Yeast as a model for amino acid transport
Yeast has been invaluable for cloning and expressing plant genes encoding amino acid transporters, and could also be a good model for investigating aspects of their regulation. A given amino acid is typically transported into yeast cells by several permeases with different specificities, affinities and regulation7
. In addition to their role in uptake, high-affinity amino acid permeases are also important for retention of intracellular amino acids, which tend to leak out of the cell8(Table 1). With the ongoing functional
analy-sis of additional likely amino acid permeases (found during se-quencing of the yeast genome9), a complete picture of individual
amino acid transport systems in this unicellular eukaryote should soon be available.
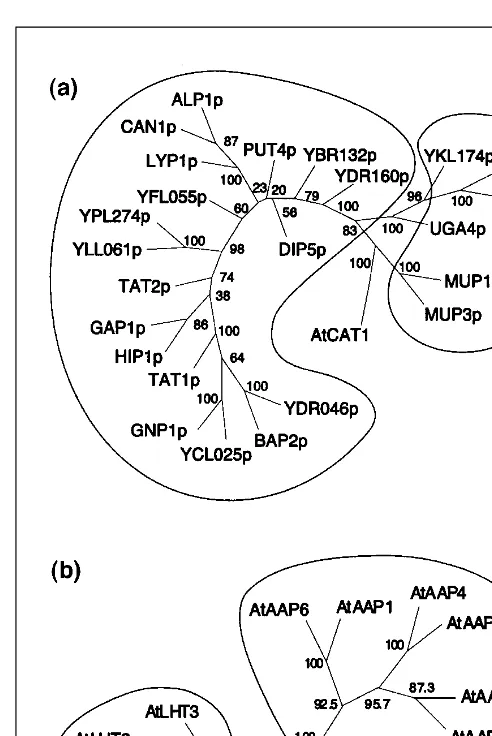
All yeast amino acid permeases that have been characterized fall into the APC (‘amino acid–polyamine–choline facilitator’) superfamily10, which may itself be subdivided (Fig. 1a). The
largest family of Gap1 (‘general amino acid permease’)-related amino acid transporters includes Gap1; ten additional, more spe-cific, permeases; and seven others found by genome sequencing (Table 1). These proteins are very similar, and are closely related to amino acid permeases found in bacteria and other fungi. Analy-sis of the predicted topological features of bacterial proteins of this family (aroP and pheP) suggests a structure of 12 membrane-spanning domains separated by hydrophilic regions with the N-and C-termini located in the cytosol11
. The second family [g -aminobutyric acid (GABA) permease-related] includes two very similar methionine permeases (Mup1 and Mup3) (Ref. 12) that are homologous to several other proteins: a Schistosoma mansoni amino acid transporter; the yeast GABA-, choline- and KAPA/ DAPA permeases13; and paralogues found by genome sequencing.
In addition to the APC superfamily, yeast cells contain seven highly related proteins that are similar to the ATF (‘amino acid transporter family’) superfamily in plants9
. However, the functional role of these proteins has yet to be tested. There might also be additional classes of amino acid transport proteins, which could, for example, be involved in compartmentalization of amino acids into the vacuole. It has been suggested that these proteins are members of a large family (31 members in yeast) of putative H+
-antiporters9
. The large ABC superfamily of transport proteins14
might also include amino acid transport systems – several members of this family from other organisms have been shown to transport amino acids15.
Regulation of amino acid transport in yeast occurs mainly by transcriptional control of amino acid permease genes and by tar-geting and turnover of the proteins. Transcription of several of the permease genes, such as GAP1, is subject to nitrogen repression: transcription is highest on media containing nitrogen sources that support only limited growth rates (urea or proline), and is down-regulated in the presence of preferred nitrogen sources (NH4
+
, glu-tamine or asparagine7). Four transcription factors of the GATA
family (Gln3, Nil1/Gat1, Uga43/Dal80 and Gzf3/Nil2/Deh1) play key functions in this global regulation and seem to be part of a complex network of auto- and cross-regulation16
. Among genes subject to nitrogen repression, some are induced in the presence of their own substrates (e.g. UGA4); expression of other amino acid permease genes (e.g. HIP1) does not appear to be influenced by the
Amino acid transport in plants
Wolf-Nicolas Fischer, Bruno André, Doris Rentsch, Sylvia Krolkiewicz,
Mechthild Tegeder, Kevin Breitkreuz and Wolf B. Frommer
quality of the nitrogen source or the pres-ence of amino acid substrates. Intracellular traffic of synthesized permeases is also subject to nitrogen control. For example, it was recently shown that on urea medium, newly synthesized Gap1 proteins are trans-ported from the Golgi complex to the plasma membrane, whereas, on glutamate medium, they are transported from the Golgi to the vacuole or lysosome, where they are degraded17
. Another level of regulation of amino acid permeases is the control of their stability at the plasma membrane. Upon ad-dition of a preferred nitrogen source, such as NH4
+
, to cells growing on a poor nitrogen medium, Gap1 proteins previously accu-mulated at the plasma membrane undergo endocytosis, polyubiquitination and degra-dation in the vacuole18
. Genetic dissection of this down-regulation showed that it in-volves a ubiquitin–protein ligase (Npi1/ Rsp5), which is essential for cell viability19. The cytosolic C-terminal extremity of the Gap1 permease contains several features, including a di-leucine motif, that are essen-tial for sensitivity to NH4
+-triggered
down-regulation20 .
