GENERAL CHEMISTRY
RAYMOND
F
IFTH
E
DITION
The Essential Concepts
RAYMOND
F
IFTH
E
DITION
The Essential Concepts
RAYMOND
F
IFTH
E
DITION
A
BOUT THEC
OVERMolecules in the upper atmosphere are constantly being bombarded by high-energy particles from the sun. As a result, these molecules either break up into atoms and/or become ionized. Eventually, the electronically excited species return to the ground state with the emission of light, giving rise to the phenomenon called aurora borealis
Raymond
CHANG
Williams College
G
ENERAL
C
HEMISTRY
The Essential Concepts
GENERAL CHEMISTRY: THE ESSENTIAL CONCEPTS, FIFTH EDITION
Published by McGraw-Hill, a business unit of The McGraw-Hill Companies, Inc., 1221 Avenue of the Americas, New York, NY 10020. Copyright © 2008 by The McGraw-Hill Companies, Inc. All rights reserved. No part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written consent of The McGraw-Hill Companies, Inc., including, but not limited to, in any network or other electronic storage or transmission, or broadcast for distance learning.
Some ancillaries, including electronic and print components, may not be available to customers outside the United States.
This book is printed on acid-free paper.
1 2 3 4 5 6 7 8 9 0 DOW/DOW 0 9 8 7
ISBN 978–0–07–304851–2 MHID 0–07–304851–8
ISBN 978–0–07–304857–4 (Annotated Instructor’s Edition) MHID 0–07–304857–7
Publisher: Thomas D. Timp
Senior Sponsoring Editor: Tamara L. Good-Hodge
Managing Developmental Editor: Shirley R. Oberbroeckling
Marketing Manager: Todd L. Turner
Senior Project Manager: Gloria G. Schiesl
Senior Production Supervisor: Kara Kudronowicz
Lead Media Project Manager: Judi David
Lead Media Producer: Daryl Bruflodt
Senior Designer: David W. Hash
Cover/Ulterior Designer: Jamie E. O’Neal
(USE) Cover Image: Northern Lights, ©Daryl Benson/Masterfile
Senior Photo Research Coordinator: John Leland
Photo Research: Tom Michaels/PhotoFind, LLC
Supplement Producer: Mary Jane Lampe
Compositor: Techbooks
Typeface: 10/12 Times Roman
Printer: R. R. Donnelley Willard, OH
The credits section for this book begins on page C-1 and is considered an extension of the copyright page.
Library of Congress Cataloging-in-Publication Data
Chang, Raymond.
General chemistry : the essential concepts / Raymond Chang. – 5th ed. p. cm.
Includes index.
ISBN 978–0–07–304851–2 — ISBN 0–07–304851–8 (hard copy : alk. paper) 1. Chemistry–Textbooks. I. Title.
QD33.2.C48 2008
540–dc22 2006102621
v
Raymond Chang
was born in Hong Kong and grew up in Shanghai and HongKong, China. He received his B.Sc. degree in chemistry from London University, England, and his Ph.D. in chemistry from Yale University. After doing postdoctoral research at Washington University and teaching for a year at Hunter College of the City University of New York, he joined the chemistry department at Williams College, where he has taught since 1968.
Professor Chang has served on the American Chemical Society Examination Committee, the National Chemistry Olympiad Examination Committee, and the Graduate Record Examinations (GRE) Committee. He is an editor of The Chemical Educator. Professor Chang has written books on physical chemistry, industrial chem-istry, and physical science. He has also coauthored books on the Chinese language, children’s picture books, and a novel for young readers.
For relaxation, Professor Chang maintains a forest garden, plays tennis, and prac-tices the violin.
