Hydrogen Production through the Water
-
Gas Shift Reaction:
Thermodynamic Equilibrium versus Experimental Results over
Supported Ni Catalysts
Agus Haryanto,
†,‡Sandun D. Fernando,*
,§S. D. Filip To,
†Philip H. Steele,
|Lester Pordesimo,
†and Sushil Adhikari
⊥Agricultural and Biological Engineering Department, and Department of Forest Products, Mississippi State UniVersity, Mississippi State, Mississippi 39762, Agricultural Engineering Department, UniVersity of Lampung, Bandar Lampung 35145, Indonesia, Biological and Agricultural Engineering Department, Texas
A&M UniVersity, College Station, Texas 77843, and Biosystems Engineering Department, Auburn UniVersity, Auburn, Alabama 36849
ReceiVed December 9, 2008. ReVised Manuscript ReceiVed March 5, 2009
This paper discusses the experimental results of the water-gas shift reaction over supported nickel catalysts
in comparison to thermodynamic equilibrium composition at the same reaction conditions. The effects of different supports on the performance of H2production over nickel-supported catalysts are also evaluated at both low
and high temperatures. Ceria-promoted nickel catalyst supported on powder alumina (Ni/CeO2-Al2O3)
demonstrated excellent performance. The catalyst was not stable at low temperature (250 °C) but showed good stability at high temperature (450°C). At 450°C, with a catalyst loading of 0.05 g, CO/S (S)steam) ratio of 1:3, and gas hourly space velocity (GHSV)≈200 L h-1g
cat-1, the activity of Ni/CeO2-Al2O3was
95% with a H2yield of 52% and a H2selectivity of 73%.
Introduction
The water-gas shift (WGS) reaction is an established industrial technology, in which water (H2O), in the form of
steam, is reacted with carbon monoxide (CO) to produce hydrogen (H2) and carbon dionoxide (CO2). The reaction is
presented below.1-3
Recently, the WGS reaction has attracted renewed interest because of rapid innovations in fuel cell technology. CO existent in the synthesis gas produced via steam reforming of hydro-carbons (e.g., natural gas, petroleum, or renewable resources) and gasification of coal or biomass is poisonous to the catalysts used in fuel cells. The benefit of using the WGS reaction is that it reduces the CO concentration while producing extra H2,
which is a fuel for hydrogen fuel cells.
In industrial processes, it is important to have high reaction rates and to obtain maximum concentrations of the desired
products. To produce high-purity hydrogen at the highest possible CO conversions, two-stage adiabatic reactors, with cooling in between, are used. These reactors consist of a high-temperature shift (HTS) section operated at 320-450°C with a catalyst based on iron oxide structurally promoted with chromium oxide (Fe2O3-Cr2O3) and a low-temperature shift
(LTS) section operating in a temperature range of 200-250°C with copper-zinc oxide supported on an alumina (Cu-ZnO/ Al2O3) catalyst.4-6Typical designs of HTS WGS reactors with
Fe2O3-Cr2O3catalysts reduce the CO content from 8-10 to
3-5%, while a LTS reactor with Cu-ZnO/Al2O3 catalysts
further decrease the CO level to less than 1%.
In previous works,7,8 we demonstrated that noble metal
catalysts, especially platinum (Pt) and ruthenium (Ru), and transition-metal catalysts [nickel (Ni)] showed good performance for the WGS reaction at high temperatures (700°C). Because of its low price compared to noble metals, it is reasonable to develop further the Ni catalyst. Moreover, our observation was
* To whom correspondence should be addressed. Telephone:+ 1-979-845-9793. Fax:+1-979-845-3932. E-mail: [email protected].
†Agricultural and Biological Engineering Department, Mississippi State University.
‡University of Lampung. §Texas A&M University.
|Department of Forest Products, Mississippi State University. ⊥Auburn University.
(1) Panagiotopoulou, P.; Kondarides, D. I. Effect of morphological characteristics of TiO2-supported noble metal catalysts on their activity for the water-gas shift reaction.J. Catal.2004,225, 327–336.
(2) Wheeler, C.; Jhalani, A.; Klein, E. J.; Tummala, S.; Schmidt, L. D. The water-gas shift reaction at a short contact time.J. Catal.2004,223, 191–199.