The lifestyle of yeast is very different from that of most plants. Saccharomyces spp. grow as saprophytes under varying sub-strate conditions, and therefore must adapt rapidly to a changing nutrient supply. For plant cells, the situation is usually very dif-ferent, because cells do not normally experi-ence dramatic changes in the extracellular composition of amino acids. Thus, the spec-trum of amino acid transporter genes from yeast, though similar to that found in higher plants, is also likely to have important differences.
A myriad of plant amino acid transporter genes
Biochemical approaches for identifying and isolating transport proteins are problematic and, to circumvent this, a technique has been devised to exploit Saccharomyces as a model system. Yeast mutants lacking uptake systems for certain amino acids, and which therefore cannot grow on media containing such amino acids as the sole nitrogen source, were chosen as a complemen-tation system. The mutants were transformed with plant cDNA libraries in order to screen for transporters that would be able to suppress the growth-deficient phenotype. Using various yeast amino acid transport mutants, many plant amino acid transporter genes were identified (>13 genes from Arabidopsis). Based on sequence similarities, the plant transporters can be classified into two major superfamilies: the ATFs and the APCs (Table 2).
The ATF superfamily is so far the largest and best-character-ized family. Detailed computer analysis indicates that the trans-porters are built up from ten membrane-spanning domains, whereas experimental evidence suggests the presence of 11 hydrophobic segments, with the N-terminus exposed to the cytosol21,22
. Several members of this family were shown to func-tion as proton symporters23,24. Based on sequence similarities and substrate specificity, four families can be distinguished: ProTs, AAPs; LHT-related proteins and AUX1-related proteins (Fig. 1b).
The two members of the ProT family found in Arabidopsis have a strong preference for proline25
. In contrast, the members of the AAP family recognize a large spectrum of different amino acids, includ-ing amides and ureides26,23,24
. The AtAAP1, AtAAP2, AtAAP4 and AtAAP6 proteins have a preference for neutral and acidic amino acids, AtAAP6 displaying the highest affinity for most substrates. AtAAP3 and AtAAP5, in addition to transporting neutral and acidic amino acids, transport basic amino acids with high efficiency. Therefore, the AAPs are classified either as neutral and acidic amino acid transporters or as general amino acid transporters21
. Individual carriers belonging to one subgroup might also differ with respect to their affinity for some amino acids (e.g. for large hydrophobic amino acids); the physiological relevance of these dif-ferences remains to be demonstrated. New members of the AAP family from Arabidopsis are being identified by the genome se-quencing project (Table 2). The family of LHT-related transporters was found by homology with known sequences and comprises ex-pressed sequence tags (ESTs) and genomic clones distantly related to the AAPs. So far only one member of this family (LHT1) has been functionally characterized, and was shown to transport lysine and histidine27. A member of the fourth family, the AUX1-related proteins, was identified as being defective in an auxin-resistant mutant of Arabidopsis28. Auxins are structurally very similar to
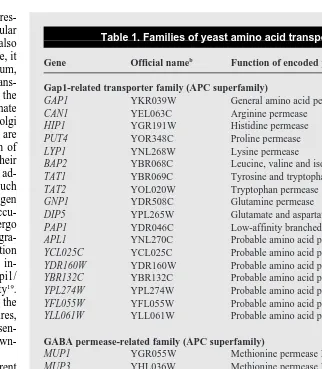
Table 1. Families of yeast amino acid transportersa
Gene Official nameb
Function of encoded protein
Gap1-related transporter family (APC superfamily)
GAP1 YKR039W General amino acid permease
CAN1 YEL063C Arginine permease
HIP1 YGR191W Histidine permease
PUT4 YOR348C Proline permease
LYP1 YNL268W Lysine permease
BAP2 YBR068C Leucine, valine and isoleucine permease
TAT1 YBR069C Tyrosine and tryptophan permease
TAT2 YOL020W Tryptophan permease
GNP1 YDR508C Glutamine permease
DIP5 YPL265W Glutamate and aspartate permease
PAP1 YDR046C Low-affinity branched amino acid permease
APL1 YNL270C Probable amino acid permease
YCL025C YCL025C Probable amino acid permease
YDR160W YDR160W Probable amino acid permease
YBR132C YBR132C Probable amino acid permease
YPL274W YPL274W Probable amino acid permease
YFL055W YFL055W Probable amino acid permease
YLL061W YLL061W Probable amino acid permease
GABA permease-related family (APC superfamily)
MUP1 YGR055W Methionine permease I
MUP3 YHL036W Methionine permease III
UGA4 YDL210W GABA permease
HNM1/CTR1 YGL077C Choline permease
BIO5 YNR056C Permease of keto-aminopelargonic acid and diaminopelargonic acid
YKL174C YKL174C Protein of unknown function
a
These families were formed based on functional analyses and data from the yeast genome project. bOfficial names are the open reading frame (ORF) designations according to the yeast genome project.
Additional genes that belong to the ATF family include: YNL101W, YKL146W, YJR001W, YER119C,
YEL064C, YIL088C and YBL089W. The official ORF names of all these are simply the non-italicized
tryptophan, and thus AUX1 might have developed from an AAP amino acid transporter into an auxin transporter. Alternatively, AUX1 might function as an auxin receptor, as in the case of yeast hexose transporters, where at least two members of the hexose transporter family serve as glucose receptors involved in the acti-vation of hexose uptake29
.