vii
List of Animations xvii Preface xix
A Note to the Student xxvi
1
Introduction 12
Atoms, Molecules, and Ions 283
Stoichiometry 584
Reactions in Aqueous Solutions 945
Gases 1326
Energy Relationships in Chemical Reactions 1717
The Electronic Structure of Atoms 2068
The Periodic Table 2459
Chemical Bonding I: The Covalent Bond 27910
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 31211
Introduction to Organic Chemistry 35512
Intermolecular Forces and Liquids and Solids 39013
Physical Properties of Solutions 42514
Chemical Kinetics 45415
Chemical Equilibrium 49616
Acids and Bases 52917
Acid-Base Equilibria and Solubility Equilibria 57418
Thermodynamics 61019
Redox Reactions and Electrochemistry 64220
The Chemistry of Coordination Compounds 68421
Nuclear Chemistry 70822
Organic Polymers—Synthetic and Natural 739A
PPENDIX1
Units for the Gas Constant A-1A
PPENDIX2
Selected Thermodynamic Data at 1 atm and 25⬚C A-2A
PPENDIX3
Mathematical Operations A-6A
PPENDIX4
The Elements and the Derivation of Their Names and Symbols A-9Glossary G-1
Answers to Even-Numbered Problems AP-1 Credits C-1
Index I-1
ix LIST OFANIMATIONS xvii
PREFACE xix
A NOTE TO THESTUDENT xxvi
Introduction 1
1.1
The Study of Chemistry 21.2
The Scientific Method 21.3
Classifications of Matter 41.4
Physical and Chemical Properties of Matter 71.5
Measurement 81.6
Handling Numbers 131.7
Dimensional Analysis in Solving Problems 18KEYEQUATIONS22
SUMMARY OFFACTS ANDCONCEPTS22 KEYWORDS22
QUESTIONS ANDPROBLEMS 23
Atoms, Molecules, and Ions 28
2.1
The Atomic Theory 292.2
The Structure of the Atom 302.3
Atomic Number, Mass Number, and Isotopes 352.4
The Periodic Table 362.5
Molecules and Ions 382.6
Chemical Formulas 392.7
Naming Compounds 432.8
Introduction to Organic Compounds 52SUMMARY OFFACTS ANDCONCEPTS52 KEYWORDS53
QUESTIONS ANDPROBLEMS 53
Stoichiometry 58
3.1
Atomic Mass 593.2
Avogadro’s Number and the Molar Mass of an Element 603.3
Molecular Mass 643.4
The Mass Spectrometer 663.5
Percent Composition of Compounds 671
2
C H A P T E R
C H A P T E R
3
C H A P T E R
x Contents
4
5
C H A P T E R
C H A P T E R
6
C H A P T E R
3.6
Experimental Determination of Empirical Formulas 703.7
Chemical Reactions and Chemical Equations 733.8
Amounts of Reactants and Products 773.9
Limiting Reagents 813.10
Reaction Yield 83KEYEQUATIONS85
SUMMARY OFFACTS ANDCONCEPTS 85 KEYWORDS86
QUESTIONS ANDPROBLEMS 86
Reactions in Aqueous Solutions 94
4.1
General Properties of Aqueous Solutions 954.2
Precipitation Reactions 974.3
Acid-Base Reactions 1014.4
Oxidation-Reduction Reactions 1064.5
Concentration of Solutions 1144.6
Solution Stoichiometry 118KEYEQUATIONS123
SUMMARY OFFACTS ANDCONCEPTS 123 KEYWORDS124
QUESTIONS ANDPROBLEMS 124
Gases 132
5.1
Substances That Exist as Gases 1335.2
Pressure of a Gas 1345.3
The Gas Laws 1365.4
The Ideal Gas Equation 1425.5
Dalton’s Law of Partial Pressures 1485.6
The Kinetic Molecular Theory of Gases 1535.7
Deviation from Ideal Behavior 159KEYEQUATIONS162
SUMMARY OFFACTS ANDCONCEPTS 163 KEYWORDS163
QUESTIONS ANDPROBLEMS 163
Energy Relationships in Chemical
Reactions 171
Contents xi
6.6
Standard Enthalpy of Formation and Reaction 191KEYEQUATIONS197
SUMMARY OFFACTS ANDCONCEPTS 197 KEYWORDS198
QUESTIONS ANDPROBLEMS 198
The Electronic Structure of Atoms 206
7.1
From Classical Physics to Quantum Theory 2077.2
The Photoelectric Effect 2117.3
Bohr’s Theory of the Hydrogen Atom 2127.4
The Dual Nature of the Electron 2177.5
Quantum Mechanics 2197.6
Quantum Numbers 2217.7
Atomic Orbitals 2227.8
Electron Configuration 2267.9
The Building-Up Principle 233KEYEQUATIONS237
SUMMARY OFFACTS ANDCONCEPTS 237 KEYWORDS238
QUESTIONS ANDPROBLEMS 238
The Periodic Table 245
8.1
Development of the Periodic Table 2468.2
Periodic Classification of the Elements 2478.3
Periodic Variation in Physical Properties 2508.4
Ionization Energy 2568.5
Electron Affinity 2598.6
Variation in Chemical Properties of the Representative Elements 261SUMMARY OFFACTS ANDCONCEPTS 272 KEYWORDS272
QUESTIONS ANDPROBLEMS 272
Chemical Bonding I: The
Covalent Bond 279
9.1
Lewis Dot Symbols 2809.2
The Ionic Bond 2819.3
Lattice Energy of Ionic Compounds 2839.4
The Covalent Bond 2859.5
Electronegativity 2879.6
Writing Lewis Structures 2919.7
Formal Charge and Lewis Structures 2937
8
C H A P T E R