(3) Jacobs, G.; Williams, L; Graham, U; Sparks, D.; Davis, B. H. Low-temperature water-gas shift: In-situ DRIFTS-reaction study of a Pt/CeO2 catalyst for fuel cell reformer applications.J. Phys. Chem. B2003,107, 10398–10404.
(4) Qi, X; Flytzani-Stephanopoulos, M. Activity and stability of Cu -CeO2catalysts in high-temperature water-gas shift for fuel-cell applications.
Ind. Eng. Chem. Res.2004,43, 3055–3062.
(5) Quadro, E. B.; de Lourdes, M.; Dias, R.; Amorim, A. M. M.; do Carmo Rangel, M. Chromium and copper-doped magnetite catalysts for the high temperature shift reaction.J. Braz. Chem. Soc.1999,10(1), 51– 59.
(6) Bustamante, F.; Enick, R. M.; Killmeyer, R. P.; Howard, B. H.; Rothenberger, K. S.; Cugini, A. V.; Morreale, B. D.; Ciocco, M. V. Uncatalyzed and wall-catalyzed forward water-gas shift reaction kinetics.
AIChE J.2005,51(5), 1440–1454.
(7) Haryanto, A.; Fernando, S.; Adhikari, S. Ultrahigh temperature water gas shift catalysts to increase hydrogen yield from biomass gasification.
Catal. Today2007,129, 269–274.
(8) Haryanto, A.; Fernando, S.; Adhikari, S. H2yield from water gas shift reaction over bimetallic Pt-Ru and Ni catalysts supported on ceria-alumina at high temperatures. Presented at the Institute of Biological Engineering (IBE) Meeting, St. Louis, MO, March 29-April 1, 2007.
10.1021/ef801076r CCC: $40.75 2009 American Chemical Society Published on Web 04/27/2009
reinforced by a recent review work by Davda et al. that reported that Ni catalysts had higher activity in WGS reactions than noble metals, such as Pt or Rh (rhodium).9
Nickel catalysts are commonly used in steam reforming of natural gas. However, Ni also plays an important role in the WGS reaction. For example, Willms reported that Ni was a good catalyst for producing H2either through the WGS reaction or
the steam-reforming process.10Cooper also noted that Ni, which
forms a part of the anode composition, facilitated the WGS reaction to take place on the surface of the anode of solid oxide fuel cells.11The existence of Ni in the CeO
2-supported bimetallic
Ni-Rh catalyst reportedly helped conversion of CO into CO2
and H2by the WGS reaction.12
Chu et al. observed that Ni/ceria had a higher activity for the WGS reaction than Fe/ceria. Nickel catalysts, however, seemed to be good for HTS than for the LTS reaction.13Li et
al. investigated the use of ceria-lantana-supported catalysts in the WGS reaction at gas hourly space velocities (GHSVs) of 8000 and 80 000 h-1and a temperature range of 150-550°C.
It was found that the Ni-Ce(La)Oxcatalyst was much superior than the support itself, i.e., Ce(La)Ox. It was also observed that, at around 350°C, the activity of the Ni-loaded catalyst surpassed the activity of the Cu-loaded one.14
The aim of this research was to compare thermodynamic equilibrium compositions and experimental results of the WGS reaction using nickel catalysts. Analysis of thermodynamic equilibrium was performed over the temperature range of 27-1227°C (300-1500 K) and CO/S molar ratios of 1:1-1: 9. Experiments were carried out at temperatures of 250 and 450°C with a CO/steam (CO/S) molar ratio of 1:3.
Experimental Section
Thermodynamic Equilibrium. Thermodynamic equilibrium composition of a reaction is accomplished when Gibb’s energy function (G) achieves a minimum point. The derivation of Gibb’s
energy function was performed previously,15and the function can
be expressed as follows:
where Gi0 is the Gibb’s free energy for species i at standard
conditions,niis the number of moles of speciesi,yiis the mole
fraction of speciesi,Ris the universal gas constant (8.3144 J mol-1
K-1),Tis the absolute temperature, andPis the pressure (in atm).