The plant APCs can be subdivided into two families, the CATs and proteins most homologous to the yeast GABA permease-related transporter family. The plant CAT family shares significant hom-ology with the mammalian CATs, and is similarly composed of 14 putative membrane-spanning domains. The CATs are also
distantly related to the yeast APC transporters, such as GAP1. So far, from plants, only a single member of this family (AtCAT1) has been identified, by complementation of a yeast histidine trans-port mutant30. However, an EST falling into the same family has been identified and is currently under investigation (Table 2). Transport studies in yeast support the hypothesis that AtCAT1 is an H+
-coupled transport system with high affinity for basic amino acids that also recognizes many other amino acids with lower efficiency30
.
A member of the GABA permease-related family of amino acid transporters, originally described in yeast, has recently been identified (Table 2). Similar to the yeast transporters, it contains 12 putative membrane-spanning domains (K. Breitkreuz et al., unpublished).
As with many other organisms, plants contain a large number of proteins that belong to the ABC superfamily of transporters14. In other organisms, these proteins are found in different subcellu-lar compartments and have been shown to transport many differ-ent substrates, including amino acids. So far, none of the members of the plant family has been shown to function in amino acid transport, and thus any role in cellular import or release of amino acids must remain speculative.
In summary, both yeast and plants contain multiple sets of amino acid transport proteins. Even before the genomic sequencing of Arabidopsis has been completed, the number of putative amino acid transporter genes from plants already exceeds the total num-ber in yeast. This is not surprising, given that the plant contains so many different cell types. There are also a large number of general amino acid transporters, which can transport many different amino acids. This might be the standard situation in a plant, where an excess of amino acids is exported into the vascular system. Seeds require the rapid import, translocation and export of large amounts of transiently stored amino acids within a short time. Transient storage occurs mainly in the form of proteins. Thus, during the life cycle of a plant, organic nitrogen stored in the form of proteins has to be remobilized, especially from vegetative stor-age proteins in leaves, for retrieval during leaf senescence, or dur-ing germination from storage in cotyledons or endosperm. In all these situations, an efficient mechanism would be to use general amino acid transport systems to mobilize as many of the amino acids released by proteolysis as possible. In contrast, more-specific systems such as the proline transporters might be required only under defined conditions such as drought or salt stress, where spe-cific amino acids have to be mobilized preferentially25.
Multiple transporters with overlapping specificity: redundancy or necessity?
If plants use predominantly general amino acid transporters, why did they develop so many similar systems? As a first step towards answering this question, expression of the different carriers was studied at the RNA level. All characterized members of the plant ATF and APC superfamily show differential expression pat-terns25,26,31
(Fig. 2; Table 3). The expression of a given transporter gene can be very specific or can overlap with other genes (e.g. AtAAP3 was found only in roots, AtAAP6 only in sink tissues and StAAP1 mainly in leaves; M. Kwart and W.B. Frommer, unpub-lished). However, the expression patterns derived from northern blots yield only limited information: as with the sucrose trans-porter, the expression of many amino acid transporters might be restricted to the vascular system, and thus the relative density of the vascular strands within the harvested material could determine the signal strength and spuriously indicate a particular tissue specificity. To address this problem, promoters were isolated for most AtAAP and AtCAT1 genes (Refs 30 and 31; W-N. Fischer Fig. 1. Computer-aided analyses of sequence homology between
and W.B. Frommer, unpublished). At the RNA level, AtAAP1 and AtAAP2 seem to be co-regulated developmentally during silique maturation, with the induction of both preceding the accumulation of 2S albumin mRNAs (Ref. 31). Promoter– GUS fusion analysis revealed that AtAAP2 is expressed in the vascular strands enter-ing the siliques, thus strongly suggestenter-ing a role in the uptake of amino acids into the seeds (Fig. 3) and in xylem-to-phloem transfer. In contrast, AtAAP1 is expressed in the endosperm and in the embryo proper, supporting a function in the import of amino acids into the endosperm and sub-sequently into the cotyledons of the de-veloping embryo. Expression of AtCAT1 was detected in roots, stems, major veins of leaves and in flowers30
.
How does the molecular analysis fit with genetic studies?
Mutant analysis is a powerful strategy for studying the function of transporters in vivo. The classical approach for identifying transport mutants is selection on media containing toxic concentrations of com-pounds or toxic analogues. Mutants with non-redundant and selective transport sys-tems exclude the toxic compounds from the interior and thus survive. Using this type of approach, at least three classes of amino acid transport systems could be differentiated in the unicellular green alga Chlorella32
. The selection of tobacco protoplasts on high concentrations of va-line, and subsequent regeneration of intact plants, led to the identification of amino acid transport mutants resistant to high external valine concentrations. Different types of mutants were identified in
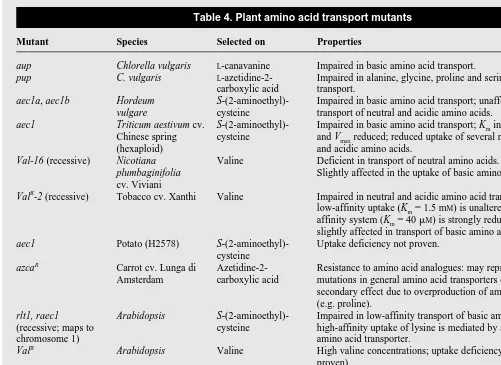
Arabid-opsis by selection of seedlings on high concentrations of valine or threonine, or on the proline analogue azetidine-2-carboxylic acid (Table 4).