C H A P T E R
9
xii Contents
9.8
The Concept of Resonance 2969.9
Exceptions to the Octet Rule 2989.10
Bond Enthalpy 302KEYEQUATION 305
SUMMARY OFFACTS ANDCONCEPTS 305 KEYWORDS306
QUESTIONS ANDPROBLEMS 306
Chemical Bonding II: Molecular
Geometry and Hybridization of
Atomic Orbitals 312
10.1
Molecular Geometry 31310.2
Dipole Moments 32210.3
Valence Bond Theory 32510.4
Hybridization of Atomic Orbitals 32810.5
Hybridization in Molecules Containing Double and Triple Bonds 33710.6
Molecular Orbital Theory 340KEYEQUATIONS348
SUMMARY OFFACTS ANDCONCEPTS 349 KEYWORDS349
QUESTIONS ANDPROBLEMS 349
Introduction to Organic
Chemistry 355
11.1
Classes of Organic Compounds 35611.2
Aliphatic Hydrocarbons 35611.3
Aromatic Hydrocarbons 37011.4
Chemistry of the Functional Groups 37411.5
Chirality—The Handedness of Molecules 381SUMMARY OFFACTS ANDCONCEPTS384 KEYWORDS384
QUESTIONS ANDPROBLEMS 385
Intermolecular Forces and
Liquids and Solids 390
12.1
The Kinetic Molecular Theory of Liquids and Solids 39112.2
Intermolecular Forces 39212.3
Properties of Liquids 39812.4
Crystal Structure 40112.5
Bonding in Solids 40510
C H A P T E R
11
C H A P T E R
12
Contents xiii
12.6
Phase Changes 40812.7
Phase Diagrams 415KEYEQUATIONS417
SUMMARY OFFACTS ANDCONCEPTS 417 KEYWORDS418
QUESTIONS ANDPROBLEMS 418
Physical Properties
of Solutions 425
13.1
Types of Solutions 42613.2
A Molecular View of the Solution Process 42613.3
Concentration Units 42913.4
Effect of Temperature on Solubility 43213.5
Effect of Pressure on the Solubilityof Gases 433
13.6
Colligative Properties 435KEYEQUATIONS447
SUMMARY OFFACTS ANDCONCEPTS 447 KEYWORDS447
QUESTIONS ANDPROBLEMS 448
Chemical Kinetics 454
14.1
The Rate of a Reaction 45514.2
The Rate Laws 45914.3
Relation Between Reactant Concentrations and Time 46314.4
Activation Energy and Temperature Dependence of Rate Constants 47114.5
Reaction Mechanisms 47714.6
Catalysis 480KEYEQUATIONS486
SUMMARY OFFACTS ANDCONCEPTS 487 KEYWORDS487
QUESTIONS ANDPROBLEMS 487
Chemical Equilibrium 496
15.1
The Concept of Equilibrium 49715.2
Ways of Expressing Equilibrium Constants 50015.3
What Does the Equilibrium Constant Tell Us? 50715.4
Factors That Affect Chemical Equilibrium 512KEYEQUATIONS519
SUMMARY OFFACTS ANDCONCEPTS 519 KEYWORDS520
QUESTIONS ANDPROBLEMS 520
13
C H A P T E R
14
C H A P T E R
15
xiv Contents
Acids and Bases 529
16.1
Brønsted Acids and Bases 53016.2
The Acid-Base Properties of Water 53116.3
pH—A Measure of Acidity 53316.4
Strength of Acids and Bases 53616.5
Weak Acids and Acid IonizationConstants 540
16.6
Weak Bases and Base Ionization Constants 55116.7
The Relationship Between Conjugate Acid-Base Ionization Constants 55316.8
Molecular Structure and the Strength of Acids 55416.9
Acid-Base Properties of Salts 55716.10
Acidic, Basic, and Amphoteric Oxides 56316.11
Lewis Acids and Bases 565KEYEQUATIONS567
SUMMARY OFFACTS ANDCONCEPTS 567 KEYWORDS567
QUESTIONS ANDPROBLEMS 568
Acid-Base Equilibria and
Solubility Equilibria 574
17.1
Homogeneous Versus Heterogeneous Solution Equilibria 57517.2
Buffer Solutions 57517.3
A Closer Look at Acid-Base Titrations 58017.4
Acid-Base Indicators 58617.5
Solubility Equilibria 58917.6
The Common Ion Effect and Solubility 59617.7
Complex Ion Equilibria and Solubility 59717.8
Application of the Solubility Product Principleto Qualitative Analysis 600
KEYEQUATIONS603
SUMMARY OFFACTS ANDCONCEPTS 603 KEYWORDS603
QUESTIONS ANDPROBLEMS 604
Thermodynamics 610
18.1
The Three Laws of Thermodynamics 61118.2
Spontaneous Processes 61118.3
Entropy 61218.4
The Second Law of Thermodynamics 61717
C H A P T E R
18
C H A P T E R
16
Contents xv
18.5
Gibbs Free Energy 62218.6
Free Energy and Chemical Equilibrium 62918.7
Thermodynamics in Living Systems 632KEYEQUATIONS634
SUMMARY OFFACTS ANDCONCEPTS 635 KEYWORDS635
QUESTIONS ANDPROBLEMS 635
Redox Reactions and
Electrochemistry 642
19.1
Redox Reactions 64319.2
Galvanic Cells 64619.3
Standard Reduction Potentials 64819.4
Spontaneity of Redox Reactions 65419.5
The Effect of Concentration on Cell Emf 65719.6
Batteries 66119.7
Corrosion 66519.8
Electrolysis 66819.9
Electrometallurgy 673KEYEQUATIONS674
SUMMARY OFFACTS ANDCONCEPTS 675 KEYWORDS675
QUESTIONS ANDPROBLEMS 675
The Chemistry of Coordination
Compounds 684
20.1
Properties of the Transition Metals 68520.2