For an equimolar reaction, as presented by eq 1, equilibrium CO conversion (X) can be calculated using eq 316 and the
compositions of output gases are determined using the mass balance principle
wherewis the number of moles of water andkeqis the equilibrium
constant and is determined using the following relations:17
and
Experimental Procedures. Nine nickel-based catalysts, with compositions as presented in Table 1, were prepared by an incipient wetness technique. Monolith alumina (from Vesuvius Hi Tech Ceramics, Champaign, IL) was crushed and sieved to obtain particle sizes of 20-60 mesh. Other supports including ceria-zirconia
(CeZrO4), ceria-yttria (CeYO5), ceria-(20%) gadolinia,
ce-ria-(15%) samaria [all from Sigma Aldrich (St. Louis, MO)], and
ceria particles (NanoScale Materials, Manhattan, KS) were used as received. Cerium nitrate hexahydrate [Ce(NO3)3·6H2O]
(Sigma-Aldrich) was used as the ceria promoter for Ni supported on alumina catalysts. The Ni precursor for this experiment was nickel nitrate hexahydrate [Ni(NO3)2·6H2O] (Sigma-Aldrich).
The Ni precursor was diluted with distilled water to make a solution with a Ni concentration of 3.85% (w/w). The solution was used for loading Ni into the supports. The Ni loading for all catalysts was 4% (w/w). The catalysts were dried at 75°C overnight. For ceria-promoted catalysts, ceria (3%, w/w) was impregnated and dried prior to Ni loading. The catalysts were calcined at 500°C in a muffle furnace for 3 h. Except for catalysts supported on monolith alumina, the catalysts were pelletized, crushed, and sieved to obtain particle sizes between 20 and 60 mesh.
(9) Davda, R. R.; Shabaker, J. W.; Huber, G. W.; Cortright, R. D.; Dumesic, J. A. A review of catalytic issues and process conditions for renewable hydrogen and alkanes by aqueous-phase reforming of oxygenated hydrocarbons over supported metal catalysts.Appl. Catal., B2005,56, 171– 186.
(10) Willms, R. S.; Wilhelm, R.; Okuno, K. Performance of a palladium membrane reactor using a Ni catalyst for fusion fuel impurities processing. Presented at the Third International Symposium on Fusion Nuclear Technology, Los Angeles, CA, June 27-July 1, 1994.
(11) Cooper, R. J.; Billingham, J.; King, A. C. Flow and reaction in solid oxide fuel cells.J. Fluid Mech.2000,411, 233–262.
(12) Kugai, J.; Velu, S.; Song, C. Low-temperature reforming of ethanol over CeO2-supported Ni-Rh bimetallic catalysts for hydrogen production.
Catal. Lett.2005,101, 255–264.
(13) Chu, D.; Lee, I. C.; Pati, R. K.; Ehrman, S. H. Ceria based nano-scale catalysts for water-gas shift (WGS). http://www.asc 2004.com/ Manuscripts/sessionM/MP-03.pdf (accessed on Jan 5, 2004).
(14) Li, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Low-temperature water-gas shift reaction over Cu- and Ni-loaded cerium oxide catalysts.
Appl. Catal., B2000,27, 179–191.
(15) Adhikari, S.; Fernando, S.; Haryanto, A. A comparative thermo-dynamic and experimental analysis on hydrogen production by steam reforming of glycerin.Energy Fuels2007,21, 2306–2310.
(16) Haryanto, A.; Fernando, S.; Adhikari, S. Ultrahigh temperature water gas shift catalysts to increase hydrogen yield from biomass gasifica-tion.Catal. Today2007,129(3-4), 269–274.
(17) Twigg, M. V.Catalyst Handbook, 2nd ed.; Wolf Publishing Ltd.: Frome, U.K., 1989; p 608.