The Arabidopsis raz1 mutant, which lacks activity of a high-affinity proline transporter, might carry a mutation in a member of the ATF superfamily, all of which are able to mediate proline transport33; a potential candidate is one of the ProT proline trans-porters. The Arabidopsis rlt1 and raec1 mutants are charac-terized by reduced uptake of basic amino acids34. Both mutations were mapped to chromosome 1. Because AtAAP5 transports argi-nine, lysine and histidine with high affinity, the AtAAP5 gene might be affected in these mutants26
. The AtLHT1 protein does not transport arginine, and thus cannot be the target; however, an-other member of the LHT-family, AtLHT3 (which maps to chromo-some I), could carry the mutation. In the case of the barley aec1 mutants, defects in orthologues of AtLHT1, AtCAT1 or AtAAP5, all representing high-affinity basic amino acid transporters, could explain the lack of basic amino acid uptake into the roots35
. The wheat aec1 mutant is deficient in the uptake of basic, neutral and acidic amino acids36
. A mutation in an AtAAP3 orthologue could lead to the described phenotype, because so far AtAAP3 is the only amino acid transporter known to be able to mediate trans-port of acidic, basic and neutral amino acids26. High AtAAP4
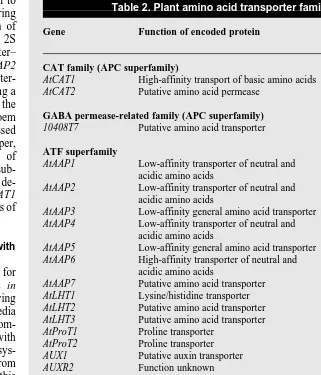
Table 2. Plant amino acid transporter familiesa
Gene Function of encoded protein Database
accession no.
CAT family (APC superfamily)
AtCAT1 High-affinity transport of basic amino acids X77502
AtCAT2 Putative amino acid permease AC000103
GABA permease-related family (APC superfamily)
10408T7 Putative amino acid transporter T22007
ATF superfamily
AtAAP1 Low-affinity transporter of neutral and L16240
acidic amino acids
AtAAP2 Low-affinity transporter of neutral and X71787
acidic amino acids
AtAAP3 Low-affinity general amino acid transporter X77499
AtAAP4 Low-affinity transporter of neutral and X77500
acidic amino acids
AtAAP5 Low-affinity general amino acid transporter X77501
AtAAP6 High-affinity transporter of neutral and X95736
acidic amino acids
AtAAP7 Putative amino acid transporter AB005244
AtLHT1 Lysine/histidine transporter T13994
AtLHT2 Putative amino acid transporter AC00103
AtLHT3 Putative amino acid transporter AC002294
AtProT1 Proline transporter X995737
AtProT2 Proline transporter X995738
AUX1 Putative auxin transporter X98772
AUXR2 Function unknown T75723
AUXR3 Function unknown Z29120
NsAAP1 Putative amino acid transporter U31932
StAAP1 Putative amino acid transporter Y009825
StAAP2 Putative amino acid transporter Y009826
RcAAP1 Putative amino acid transporter Z68759
RcAAP2 Putative amino acid transporter Y11121
aThese families were formed on the basis of sequence similarity (with data from the Arabidopsis
genome project) and functional analyses.
Fig. 2. Tissue specificity of plant amino acid transporters. The
diagram is based on results obtained from northern analyses and promoter–reporter gene fusions25,26
expression was found in mature leaves of Arabidopsis26. As AtAAP4 efficiently transports valine, a mutation in an AtAAP4 orthologue of tobacco might explain the valine resistance of this mutant37,38
. In several cases, the chromosomal location of the mutation has been mapped. Comparison of mapping data from mutants and amino acid transporter genes will be an excellent way of assigning physiological functions to the individual carriers. However, it is also conceivable that other genes involved (e.g. in the regulation of amino acid transport) will be identified, as with the yeast shr3 mutant, which is defective in targeting yeast amino acid permeases to the plasma membrane39.
How do the biochemical properties of the carriers fit with physiological studies?
Expression of plant amino acid transporters in different systems allows precise characterization of individual properties, such as substrate specificity. However, under physiological conditions, the relative composition of the intra- and extracellular fluids will determine which amino acids are actually transported. Thus, how relevant are the observed differences in substrate specificity? The intrinsic biochemical properties of both broad and general AAPs and the cationic CAT transporters might be overruled by the actual amino acid composition of the cytosol and extracellular space. For example, a transporter that has been characterized as a high-affinity transporter for cationic amino acids, but has a low affinity for glutamate, might mainly function as a glutamate car-rier in vivo, because glutamate concentrations are much higher than lysine concentrations. The problem is to identify the actual concentrations encountered by the carriers. For this, imaging tech-niques with resolution down to the subcellular level are required. Our current understanding is based almost exclusively on data obtained from whole organs, such as by grinding leaves or ana-lyzing apoplastic wash fluids. These results must be interpreted with caution, because they are averaged across the whole leaf – local differences may well be important, as was elegantly demon-strated by imaging of photosynthesis40.