Coordination Compounds 68820.3
Geometry of Coordination Compounds 69320.4
Bonding in Coordination Compounds:Crystal Field Theory 695
20.5
Reactions of Coordination Compounds 70120.6
Coordination Compounds in Living Systems 702KEYEQUATION 703
SUMMARY OFFACTS ANDCONCEPTS 703 KEYWORDS704
QUESTIONS ANDPROBLEMS 704
19
C H A P T E R
20
xvi Contents
Nuclear Chemistry 708
21.1
The Nature of Nuclear Reactions 70921.2
Nuclear Stability 71121.3
Natural Radioactivity 71621.4
Nuclear Transmutation 72021.5
Nuclear Fission 72221.6
Nuclear Fusion 72721.7
Uses of Isotopes 72921.8
Biological Effects of Radiation 732KEYEQUATIONS733
SUMMARY OFFACTS ANDCONCEPTS 733 KEYWORDS734
QUESTIONS ANDPROBLEMS 734
Organic Polymers—Synthetic
and Natural 739
22.1
Properties of Polymers 74022.2
Synthetic Organic Polymers 74022.3
Proteins 74422.4
Nucleic Acids 752SUMMARY OFFACTS ANDCONCEPTS 754 KEYWORDS755
QUESTIONS ANDPROBLEMS 755
A
PPENDIX1
Units for the Gas Constant A-1
A
PPENDIX2
Selected Thermodynamic Data at
1 atm and 25
⬚
C A-2
A
PPENDIX3
Mathematical Operations A-6
A
PPENDIX4
The Elements and the Derivation of
Their Names and Symbols A-9
GLOSSARY G-1
ANSWERS TOEVEN-NUMBEREDPROBLEMS AP-1
CREDITS C-1
INDEX I-1
22
C H A P T E R
21
xvii
The animations below are correlated to
General Chemistry within each chapter in two ways. The first is the Interactive Activity Summary found in the opening pages of every chapter. Then within the chapter are icons inform-ing the student and instructor that an animation is avail-able for a specific topic and where to find the animation for viewing on our Chang General ChemistryARIS web-site. For the instructor, the animations are also available on the Chemistry Animations Library DVD.
Chang Animations
Absorption of color (20.4) Acid-base titrations (17.3) Acid ionization (16.5) Activation energy (14.4)
Alpha, beta, and gamma rays (2.2) Alpha-particle scattering (2.2) Atomic and ionic radius (8.3) Base ionization (16.6) Buffer solutions (17.2) Catalysis (14.6) Cathode ray tube (2.2) Chemical equilibrium (15.1) Chirality (11.5)
Collecting a gas over water (5.5) Diffusion of gases (5.6)
Dissolution of an ionic and a covalent compound (13.2) Electron configurations (7.8)
Emission spectra (7.3)
Equilibrium vapor pressure (12.6) Formal charge calculations (9.5) Galvanic cells (19.2)
Gas laws (5.3) Heat flow (6.4) Hybridization (10.4) Hydration (4.1)
Ionic vs. covalent bonding (9.4) Le Châtelier’s principle (15.4) Limiting reagent (3.9) Making a solution (4.5) Millikan oil drop (2.2) Neutralization reactions (4.3) Nuclear fission (21.5) Orientation of collision (14.4) Osmosis (13.6)
Oxidation-reduction reactions (4.4 & 19.1) Packing spheres (12.4)
Polarity of molecules (10.2) Precipitation reactions (4.2)
Preparing a solution by dilution (4.5) Radioactive decay (21.3)
Resonance (9.8)
Sigma and pi bonds (10.5)
Strong electrolytes, weak electrolytes, and nonelectrolytes (4.1)
VSEPR (10.1)
McGraw-Hill Animations
Atomic line spectra (7.3) Charles’ law (5.3)
Cubic unit cells and their origins (12.4) Dissociation of strong and weak acids (16.5) Dissolving table salt (4.1)
Electronegativity (9.5) Equilibrium (15.1)
Exothermic and endothermic reactions (6.2) Formal Charge Calculations (9.7)
Formation of an ionic compound (9.3) Formation of the covalent bond in H2 (10.4) Half-life (14.3)
Influence of shape on polarity (10.2) Law of conservation of mass (2.1)
Molecular shape and orbital hybridization (10.4) Nuclear medicine (21.7)
Operation of voltaic cell (19.2)
Oxidation-reduction reaction (4.4 & 19.1) Phase diagrams and the states of matter (12.7) Reaction rate and the nature of collisions (14.4) Three states of matter (1.3)
Using a buffer (17.2)
VSEPR theory and the shapes of molecules (10.1)
Simulations
Stoichiometry (Chapter 3) Ideal gas law (Chapter 5) Kinetics (Chapter 14) Equilibrium (Chapter 15) Titration (Chapter 17) Electrochemistry (Chapter 19) Nuclear (Chapter 21)
xix
In this fifth edition of General Chemistry: The Essential Concepts, I have continued the tradition of presenting only the material that is essential to a one-year general chemistry course. As with previous editions, I have included all the core topics that are necessary for a solid foundation in general chemistry without sacrificing depth, clarity, or rigor.
