February 2007
Treating Infectious Diseases
in a Microbial World
The vast majority of microorganisms do not cause disease. In fact, human evolution has occurred in the context of a world co-inhabited by thousands of types of microorganisms, and we couldn’t survive with-out them. Although there is an acute need to pursue new drugs to ight emerging diseases and increasing antibiotic resistance, the proposed idea of developing a superdrug that kills all microorganisms is at best limited and at worst seriously lawed. Instead, more nuanced approaches should be pursued that seek to understand and mimic the complex relationships that keep human health and microbial communities in a healthy balance.
T
he emergence of new infectious diseases like SARS and West Nile virus, combined with the potential threat of bioterrorist attacks and growing antibiotic resistance among disease-causing bacteria, highlights an acute need for the development of new antimicrobialtherapeutics to ight infectious agents. One might wish to identify one solution to all microbial threats to human health—a single “gorillacillin” drug that can protect against any infectious agent. But this type of antimicrobial superdrug could counteract the ancient and complex
relationship between microorganisms and human health, in which microorganisms also contribute
to protection against infection and maintenance of human health. Therefore, it is important to recognize the potential for inding novel
ways to ight infectious diseases through
a deeper understanding of the natural
interactions between the human body and
the microorganisms that dominate our
world. To that end, possible antimicrobial
therapies include those that are directed against the pathogenic microorganisms and those that are designed to boost the
immune responses in the patient. These two approaches provide complementary
and overlapping strategies in the treatment
of infectious diseases.
Living in a microbial world
Research in ields as diverse as evolu
-tionary biology, bacteriology, ecology, im
-munology and developmental biology has
led to a growing realization that humans
exist as part of an environment full of mi
croorganisms, the vast majority of which do not cause disease and many of which actually beneit human health. In fact, every human body contains many more bacterial cells than human cells. The microorganisms that overwhelmingly dominate our world have been evolving, coexisting, and competing with each other for millions of years. Humans have learned to take advantage of this long history: for example, most of
the antimicrobial agents that have revolutionized the treatment of infectious diseases in the past several decades are derived from bacterial products that have
been used by bacteria in their interactions with each other for millions of years. However, the ability of
bacteria to develop resistance to antimicrobial agents
is also an ancient evolutionary skill—one that presents a serious and growing threat to human health.
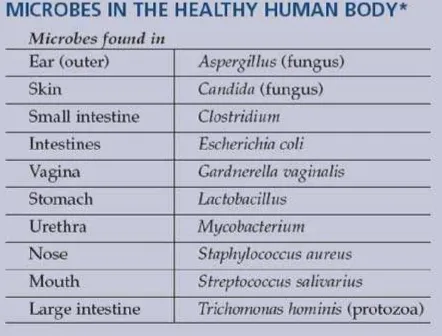
All humans live in intimate community with
thousands of microbial species—on our skins and in our guts and oral cavities—and these
microorgan-isms affect human health in many positive ways from development, to nutrition, to lowered susceptibility to disease. The human immune system has evolved in
the midst of this microbe-dominated world,
develop-ing highly nuanced and carefully regulated responses to the myriad microorganisms it encounters.
In such a microbial world, the idea of develop -ing a “gorillacillin” to eliminate all disease-caus-ing
microorganisms becomes hopelessly complicated. How would such a drug distinguish microbial friend from foe? How would it outwit the varied defense tactics developed over millions of years by thousands
of microorganisms? Would this “gorrillacillin”
im-prove or diminish the performance of the highly com
-plicated human immune system? How long would
it remain effective before harmful microorganisms evolve resistance to it? These questions are daunting,
even discouraging. At the same time, antibiotics have saved millions of lives and interventions exploiting the human immune system—especially immuniza
-tion—have vastly reduced human vulnerability to infectious disease. If “gorillacillin” is an unrealistic—
perhaps even an undesirable—goal, it is nevertheless clear that effective antimicrobial therapeutics have
been and can again be developed.
Identifying new ways of ighting disease
At the request of the National Institute of Allergy and Infectious Diseases, two committees established by the National Academies organized
workshops to help generate ideas for innovative research approaches to the development of
antimicro-bial therapeutics. One workshop focused on potential new classes of antibiotics, while the other explored the possibility of treating infectious diseases by affecting the response of the immune system.