At present, there is no efficient technique available for determin-ing the concentration of different amino acids in the subcellular com-partments of plant cells under non-invasive conditions. However, in vivo NMR might be able to clarify this situation. NMR correlation-peak imaging can resolve the spatial distribution of various amino acids and sugars in the hypocotyl of castor bean seedlings almost down to the level of individual cells. Glutamine and/or glutamate was detected in the cortex parenchyma and in the vascular bundles. Lysine and arginine were mainly present in the vascular bundles, whereas valine was observed in the cortex parenchyma, but not
in the vascular bundles41
. The finding that valine shows a different distribution to the other amino acids is surprising, because the broad-specificity AAPs are able to transport acidic and neutral amino acids, including valine, with comparable efficiency. NMR-imaging techniques are also excellent tools for determining both the direction and the velocity of fluxes in the vascular system42
. Until such tools become available for the analysis of mutants and transgenic plants, we have to rely on the whole-organ experi-ments. Nevertheless, the tools for such analyses are highly developed. By a combi-nation of non-aqueous fractiocombi-nation with apoplastic washing techniques and laser stylectomy from aphid stylets, the sucrose and amino acid compositions for vacuolar, cytosolic, apoplastic and phloem sap can be studied in the same plant43. For both sugars and amino acids, a large drop in concen-tration was observed in the apoplast. It is still a matter of debate whether the wash fluids represent the actual concentrations found at sites of phloem loading, especially because the loading occurs at the surface of only a few cells, as was shown for sucrose transporters that are localized at the small sieve elements in minor veins44
. The con-centration drop, and thus the steep gradient
Table 3. Organ-specific expression patterns of different plant amino acid transporter genesa
Tissue
Gene Sink leaf Source leaf Stem Flower Fruit Root Seedling Refs
AtAAP1 – – – – S – – 50, 51
AtAAP2 – V V – V V V 52
AtAAP3 – – – – – +++++ +++ 26
AtAAP4 – +++ ++++ ++ – – – 26
AtAAP5 – ++++ ++++ ++++ ++ ++ – 26
AtAAP6 +++ – – – – ++++ – 25
AtProT1 +++ +++ ++++ ++++ – ++++ – 25
AtProT2 – ++ + ++++ ++ ++++ – 25
AtCAT1 – ++ + ++++ ++ +++ – 30
StAAP1 – +++ – – – – – b
StAAP2 – – +++ – – – – b
AtLHT1 ++++ – – P ++++ – – 27
RcAAP1 +++ +++ – – – +++ ++++ c
RcAAP2 ++ +++ – – – ++ ++++ c
a
Amount of expression is shown by ‘plus’ symbols, from low (one symbol) to high (five symbols). Dashes indicate no detectable expression. Other abbreviations: V, expression in the vascular bundle; S, expression in the seed; P, expression in the pollen.
b
W.B. Frommer and M. Kwart, unpublished. c
See note added in proof.
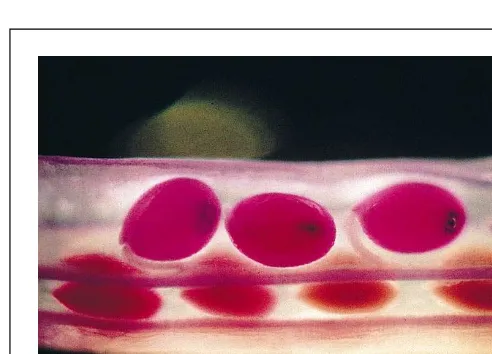
Fig. 3. Light microscope image of a silique from Arabidopsis. The
seeds are stained pink/purple with fuchsin-red. Courtesy of Brigitte
towards the phloem sap, indicates that an active transport step using proton-coupled transport systems is required for loading amino acids into the phloem. This finding supports a function for members of the AAP family in phloem loading. Interestingly, the relative concentrations of a given amino acid were found to be very similar in the cytosol of mesophyll cells, in the apoplastic wash fluids and in the phloem sap. This result is in agreement with the assumption that low-selectivity transporters such as the AAPs import those amino acids into the sieve tubes that are produced in excess and then released by the mesophyll. Such a low selectivity of the transporters involved might also ensure that none of the vari-ous amino acids can accumulate in the apoplast and serve as a source of nutrients for potential pathogens.
If fluxes can be modified, what is the aim?
Classically, it is assumed that the first enzymatic step in a pathway is rate limiting and has the highest control coefficient. However, this does not take into account that in many cases the first (or last) step in a metabolic pathway is the transport into or out of the cell or subcellular compartment. Theoretically, transporters are located at ideal strategic positions for control. The available transporter genes constitute an ideal set of tools for redirecting and modifing fluxes of amino acids in transgenic plants. Attractive long-term goals may therefore be specifically to adapt plants to local environ-ments (e.g. to improve nitrogen efficiency or salt-stress tolerance), or to alter the allocation and thus the quantity or quality of products.
Potatos are crops used for both food and starch production. Potatos accumulate a relatively low level of organic nitrogen in
tubers, but the relative contribution of soluble organic nitrogen (amino acids such as glutamate and aspartate) is approximately 50%. Thus, for food production, an optimized export of amino acids from leaves or an optimized allocation to tubers could be achieved by altering amino acid transporter gene expression. However, potato starch is also used for many non-food purposes. When potato tubers are used for starch production, the high levels of free amino acids are a serious ecological problem. Energy-con-suming techniques such as evaporation are required to concentrate the amino acids present in the tuber juice. A solution to this prob-lem would be to reduce the import of amino acids into the tubers. Besides amino acid transport, plants also use oligopeptides for rapid mobilization of protein-derived degradation products. Relevant genes have been cloned using yeast peptide-transport mutants45,46
. Antisense repression of AtPTR2a leads to severe problems during seed development, underlining the importance of oligopeptide transport in nitrogen allocation47
.