General Chemistry covers these topics in the same depth and at the same level as 1100-page texts. Therefore, this book is not a condensed version of a big text. I have written it so that an instructor can cover 95 percent of the content, instead of the two-thirds or three-quarters that in my experience is typical of the big books. My hope is that this concise-but-thorough approach will appeal to efficiency-minded instructors and will please value-conscious students. The responses I have received from users over the years convince me that there is a strong need for such a text.
What’s New in This Edition?
Many sections have been revised and updated based on the comments from reviewers and users. Some examples are:
• An introduction to organic compounds has been added to Section 2.8.
• Ionic bonding has been added to Section 9.2. • Section 14.3 now also discusses zero-order reactions
in addition to first- and second-order reactions. • Section 16.3 compares the definition of pH using
concentration and activity.
• Many new problems have been added under the Special Problems section in each chapter.
• The ARIS electronic homework system is available for the fifth edition. ARIS will enhance the student learning experience, administer assignments, track student progress, and administer an instructor’s course. The students can locate the animations and interactives noted in the text margins in ARIS. Quizzing and homework assigned by the instructor is available in the ARIS electronic homework program.
Art
As always, I strive for a clean but visual design. For example, the following diagram shows the conversion of molecular hydrogen chloride to hydrochloric acid.
I have also added new molecular art to line drawings and photos and to a number of end-of-chapter problems. In addition, we have updated the photo program to complement the visual layout of the design. Finally, we have updated the format of the periodic table throughout the text.
All key equations and answers to many Worked Examples have been shaded for easy visual access. The key equations are also listed at the end of each chapter.
Problems
The development of problem-solving skills has always been a major objective of this text. For example, in Section 3.8 the general approach for solving stoichiom-etry problems is broken down in a numbered step-by-step process. Immediately following is Example 3.13 using this approach. Example 3.14 then requires the students to use this same type of process on their own.
(10.2) bond order⫽1
2a
number of electrons in bonding MOs ⫺
number of electrons in antibonding MOsb
Cl–
HCl
H3O+
SolutionWe proceed as follows.
Step 1:The major species in solution are HCOOH, H⫹, and the conjugate base HCOO⫺.
Step 2:First we need to calculate the hydrogen ion concentration from the pH value
Taking the antilog of both sides, we get
Next we summarize the changes:
HCOOH(aq) H⫹(aq) ⫹ HCOO⫺(aq)
Initial (M): 0.10 0.00 0.00
Change (M): Equilibrium (M):
Note that because the pH and hence the H⫹ion concentration is known, it
follows that we also know the concentrations of HCOOH and HCOO⫺at
equilibrium.
Step 3:The ionization constant of formic acid is given by
CheckThe Kavalue differs slightly from the one listed in Table 16.3 because of the
rounding-off procedure we used in the calculation.
Practice ExerciseThe pH of a 0.060 Mweak monoprotic acid is 3.44. Calculate the
Kaof the acid.
⫽1.8⫻10⫺4 ⫽(4.1⫻10
⫺3)(4.1 ⫻10⫺3)
(0.10⫺4.1⫻10⫺3)
Ka⫽
[H⫹][HCOO⫺]
[HCOOH]
4.1⫻10⫺3
4.1⫻10⫺3
(0.10⫺4.1⫻10⫺3)
⫹4.1⫻10⫺3 ⫹4.1⫻10⫺3
⫺4.1⫻10⫺3 ∆
[H⫹]⫽10⫺2.39
⫽4.1⫻10⫺3M
2.39⫽ ⫺log[H⫹]
pH⫽ ⫺log[H⫹]
Marginal references enable students to apply new skills to other, similar problems at the end of the chapter. Each Worked Example is followed by a Practice Exercise that asks the students to solve a similar problem on their own. The answers to the Practice Exercises are provided after the end-of-chapter problems in each chapter.
xx Preface
Example 16.9
The pH of a 0.10 Msolution of formic acid (HCOOH) is 2.39. What is the Kaof the acid? StrategyFormic acid is a weak acid. It only partially ionizes in water. Note that the concentration of formic acid refers to the initial concentration, before ionization has started. The pH of the solution, on the other hand, refers to the equilibrium state. To calculate Ka, then, we need to know the concentrations of all three species: [H ],
[HCOO ], and [HCOOH] at equilibrium. As usual, we ignore the ionization of water. The following sketch summarizes the situation.