The road from a brilliant idea to a clinically available treatment is long and full of pitfalls. Dif -fering approaches to antibiotic use in different
coun-tries, declining investment in antimicrobials by large
pharmaceutical companies, increasing costs of clinical
trials, and complicated regulatory and legal environ -ments are just a few of the obstacles to bringing new
compounds from the laboratory to the clinic. Interest -ing and important as these issues are, the workshops
were not designed to address them—rather they focused on the scientiic possibilities. Readers inter
-ested in the non-scientiic aspects of drug develop -ment are referred to related reports from the National
Academies and other sources.
At each workshop, participants considered the
current state of knowledge, identiied approaches that
have been successful in the past, and brainstormed
about ways in which new areas of research could revolutionize the treatment of infectious disease. The recommendations put forward by each committee emerged independently from their respective work
-shop discussions.
Related publications from the National Academies are available
at www.nap.edu. The interested reader is also referred to Infectious Diseases Society of America, Bad Bugs, No Drugs: As Antibiotic Dis-covery Stagnates…A Public Health Crisis Brews (http://www.idsociety. org/badbugsnodrugs).
If “gorillacillin” is an unrealistic—
perhaps even an undesirable—goal, it is
nevertheless clear that effective
antimi-crobial therapeutics have been and can
again be developed.
Short-term gains: improving current
approaches
Some of the committees’ recommendations
relect ways in which current approaches to develop
-ing antibiotics and ways of inluenc-ing the immune system’s response to pathogens could be improved. Implementation of recommendations of this type is most likely to provide improved therapeutics in the short term. For example,
• Many successful antibiotics have been dis
-covered by studying natural products. The emerging ield of metagenomics offers the possibility of discov
-ering gene products with antibiotic activity without having to culture individual organisms.
• Generating slight chemical variations of
compounds with promising activity frequently results in more effective drugs; new chemical synthesis ap
-proaches that allow the rapid synthesis of more varied structures could speed this process.
• Immunization, both passive and active, has been hugely successful, but could be improved with enhanced understanding of exactly how different anti
-bodies function and how they interact with the innate immune system (the non-speciic or general immune system).
Long-term gains: basic research and novel
approaches
Other recommendations relect the committees’
judgments as to which areas of basic research are most
likely to lead to genuinely novel approaches to infec
-tious disease treatment. Because the outcome of such basic research is dificult to predict, these approaches
might be labeled “high-risk,” but have the potential
also to yield great reward in the long term.
• Current antibiotic development concentrates on targets that are essential for bacterial metabo-lism; research into how bacteria communicate with
each other may allow the development of drugs that confuse, rather than kill, bacteria. Such drugs might
be less likely to provoke resistance, because bacteria with the ability to resist such drugs would not neces
-sarily be any more likely to survive than non-resistant bacteria.
• The human immune system is constantly
interacting with the thousands of bacterial species
naturally living within the human body; understand -ing how these bacteria communicate with the immune
system and how the immune system singles out harm -ful microorganisms could lead to drugs that help the
bacteria in our bodies outcompete pathogens. • Once considered primitive, the innate im
-mune system (the non-speciic or general im-mune system) is increasingly regarded as highly complex, regulated and intimately intertwined with the acquired (speciic or targeted) immune system and the nervous system. Understanding innate immune system regula
-tory pathways and active molecules may lead to drugs that are effective against a wide array of infectious agents.
• Development of techniques that affect the re
-sponse of the immune system must be conducted care
-fully to avoid unintended harmful effects of artiicially boosting natural immune responses. Overstimulation of the immune system can disrupt natural balances and present a danger to the host.
Improving diagnostic tools
Both workshops highlighted the value of im -proved diagnostics and recommended new approaches
to identifying disease-causing agents.
• Rapid diagnostic tools to allow identiica -tion of disease-causing microorganisms would make
it possible for physicians to reduce the use of
broad-spectrum antibiotics and to encourage the use and
development of narrowly-targeted therapeutics. • Diagnostic proiles that describe the immune
status of the patient could make it possible to predict how effective different treatments will be and to target
drugs that affect the response of the immune system to the right patients at the right time.