Future prospects
The remaining transporters will now mainly be identified by the Arabidopsis genome project, with the function of new genes iden-tified by sequence homology tested in yeast mutants and Xenopus laevis oocytes. Furthermore, comparison of the map positions of the genes isolated and of the mutations, together with detailed expression and localization studies, will provide a better under-standing of amino acid transport in plants. Systematic searches for knockout mutants will, however, be necessary to obtain a full under-standing of the role of the individual transporter genes48. Another
Table 4. Plant amino acid transport mutants
Mutant Species Selected on Properties Refs
aup Chlorella vulgaris L-canavanine Impaired in basic amino acid transport. 32
pup C. vulgaris L-azetidine-2- Impaired in alanine, glycine, proline and serine 32
carboxylic acid transport.
aec1a, aec1b Hordeum S-(2-aminoethyl)- Impaired in basic amino acid transport; unaffected in 35
vulgare cysteine transport of neutral and acidic amino acids.
aec1 Triticum aestivum cv. S-(2-aminoethyl)- Impaired in basic amino acid transport; Kmincreased 36
Chinese spring cysteine and Vmaxreduced; reduced uptake of several neutral
(hexaploid) and acidic amino acids.
Val-16 (recessive) Nicotiana Valine Deficient in transport of neutral amino acids. 37
plumbaginifolia Slightly affected in the uptake of basic amino acids.
cv. Viviani
ValR
-2 (recessive) Tobacco cv. Xanthi Valine Impaired in neutral and acidic amino acid transport; 38
low-affinity uptake (Km= 1.5 mM) is unaltered;
high-affinity system (Km= 40 mM) is strongly reduced;
slightly affected in transport of basic amino acids.
aec1 Potato (H2578) S-(2-aminoethyl)- Uptake deficiency not proven. 53 cysteine
azcaR Carrot cv. Lunga di Azetidine-2- Resistance to amino acid analogues: may represent 54
Amsterdam carboxylic acid mutations in general amino acid transporters or a secondary effect due to overproduction of amino acids (e.g. proline).
rlt1, raec1 Arabidopsis S-(2-aminoethyl)- Impaired in low-affinity transport of basic amino acids; 34, 55
(recessive; maps to cysteine high-affinity uptake of lysine is mediated by a general chromosome 1) amino acid transporter.
ValR Arabidopsis Valine High valine concentrations; uptake deficiency (not 56
proven).
raz1 Arabidopsis Azetidine-2- Affected in the high-affinity uptake of proline. 33
(semidominant; maps carboxylic acid to chromosome 5 at
important field is the analysis of the regulation of amino acid transport. Important questions waiting to be resolved can be approached now, such as whether AAPs involved in phloem load-ing of amino acids might be localized in sieve elements [similar to what has been described for the sucrose transporter SUT1 (Ref. 44)], or which transporters play a role in the uptake of amino acids into the endosperm or transfer cells in developing seeds29,49
. Yeast will continue to serve as a model system and perhaps as a tool for obtaining a better understanding of the regulation of plant amino acid transport. Knowledge gained from studies with Arabidopsis might eventually provide sufficient information for engineering improvements in crop species.
Acknowledgements
We would like to thank John M. Ward for criticism of this manuscript. This work was supported by grants from Deutsche Forschungsgemeinschaft to W.B.F. (Aminosäuretransport and SFB 446).
References
01 von Wirén, N., Gazzerini, S. and Frommer W.B. (1998) Regulation of mineral
nitrogen uptake in plants, Plant Soil, 196, 191–199
02 Andrews, M. (1986) The partitioning of nitrate assimilation between the root
and shoot of higher plants, Plant Cell Environ. 9, 511–519
03 Rosnick-Shimmel, I. (1985) The influence of nitrogen nutrition on the
accumulation of free amino acids in root tissue of Urtica dioica and their apical transport in xylem sap, Plant Cell Physiol. 26, 215–219
04 Lohaus, G., Burba, M. and Heldt, H.W. (1994) Comparison of the contents of
sucrose and amino acids in the leaves, phloem sap and taproots of high and low sugar-producing hybrids of sugar beet (Beta vulgaris L.), J. Exp. Bot. 45, 1097–1101
05 Pate, J.S. (1980) Transport and partioning of nitrogenous solutes, Annu. Rev. Plant Physiol. 31, 313–340
06 Bush, D.R. (1993) Proton-coupled sugar and amino acid transporters in plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 44, 513–542
07 Grenson, M. (1992) Amino acid transporters in yeast: structure, function and
regulation, in Molecular Aspects of Transport Proteins (De Pont, J.J.L.L.M., ed.), pp. 219–245, Elsevier
08 Grenson, M. (1973) Specificity and regulation of the uptake and retention of
amino acids and pyrimidines in yeast, in Genetics of Industrial
Microorganisms (Vanek, Z., Hostalek, Z. and Cudlin, J., eds), Prague
Academia
09 André, B. (1995) An overview of membrane transport proteins in Saccharomyces cerevisiae, Yeast 11, 1575–1611
10 Reizer, J. et al. (1993) Mammalian integral membrane receptors are
homologous to facilitators and antiporters of yeast, fungi, and eubacteria,
Protein Sci. 2, 20–30
11 Cosgriff, A.J. and Pittard, A.J. (1997) A topological model for the general
aromatic amino acid permease, AroP, of Escherichia coli, J. Bacteriol. 179, 3317–3323
12 Cherest, H. et al. (1997) Molecular characterization of two high affinity