(C ti d)
As an instructor, I often tell my students that a good learning tool is to sketch out the inner workings of a problem. In some of the Worked Examples, I have included this type of drawing (for example, see Example 16.9 on p. 545). It is what a scientist would do as he or she works out a problem.
Step 2:To convert grams of C6H12O6to moles of C6H12O6, we write
Step 3:From the mole ratio, we see that 1 mol C6H12O6∞6 mol CO2. Therefore, the
number of moles of CO2formed is
Step 4:Finally, the number of grams of CO2formed is given by
After some practice, we can combine the conversion steps
into one equation:
CheckDoes the answer seem reasonable? Should the mass of CO2produced be larger
than the mass of C6H12O6reacted, even though the molar mass of CO2is considerably
less than the molar mass of C6H12O6? What is the mole ratio between CO2and
C6H12O6?
Practice ExerciseMethanol (CH3OH) burns in air according to the equation
If 209 g of methanol are used up in a combustion process, what is the mass of H2O
produced?
2CH3OH⫹3O2¡2CO2⫹4H2O ⫽1.25⫻103 g CO
2
mass of CO2⫽856 g C6H12O6⫻
1 mol C6H12O6
180.2 g C6H12O6⫻
6 mol CO2
1 mol C6H12O6⫻
44.01 g CO2
1 mol CO2
grams of C6H12O6¡moles of C6H12O6¡moles of CO2¡grams of CO2
28.50 mol CO2⫻44.01 g CO1 mol CO2 2 ⫽
1.25⫻103 g CO 2
4.750 mol C6H12O6⫻
6 mol CO2
1 mol C6H12O6⫽
28.50 mol CO2
856 g C6H12O6⫻
1 mol C6H12O6
180.2 g C6H12O6⫽
4.750 mol C6H12O6
The food we eat is degraded, or broken down, in our bodies to provide energy for growth and function. A general overall equation for this very complex process repre-sents the degradation of glucose (C6H12O6) to carbon dioxide (CO2) and water (H2O):
If 856 g of C6H12O6is consumed by a person over a certain period, what is the mass
of CO2produced?
StrategyLooking at the balanced equation, how do we compare the amount of C6H12O6and CO2? We can compare them based on the mole ratiofrom the balanced
equation. Starting with grams of C6H12O6, how do we convert to moles of C6H12O6?
Once moles of CO2are determined using the mole ratio from the balanced equation
how do we convert to grams of CO2?
SolutionWe follow the preceding steps and Figure 3.8.
Preface xxi
Pedagogy
The Interactive Activity Summary shows the avail-able media to further enhance students’ ability to understand a concept.
The Essential Conceptsin each chapter opener summarizes the main topics to be discussed in the chapter.
Marginal notes provide additional informa-tion to students regarding quick facts, referring students to a section in which the concept will be further detailed or linking back to a section they can use to review the material.
There is a plethora of molecular art in the margin, enabling students to “see” the molecule under discussion.
The periodic table icon in the margin illus-trates the properties of elements according to their positions in the periodic table.
Also in the margin, students will find the icon highlighting the media (animations and interac-tives) that can be used to understand the concept presented.
The end of the chapter provides further study aids with the Key Equations, Summary of Facts and Concepts, and also the Key Words. They give students a quick snapshot of the chapter in review.
Molecular models are used to study complex biochemical reactions such as those between protein and DNA molecules.
C H A P T E R
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
ESSENTIALCONCEPTS
Molecular GeometryMolecular geometry refers to the three-dimensional arrangement of atoms in a molecule. For relatively small molecules, in which the central atom contains two to six bonds, geometries can be reliably predicted by the valence-shell electron-pair repulsion (VSEPR) model. This model is based on the assumption that chemical bonds and lone pairs tend to remain as far apart as possible to minimize repulsion.
Dipole MomentsIn a diatomic molecule the difference in the electronegativities of bonding atoms results in a polar bond and a dipole moment. The dipole moment of a molecule made up of three or more atoms depends on both the polarity of the bonds and molecular geometry. Dipole moment measurements can help us distinguish between different possible geometries of a molecule.