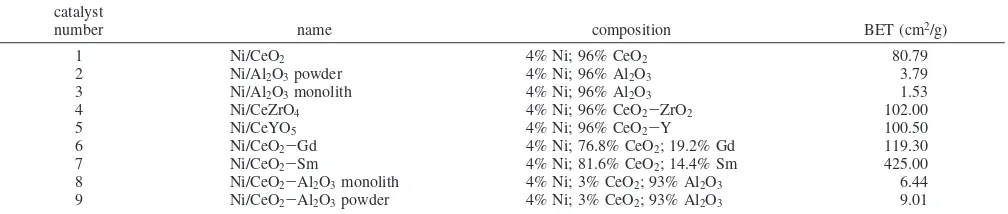
Table 1. Composition of Prepared Nickel-Based Catalysts and Their BET Surface Areas
catalyst
number name composition BET (cm2/g)
1 Ni/CeO2 4% Ni; 96% CeO2 80.79
2 Ni/Al2O3powder 4% Ni; 96% Al2O3 3.79
3 Ni/Al2O3monolith 4% Ni; 96% Al2O3 1.53
4 Ni/CeZrO4 4% Ni; 96% CeO2-ZrO2 102.00
5 Ni/CeYO5 4% Ni; 96% CeO2-Y 100.50
6 Ni/CeO2-Gd 4% Ni; 76.8% CeO2; 19.2% Gd 119.30
7 Ni/CeO2-Sm 4% Ni; 81.6% CeO2; 14.4% Sm 425.00
8 Ni/CeO2-Al2O3monolith 4% Ni; 3% CeO2; 93% Al2O3 6.44
9 Ni/CeO2-Al2O3powder 4% Ni; 3% CeO2; 93% Al2O3 9.01
G )
∑
3098 Energy & Fuels, Vol. 23, 2009 Haryanto et al.
Catalyst surface areas were measured using an Autosorb-1C (Quantachrome, Boynton Beach, FL) surface area analyzer. The surface areas were measured by the Brunauer-Emmett-Teller
(BET) method using nitrogen adsorption isotherms. Prior to surface area measurements, samples were degassed at 300°C for 3 h. In addition, to measure carbon deposits on the spent catalysts, a thermogravimetric analysis (TGA) were performed on a TG/DTA 6300 instrument (Perkin-Elmer Instrument, Wellesley, MA). The system was capable of measuring the change in mass of a sample as a function of the temperature up to 1100 °C. Temperature ramping of 20 °C/min with an air flow rate of 100 cc m-1
was used.
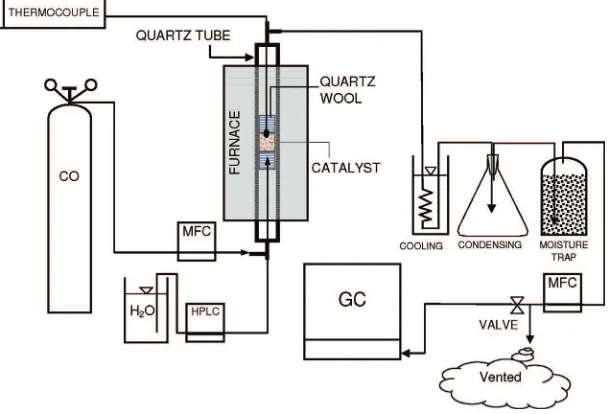
The catalysts test setup is shown in Figure 1. The reactor consisted of a quartz tube from Technical Glass Products (Paines-ville Township, OH) with 12.51 mm outside diameter, 10.01 mm inside diameter, and 50 cm length. Bids of quartz wool were used to hold the catalyst in the tube. A tube furnace (Lindberg Blue M 33850-00, Cole Parmer, Vernon Hill, IL) capable of reaching 1100 °C was used as a heat source for the reactor. A thermocouple was inserted inside the tube to measure the actual reaction temperature. The testing was conducted at a low temperature (250°C) and a high temperature (450°C), at a CO flow rate of 15 cm3at a steam
flow rate of 0.04 mL/min, and at a catalyst loading of 1 g diluted in 1.5 g of fused SiO2(Sigma Aldrich). Water was delivered using
a high-pressure liquid chromatograph (HPLC) pump (LC- 20AT, Shimadzu Scientific Instrument, Columbia, MD), while the CO flow rate was maintained using a mass flow controller (MFC, Cole Parmer). To avoid condensation of unreacted steam in the reactor tube, the section of the quartz tube protruding out from the furnace was wrapped with a heating tape and insulated.
The output gas was cooled, dried, and then measured with another MFC. A large portion of the gas was vented, and a smaller portion was routed to the gas chromatograph (GC, GC6890N, Agilent Technologies, Inc., Palo Alto, CA). The GC used a thermal conductivity detector (TCD) to detect H2and a flame ionization
detector (FID) to detect CO, CH4, and CO2. Helium and nitrogen
were used as carrier gases for the FID and TCD, respectively. Three columns including HP-Molsiv (30×0.53), HP-Plot Q (30×0.53),
and HP-Plot Q (15×0.53) were used in the GC.