sulfate transporters in Saccharomyces cerevisiae, Genetics 145, 627–635
13 André, B. et al. (1993) Cloning and expression of the UGA4 gene coding for
the inducible GABA-pecific transport protein of Saccharomyces cerevisiae,
Mol. Gen. Genet. 237, 17–25
14 Decottignies, A. and Goffeau, A. (1997) Complete inventory of the yeast ABC
proteins, Nat. Genet. 15, 137–145
15 Montesinos, M.L., Herrero, A. and Flores, E. (1997) Amino acid transport in
taxonomically diverse cyanobacteria and identification of two genes encoding elements of a neutral amino acid permease putatively involved in recapture of leaked hydrophobic amino acids, J. Bacteriol. 179, 853–862
16 Soussi Boudekou, S. et al. (1997) Gzf3p, a fourth GATA factor involved in
nitrogen-regulated transcription in Saccharomyces cerevisiae, Mol. Microbiol. 23, 1157–1168
17 Roberg, K.J., Rowley, N. and Kaiser, C.A. (1997) Physiological regulation of
membrane protein sorting late in the secretory pathway of Saccharomyces
cerevisiae, J. Cell Biol. 137, 1469–1482
18 Springael, J.Y. and André, B. Nitrogen-regulated ubiquitination of the Gap1
permease of Saccharomyces cerevisiae, Mol. Cell. Biol. (in press)
19 Hein, C. et al. (1995) NPI1, an essential yeast gene involved in induced
degradation of Gap1 and Fur4 permeases, encodes the Rsp5 ubiquitin–protein ligase, Mol. Microbiol. 18, 77–87
20 Hein, C. and André, B. (1997) A C-terminal di-leucine motif and nearby
sequences are required for NH4(
+)-induced inactivation and degradation of the
general amino acid permease, Gap1p, of Saccharomyces cerevisiae, Mol.
Microbiol. 24, 607–616
21 Rentsch, D., Boorer, K.J. and Frommer, W.B. Molecular biology of sucrose,
amino acid and oligopeptide transporters at the plasma membrane of plant cells, J. Membr. Biol. (in press)
22 Chang, H.C. and Bush, D.R. (1997) Topology of NAT2: a prototypical
example of a new family of amino acid transporters, J. Biol. Chem. 272, 30552–30557
23 Boorer, K.J. et al. (1996) Kinetics and specificity of a H+
/amino acid transporter from Arabidopsis thaliana, J. Biol. Chem. 271, 2213–2220
24 Boorer, K.J. and Fischer, W-N. (1997) Specificity and stoichiometry of the Arabidopsis H+/amino acid transporter AAP5, J. Biol. Chem. 272, 13040–13046
25 Rentsch, D. et al. (1996) Salt induced proline transporters and salt
stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant, Plant Cell 8, 1437–1446
26 Fischer, W-N. et al. (1995) Substrate specificity and expression profile of amino
acid transporters (AAPs) in Arabidopsis, J. Biol. Chem. 270, 16315–16320
27 Chen, L. and Bush, D.R. (1997) LHT1, a lysine and histidine specific amino
acid transporter in Arabidopsis, Plant Physiol. 115, 1127–1134
28 Bennett, M.J. et al. (1996) The Arabidopsis AUX1 gene: a permease-like
regulator of root gravitropism, Science 273, 948–950
29 Ozcan, S. et al. (1996) Two glucose transporters in Saccharomyces cerevisiae
are glucose sensors that generate a signal for induction of gene expression,
Proc. Natl. Acad. Sci. U. S. A. 93, 12428–12432
30 Frommer, W.B. et al. (1995) Seed and vascular expression of a high affinity
transporter for cationic amino acids in Arabidopsis, Proc. Natl. Acad. Sci.
U. S. A. 92, 12036–12040
31 Hirner, B. et al. Developmental control of H+
/amino acid permease gene expression during seed development of Arabidopsis, Plant J. (in press)
32 Sauer, N. and Tanner, W. (1985) Selection and characterization of Chlorella
mutants deficient in amino acid transport, Plant Physiol. 79, 760–764
33 Verbruggen, N. et al. (1996) The raz1 mutant of Arabidopsis thaliana lacks
the activity of a high-affinity amino acid transporter, Planta 200, 247–253
34 Heremans, B., Borstlap, A.C. and Jacobs, M. (1997) The rlt11 and raec1
mutants of Arabidopsis thaliana lack the activity of a basic-amino-acid transporter, Planta 201, 219–226
35 Bright, S.W.J., Kueh, J.S.H. and Rognes, S.E. (1983) Lysine transport in two
barley mutants with altered uptake of basic amino acids, Plant Physiol. 72, 821–824
36 Kumpaisal, R., Hashimoto, T. and Yamada, Y. (1989) Uptake of lysine by
wild-type and S-(2-aminoethyl)-L-cysteine-resistant suspension-cultured cells
of Triticum aestivum, Plant Cell Physiol. 30, 1099–1106
37 Marion-Poll, A. et al. (1988) Isolation and characterization of valine-resistant
mutants of Nicotiana plumbaginifolia, Theor. Appl. Genet. 75, 272–277
38 Borstlap, A.C. and Schuurmans, J. (1988) Kinetics of L-valine uptake in
tobacco leaf discs; comparison of wild type, the digenic mutant Valr -2 and its
monogenic derivatives, Planta 176, 42–50
39 Kuehn, M.J., Schekman, R. and Ljungdahl, P.O. (1996) Amino acid
permeases require COPII components and the ER resident membrane protein Shr3p for packaging into transport vesicles in vitro, J. Cell Biol. 135, 585–595
40 Siebke, K. and Weis, E. (1995) Assimilation images of leaves of Glechoma hederacea: analysis of non-synchronous stomata related oscillations, Planta
L
earning about the physical orga-nization of genes and repetitive sequences, regarded by some as ‘junk’, and seeing where the sequences lie, is a critical element for understanding genome organization and evolution in plants. Theapproach enables data to be linked from
Arabidopsis and the handful of smaller
genomes for which sequencing is under way with other genomes that are too large and too numerous to sequence at the present time (Fig. 1).