Hybridization of Atomic OrbitalsHybridization is the quantum mechanical description of chemical bonding. Atomic orbitals are hybridized, or mixed, to form hybrid orbitals. These orbitals then interact with other atomic orbitals to form chemical bonds. Various molecular geometries can be generated by different hybridizations. The hybridization concept accounts for the excep-tion to the octet rule and also explains the formaexcep-tion of double and triple bonds.
Molecular Orbital TheoryMolecular orbital theory describes bonding in terms of the combination of atomic orbitals to form orbitals that are associated with the molecule as a whole. Molecules are stable if the number of electrons in bonding molecular orbitals is greater than that in antibonding molecular orbitals. We write electron configurations for molecular orbitals as we do for atomic orbitals, using the Pauli exclusion principle and Hund’s rule.
Activity Summary 1. Animation: VSEPR (10.1)
2. Interactivity: Determining Molecular Shape (10.1) 3. Animation: Polarity of Molecules (10.2) 4. Interactivity: Molecular Polarity (10.2) 5. Animation: Hybridization (10.4)
6. Interactivity: Determining Orbital Hybridization (10.4) 7. Animation: Sigma and Pi Bonds (10.5) 8. Interactivity: Energy Levels of Bonding—
Homonuclear Diatomic Molecules (10.6)
CHAPTEROUTLINE
10.1 Molecular Geometry 313
Molecules in Which the Central Atom Has No Lone Pairs• Molecules in Which the Central Atom Has One or More Lone Pairs•Geometry of Molecules with More Than One Central Atom •Guidelines for Applying the VSEPR Model
10.2 Dipole Moments 322
10.3 Valence Bond Theory 325
10.4 Hybridization of Atomic Orbitals 328
sp3Hybridization•spHybridization•sp2Hybridization•
Procedure for Hybridizing Atomic Orbitals•Hybridization ofs, p, and dOrbitals
10.5 Hybridization in Molecules Containing Double and Triple Bonds 331
10.6 Molecular Orbital Theory 340
Bonding and Antibonding Molecular Orbitals •Molecular Orbital Configurations
in which CH3COO is called the acetate ion. (In this book we will use the term disso-ciationfor ionic compounds and ionizationfor acids and bases.) By writing the formula of acetic acid as CH3COOH we indicate that the ionizable proton is in the COOH group. The double arrow 34in an equation means that the reaction is reversible;that is, the reaction can occur in both directions.Initially, a number of CH3COOH
mol-ecules break up to yield CH3COO and H ions. As time goes on, some of the
CH3COO and Hions recombine to form CH3COOH molecules. Eventually, a state is reached in which the acid molecules break up as fast as the ions recombine. Such a chemical state, in which no net change can be observed(although continuous activ-ity is taking place on the molecular level), is called chemical equilibrium.Acetic acid, then, is a weak electrolyte because its ionization in water is incomplete. By contrast, in a hydrochloric acid solution, the H and Cl ions have no tendency to recombine to form molecular HCl. We use the single arrow to represent complete ionizations.
In Sections 4.2– 4.4 we will study three types of reactions in the aqueous medium (precipitation, acid-base, and oxidation-reduction) that are of great importance to industrial, environmental, and biological processes. They also play a role in our daily experience.
4.2Precipitation Reactions
One common type of reaction that occurs in aqueous solution is the precipitation reaction,which results in the formation of an insoluble product, or precipitate.A
precipitateis an insoluble solid that separates from the solution.Precipitation reac-tions usually involve ionic compounds. For example, when an aqueous solution of lead(II) nitrate [Pb(NO3)2] is added to an aqueous solution of potassium iodide (KI),
a yellow precipitate of lead iodide (PbI2) is formed:
Potassium nitrate remains in solution. Figure 4.3 shows this reaction in progress. The preceding reaction is an example of a metathesis reaction(also called a dou-ble displacement reaction), a reaction that involves the exchange of parts between two
Pb(NO3)2(aq) 2KI(aq) ° PbI2(s) 2KNO3(aq)
4.2 Precipitation Reactions 97
There are different types of chemical equilibrium. We will return to this very important topic in Chapter 15.
Animation:
Precipitation Reactions ARIS, Animations
Figure 4.3
Formation of yellow PbI2precipitate as a solution of Pb(NO3)2is added to a solution of KI. K I Pb2 NO3 NO3 K I Pb2 88n
xxii Preface
Interactives
Two sets of interactives are available with General Chemistry. The interactives enable students to manipu-late several variables. Students can “see” how changes affect the topic being studied. The seven topics include stoichiometry, the gas laws, kinetics, equilibrium, acid/base reactions, nuclear reactions and radioactivity, and the electrochemical cell. The other set of interactives are simple and fun learning tools that encompass a broad range of topics. All of these interactives are marked by the Interactive Activity icon.