The catalyst performance is demonstrated by catalyst activity, H2 yield (vol %), and H2 selectivity. The catalyst activity is
presented as CO conversion (XCO) and defined as follows:
Hydrogen selectivity (SH2) is defined as follows:
Here, [H2]maxis the maximum H2yield based on thermodynamic
equilibrium at the respected temperature and CO/S ratio.
Results and Discussion
Figure 2 presents thermodynamic CO conversion between 27 and 1227°C for different CO/S molar ratios (1:1-1:9). On the basis of eq 1, it can be inferred that H2yield (vol %, dry)
can be related to CO conversion (XCO) as follows:
This means a 100 and 50% CO conversion results in 50 and 33% (v/v, dry) H2yield, respectively.
From the figure, it can be observed that increasing the reaction temperature resulted in decreasing CO conversion and, therefore, H2 yield. This provides evidence that the WGS reaction is
favorable at low temperature. For instance, with the CO/S molar ratio of 1:3, the CO conversion was 99% at 250°C and 94% at 450°C. It can also be observed that increasing the number of moles of steam positively influenced CO conversion at the same temperature. For example, decreasing steam from 3 to 1 mol reduced the CO conversion from 99 to 90% and from 94 to 73%, respectively, at 250 and 450°C. Increasing the steam flow
Figure 1.Schematic of the experimental setup for the WGS reaction.
Figure 2.Equilibrium CO conversion (XCO) of the WGS reaction at atmospheric pressure, at temperatures of 27-1227°C, and at CO/steam molar ratios of 1:1-1:9.
XCO) [CO]in
-[CO]out
[CO]in ×100% (6)
SH2) [H2]yield
[H2]max ×100% (7)
H2yield (%, v/v)) XCO
1+XCO (8)
rate, however, has resulted in little effect on CO conversion. Besides, increasing the steam flow rate implies that more energy is required to evaporate water. Therefore, we considered the CO/S ratio of 1:3 to be reasonable for use in our experiment. Figure 3 shows the initial CO conversion for the prepared Ni catalysts at low temperature (250°C). It can be seen that Ni supported on powder alumina, with or without ceria promoter, showed the highest activity. With a catalyst loading of 1 g and GHSV of 7500 cm3h-1g
cat-1, the catalysts had activities of
96 and 92%, with and without ceria promoter, respectively. The
activity of ceria-promoted Ni catalyst supported on powder alumina (96%) was quite close to the equilibrium CO conversion (99.6%) at the same temperature (250°C) and CO/S molar ratio (1:3).Allofceria-supportedcatalysts(includingceria,ceria-zirconia, ceria-yttria, ceria-gadolinia, and ceria-samaria) showed a comparable activity of around 80%. The use of the monolith alumina as the support resulted in catalysts having the lowest activities (around 60%).
As presented in Figure 4, the catalyst activity was related closely to the BET surface area. The higher the BET surface area, the more active the catalyst. The BET surface area, however, was not the only factor affecting catalyst activity. With a lower BET surface area, Ni supported on powder alumina still produced the highest activity. Atomic structure, which was beyond our focus, was likely responsible for the behavior of these catalysts.