Species from wide taxonomic groupings have similar genes and arrangements of genes along the chromosomes – they show conserved synteny. However, knowledge of synteny – provided by high-density, marker-saturated genetic maps and genomic DNA sequence data – tells us relatively little about the large-scale physical organization of the chromosomes and the repetitive DNA el-ements that make up the bulk of most genomes. When a chromosome of an organ-ism such as wheat or pine is dissected at the molecular level, stretches of nucleotide se-quence that occur once or only a few times in the genome represent as little as 5% of the DNA. Most plant and animal genomes con-sist largely of repetitive DNA – perhaps 30 sequence motifs, typically one to 10 000 nucleotides long, present many hundreds or thousands of times in the genome – which may be located at a few defined chromo-somal sites or widely dispersed. However, this repetitive DNA, with different selective pressures from those acting on genes and evo-lutionarily successful multigene modules, can show extensive differences in sequence
motifs and abundance1–3even between closely
related species. The repetitive DNA in the genome is also important for evolutionary, genetic, taxonomic and applied studies.
41 Metzler, A. et al. (1995) Plant histochemistry by correlation peak imaging, Proc. Natl. Acad. Sci. U. S. A. 92, 11912–11915
42 Köckenberger, W. et al. (1997) A non-invasive measurement of phloem and
xylem water flow in castor bean seedlings by nuclear magnetic resonance microimaging, Planta 201, 53–63
43 Lohaus, G. et al. (1995) Further studies of the phloem loading process in
leaves of barley and spinach. The comparison of metabolite concentrations in the apoplastic compartment with those in the cytosolic compartment and in the sieve tubes, Bot. Acta 108, 270–275
44 Kühn, C. et al. (1997) Macromolecular trafficking indicated by localization
and turnover of sucrose transporters in enucleate sieve elements, Science 275, 1298–1300
45 Steiner, H.Y. et al. (1994) An Arabidopsis peptide transporter is a member of
a novel family of membrane transport proteins, Plant Cell 6, 1289–1299
46 Rentsch, D. et al. (1995) NTR1 encodes a high affinity oligopeptide
transporter in Arabidopsis, FEBS Lett. 370, 264–268
47 Song, W. et al. (1997) Antisense expression of the peptide transport gene AtPTR2-B delays flowering and arrests seed development in transgenic Arabidopsis plants, Plant Physiol. 114, 927–935
48 Krysan, P.H. et al. (1996) Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport, Proc. Natl. Acad Sci. U. S. A. 93, 8145–8150
49 Patrick, J.W. (1997) Sieve element unloading and post-sieve element
transport, Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 191–222
50 Frommer, W.B., Hummel, S. and Riesmeier, J.W. (1993) Expression cloning
in yeast of a cDNA encoding a broad specificity amino acid permease from
Arabidopsis thaliana, Proc. Natl. Acad. Sci. U. S. A. 90, 5944–5948 51 Hsu, L. et al. (1993) Cloning a plant amino acid transporter by functional
complementation of a yeast amino acid transport mutant, Proc. Natl. Acad.
Sci. U. S. A. 90, 7441–7445
52 Kwart, M. et al. (1993) Differential expression of two related amino acid
transporters with differing substrate specificity in Arabidospsis thaliana, Plant
J. 4, 993–1002
53 Jacobsen, E. (1986) Isolation, characterization and regeneration of an
S-(2-aminoethyl)cysteine resistant cell line of dihaploid potato, J. Plant Physiol. 123, 307–315
54 Cella, R., Parisi, B. and Nielsen, E. (1982) Characterization of carrot cell line
resistant to azetidine-2-carboxylic acid, Plant Sci. Lett. 24, 125–135
55 Heremans, B. and Jacobs, M. (1994) Selection to Arabidopsis thaliana (L.)
Hehnh. mutants resistant of aspartate-derived amino acid analogues, Plant Sci. 101, 151–162
56 Wu, K., Mourad, G. and King, J. (1994) A valine resistant mutant of Arabidopsis thaliana displays an acetolactate synthase with altered feedback
control, Planta 192, 249–255
Note added in proof
Information on two putative amino acid transporter cDNAs (RcAAP1 and
RcAAP2) from Ricinus communis has recently been published [Bick, J.A. et al.
(1998) Plant Mol. Biol. 36, 377–385].
Wolf-Nicolas Fischer, Doris Rentsch, Sylvia Krolkiewicz, Mechthild Tegeder, Kevin Breitkreuz and Wolf B. Frommer*are at the Botanical Institute, Eberhard Karls University, Auf der Morgenstelle 1, D-72076 Tübingen, Germany; Bruno André is at the Université Libre de Bruxelles, Laboratoire de Physiologie Cellulaire et de Genetique des Levures, Campus Plaine, CP244, Belgium.
*Author for correspondence (tel +49 7071 29 72605; fax +49 7071 29 3287; e-mail [email protected]).