Addressing challenges of clinical
development
Both workshops identiied imperfections in many of the techniques currently used to evalu
-ate antimicrobial compounds. For example, testing
antimicrobial compounds against microorganisms
grown under ideal laboratory conditions does not relect the reality of pathogens competing against the
natural microbiota in human tissue under pressure
from the immune system. Participants also identiied “Click chemistry”: a method of synthetically building
new compounds by joining small units together. This technique represents one approach to the rapid syn-thesis of promising antimicrobial compounds.
Metagenomics: an emerging ield in which the power
Committee on New Directions in the Study of Antimicrobial Therapeutics: New Classes of Antimicrobials Christopher T. Walsh - (Chair), Harvard Medical School; Bonnie L. Bassler, Princeton University; Carl F. Nathan, Weill Medical College of Cornell University; Thomas F. O’Brien, Brigham and Women’s Hospital,
Boston; Margaret Riley, University of Massachusetts, Amherst; Richard J. White, Vicuron Pharmaceuticals; Gerard D. Wright, McMaster University; Adam P. Fagen (Study Director), National Research Council.
Committee on New Directions in the Study of Antimicrobial Therapeutics: Immunomodulation
Arturo Casadevall - (Chair), Albert Einstein College of Medicine; Rita R. Colwell, University of Maryland,
College Park; R.E.W. (Bob) Hancock, University of British Columbia; Margaret Jean McFall-Ngai, Uni
-versity of Wisconsin, Madison; Carl F. Nathan, Weill Medical College of Cornell University; Liise-Anne Pirofski, Albert Einstein College of Medicine; Arthur Tzianabos, Harvard Medical School and Brigham and
Women’s Hospital, Boston; Dennis M. Zaller, Merck Research Laboratories; Ann H. Reid (Study Director),
National Research Council.
This report brief was prepared by the National Research Council based on the committees’ report. For
more information, contact the Board on Life Sciences at bls@nas.edu or visit http://nationalacademies.org/ bls. Treating Infectious Disesases in a Microbial World: Report of Two Workshops on Novel Antimicrobial Therapeuticsis available from the National Academies Press, 500 Fifth Street, NW, Washington, D.C.
20001; (800) 624-6242; www.nap.edu. Support for this publication was provided by the Presidents’ Circle Communications Initiative of the National Academies.
© 2007 The National Academy of Sciences
the challenges in developing and validating animal
models for studying diseases. It is also dificult
to design and evaluate clinical trials of drugs that are designed to affect the response of the immune
system—compounds that affect the highly complex and individually variable immune system because such “personalized” therapies do not easily it
within the traditional model of drug development
and testing.
Conclusions
Many of the most effective solutions will
require the integration of antimicrobials and
ap-proaches that inluence the response of the immune system. For example, many immune-system affect
-ing drugs may not be able to cure disease directly,
but could be effective in combination with
tradi-tional antimicrobials. Similarly, it is possible that
researchers could develop antibiotics that would be
selectively activated through interaction with the compounds used by the immune system to signal damage. Future discussions of infectious disease
treatments that target the disease-causing agent and enhance the immune response at the same time
could generate even more promising ideas.
The committees’ recommendations address
new approaches to ighting disease from a variety
of angles, from improved diagnostics to greater
utilization of the natural immune system to devel
-opment and testing of antibiotics. New technologi -cal advances such as high-throughput screening
and innovative chemistry techniques can allow
rapid generation and testing of multiple variants
of promising antimicrobial compounds. Emerg -ing areas of research such as metagenomics offer
insights into a variety of microbial communities previously inaccessible to medical research. Basic
research in areas such as bacterial communication
and the workings of the natural immune system can provide the basis for novel approaches to ighting pathogens.
The incredible array of microorganisms that is found in every corner of our world—including inside the human body—offers a huge potential for developing ways to use beneicial microorgan
-isms and the long history of microbial evolution to our advantage in treating infectious diseases. By exploring the complex relationships between
human health and microorganisms, we can hope to discover new approaches that avoid the pitfalls
of traditional approaches to ighting infection by killing all microbes, such as antibiotic resistance. A
deeper understanding of the microbial communities within our bodies and in our environment would