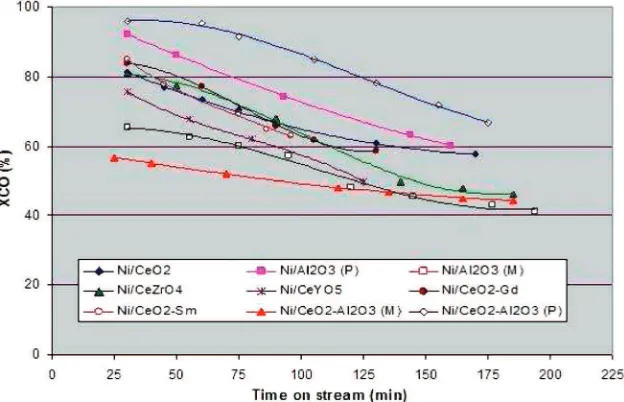
All of the prepared Ni catalysts, however, were not stable at low temperature (250 °C). Figure 5 shows that the catalyst activity decreased quickly. The decrease in CO conversion led to a conjecture that the catalyst was deactivated because of carbon deposition. Therefore, a TGAs were performed to test the above supposition. The weight profiles resulting from TGAs (Figure 6), however, revealed that there were no dramatic mass changes above 400°C, where carbon burned, for most catalysts. The only case for the presence of carbon deposits occurred with catalyst number 7 (Ni/CeO2-Sm). For this catalyst, carbon
deposition was 4.77% (by weight). This implied that the deactivation for most catalysts was not due to carbon deposition. A better reason for catalyst deactivation was chemical changes at the catalysts surface because of CO binding. This happened, for example, with platinum catalysts, which had a higher affinity toward CO at low temperatures, resulting in a binding of the catalyst active sites with CO.18,19
We observed that with 1 g of catalyst loading, some catalysts initially produced an extremely high activity. The activity was expected to be higher during the high-temperature (450°C) test. For this reason, when testing at 450 °C, the catalyst loading was reduced to 50 mg, while other conditions were kept unchanged. The results of that experiment are presented in Figure 7 (averaged over around 12 h).
It can be observed that at high temperature (450°C) all catalysts showed better performance than at low temperature (250°C). Four catalysts including Ni/CeO2-Al2O3(powder), Ni/CeO2, Ni/CeYO5,
Figure 3.Initial CO conversion with different nickel catalysts at 250°C (conditions: catalyst loading, 1 g; CO/steam molar ratio, 1:3; and GHSV, 7500 cm3h-1gcat-1).
Figure 4.Initial CO conversion versus BET surface area of different Ni catalysts at 250°C (conditions: catalyst loading, 1 g; CO/steam molar ratio, 1:3; and GHSV, 7500 cm3h-1gcat-1).
Figure 5.CO conversion versus time on stream for different Ni catalysts at 250°C (conditions: catalyst loading, 1 g; CO/steam molar ratio, 1:3; and GHSV, 7500 cm3h-1gcat-1).
3100 Energy & Fuels, Vol. 23, 2009 Haryanto et al.
and Ni/CeO2-Gd exhibited very good activity. Except for Ni/
CeO2-Gd, the other three catalysts demonstrated extremely high
H2yield and good stability over the 12 h test period. As can be
observed from Figure 8, Ni/CeO2-Gd also took a longer time to
reach steady state. Two catalysts, Ni/CeO2-Al2O3(powder) and
Ni/CeO2, had H2selectivity>70%.
In general, the ceria-promoted Ni catalyst supported on alumina powder demonstrated the best performance in the HTS WGS reaction. At 450°C and a CO/S ratio of 1:3, the catalyst
had an average activity of 95%, H2yield of 52% (v/v), and H2
selectivity of 73%. At the same conditions, the equilibrium CO conversion was 94% with a H2yield of 50%. The differences
may have been the result of inaccuracies with flow rate readings, which, in fact, fluctuated from the set point during the experiment. Our results, with an acceptable error, suggested that the performance of the Ni/CeO2-Al2O3catalyst was extremely
active in the HTS WGS reaction and achieved equilibrium CO conversion even at very little loading (0.05 g).
It can also be observed that catalysts without ceria [catalyst number 2 (Ni/Al2O3, powder) and catalyst number 3 (Ni/Al2O3,
monolith)] had the lowest H2 yield, CO conversion, and H2
selectivity compared to those supported on or promoted with ceria. This observation provides evidence that the presence of ceria was advantageous for WGS catalysts. The beneficial role of ceria for WGS catalyst has been reported, among others, by Hilaire et al.,20Gorte and Zhao,21and Swartz et al.22
Most catalysts, however, produced unwanted CH4. The evolution
of CH4formation during the WGS reaction is presented below.
(18) Ralph, R.; Hogarth, M. P. Catalysis for low temperature fuel cells. Part II: The anode challenges.Platinum Met. ReV.2002,46, 117–135.
(19) Si, Y.; Jiang, R.; Lin, J.-C.; Kunz, H. R.; Fenton, J. M. CO tolerance of carbon-supported platinum-ruthenium catalyst at elevated temperature and atmospheric pressure in a PEM fuel cell.J. Electrochem. Soc.2004,
151, A1820–A1824.
(20) Hilaire, S.; Wang, X.; Luo, T.; Gorte, R. J.; Wagner, J. A comparative study of water-gas-shift reaction over ceria-supported metallic catalysts.Appl. Catal., A2004,258, 271–276.
(21) Gorte, R. J.; Zhao, S. Studies of the water-gas-shift reaction with ceria-supported precious metals.Catal. Today2005,104, 18–24.
Figure 6.Catalyst weight profiles resulting from TGAs [catalysts are numbered as follows: (1) Ni/CeO2, (2) Ni/Al2O3powder, (3) Ni/Al2O3monolith, (4) Ni/CeZrO4, (5) Ni/CeYO5, (6) Ni/CeO2-Gd, (7) Ni/CeO2-Sm, (8) Ni/CeO2-Al2O3monolith, and (9) Ni/CeO2-Al2O3powder].
Figure 7.Average performance of Ni catalysts for the WGS reaction at 450°C (conditions: catalyst loading, 0.05 g; CO/steam molar ratio, 1:3; and GHSV, 207 L h-1gcat-1). Note that the H2yield is expressed in vol/vol.
Figure 8.Catalyst stability at 450°C for about 12 h (conditions: catalyst loading, 0.05 g; CO/steam molar ratio, 1:3; and GHSV, 207 L h-1 gcat-1).
Figure 9. Average CH4 yield for different catalysts at 450 °C (conditions: catalyst loading, 0.05 g; CO/steam molar ratio, 1:3; and GHSV, 207 L h-1gcat-1).
Tanaka and Iizuka23suggested that, after the WGS reaction,
the formation of CH4occurred through the hydrogenation of
carbonaceous species formed by the dissociation of CO or CO2.
All of the aforementioned methanation routes require H2.
Therefore, the higher the CH4yield, the lower the H2yield.
Figure 9 shows that the monolith alumina-supported catalysts (with or without ceria promotion) produced the highest CH4
yield, around 5 vol % on average. This was another indicator
of a potential drawback of using monolith alumina as a support for Ni catalysts.
Conclusions
It can be concluded that ceria-promoted Ni catalyst supported on alumina powder (Ni/CeO2-Al2O3, Ni/CeO2, and Ni/CeYO5)
demonstrated the best performance for the WGS reaction in terms of activity, H2yield, and H2selectivity. The catalysts were
stable at high temperature (450 °C) but unstable at low temperature (250°C). With catalyst loading of 0.05 g and GHSV of 207 L h-1g
cat-1, 4% Ni/CeO2-Al2O3showed the highest
performance, with activity of 95%, H2yield of 52% (v/v), and
H2selectivity of 73% (averaged over 12 h).
EF801076R
(22) Swartz, S.; Azad, A.-M.; Seabaugh, M. Ceria-based water-gas-shift catalysts. Presented at The 2002 Fuel Cell Seminar and Exposition, Palm Springs, CA, Nov 18-21, 2002; pp 587-590.
(23) Tanaka, Y.; Iizuka, T. Methanation of carbon monoxide with water over supported rhodium catalysts.Aust. J. Chem.1985,38, 293–296. CO+3H2hCH4+H2O ∆H298)
-206 kJ/mol ∆G298) -142.2 kJ (9)
CO2+4H2hCH4+2H2O ∆H298)
-165 kJ/mol ∆G298) -113.7 kJ (10)
2CO+2H2hCH
4+CO2 ∆H 298 )
-247 kJ/mol ∆G298) -170.8 kJ (11)
3102 Energy & Fuels, Vol. 23, 2009 Haryanto et al.
![Figure 6. Catalyst weight profiles resulting from TGAs [catalysts are numbered as follows: (1) Ni/CeO2, (2) Ni/Al2O3 powder, (3) Ni/Al2O3 monolith,(4) Ni/CeZrO4, (5) Ni/CeYO5, (6) Ni/CeO2-Gd, (7) Ni/CeO2-Sm, (8) Ni/CeO2-Al2O3 monolith, and (9) Ni/CeO2-Al2O3 powder].](https://thumb-ap.123doks.com/thumbv2/123dok/3984707.1928127/5.589.45.283.480.611/figure-catalyst-proles-resulting-catalysts-numbered-monolith-monolith.webp)