Instructor Resources
Annotated Instructor’s Edition
By Raymond Chang. The Annotated Instructor’s Edition includes all resources available to instructors marked by icons located in the margins of the text. Information is
Media
The Interactive Activity Summary in the chapter opening pages enables students and instructors to see at a glance the media that can be incorporated into the learn-ing process. Within the text, an icon shows students where the concept in the animation or interactive is introduced. The icon directs students to the ARIS website for viewing. For instructors, there are also directions for finding the animation or interactive in the instructor materials.
Animations
Preface xxiii
provided with regard to the integration of media (anima-tions, interactives, ARIS) and instructions as to where the instructor will find the various media. The difficulty level of the end-of-chapter problems and the various chemical disciplines to which the problems are related to is indi-cated. Information on quality demonstration videos, tips for instructors, and the icons marking the digital assets available on the ARIS Presentation Center are provided.
ARIS
McGraw-Hill’s ARIS—Assessment, Review, and In-struction System—for General Chemistry is a complete electronic homework and course management system. Instructors can create and share course materials and assignments with colleagues with a few clicks of the mouse. Instructors can edit questions and algorithms,
import their own content, and create announcements and due dates for assignments. ARIS has automatic grading and reporting of easy-to-assign algorithmically generated homework, quizzing, and testing. Once a student is regis-tered in the course, all student activity within McGraw-Hill’s ARIS is automatically recorded and available to the instructor through a fully integrated grade book that can be downloaded to Excel.®
Go to www.aris.mhhe.com to learn more, or go directly to General ChemistryARIS site at www.mhhe.com/chang.
Presentation Center
enhanced tests and quizzes, compelling course websites, or attractive printed support materials. The McGraw-Hill Presentation Center library includes thousands of assets from many McGraw-Hill titles. This ever-growing resource gives instructors the power to utilize assets specific to an adopted textbook as well as content from all other books in the library. The Presentation Center can be accessed from the instructor side of your textbook’s ARIS website, and the Presentation Center’s dynamic search engine allows you to explore by discipline, course, textbook chapter, asset type, or keyword. Simply browse, select, and download the files you need to build engaging course materials. All assets are copyright McGraw-Hill Higher Education but can be used by instructors for classroom purposes.
Instructor’s Testing and Resource
CD-ROM
The Test Bankis written by John Adams (University of Missouri) and the Instructor’s Solution Manual by Brandon J. Cruickshank (Northern Arizona University) and Raymond Chang. The Test Bank contains over 2000 multiple choice and short-answer questions. The ques-tions, which are graded in difficulty, are comparable to the problems in the text. The Test Bank is formatted for integration into the following course management systems: WebCT and Blackboard.
The Instructor’s Testing and Resource CD-ROM also contains the electronic file of the Instructor’s Solution Manual. The solutions to all of the end-of-chapter problems are given in the manual. This manual is included on the Instructor’s Testing and Resource CD-ROM.
Overhead Transparencies
Approximately 260 full-color text illustrations are repro-duced on acetate for overhead projection.
eInstruction
McGraw-Hill has partnered with eInstruction to provide the RF (radio frequency) Classroom Performance System(CPS), to bring interactivity into the classroom. CPS is a wireless response system that gives the instruc-tor and students immediate feedback from the entire class. The wireless response pads are essentially remotes that are easy to use and engage students. CPS enables you to motivate student preparation, interactiv-ity, and active learning so you can receive immediate
feedback and know what students understand. A text-specific set of questions, formatted for PowerPoint, is available via download from the Instructor area of the ARIS textbook website.
Cooperative Chemistry
Laboratory Manual
By Melanie Cooper (Clemson University). This innova-tive guide features open-ended problems designed to simulate experience in a research lab. Working in groups, students investigate one problem over a period of several weeks, so that they might complete three or four projects during the semester, rather than one preprogrammed experiment per class. The emphasis here is on experi-mental design, analysis problem solving, and communi-cation.
Student Resources
Problem-Solving Workbook
with Solutions
By Brandon J. Cruickshank (Northern Arizona University) and Raymond Chang is a success guide writ-ten for use with General Chemistry. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. Solutions for all of the text’s even-numbered problems are included.
ARIS
For students, ARIS contains the animations and interac-tivities listed in the Interactive Activity list at the begin-ning of each chapter. ARIS also features interactive quizzes for each chapter of the text. This program enables students to complete their homework online, as assigned by their instructors.
Chang Chemistry Resource Card
Our resource card is an easy, quick source of information on general chemistry. The student will find the periodic table, basic tables, and key equations within reach with-out having to consult the text.
Schaum’s Outline of College Chemistry
By Jerome Rosenberg, Michigan State University, and Lawrence Epstein, University of Pittsburgh. This helpful study aid provides students with hundreds of solved and supplementary problems for the general chemistry course.
Acknowledgments
Reviewers
I would like to thank the following individuals who reviewed or participated in various McGraw-Hill symposia on general chemistry. Their insight into the needs of students and instructors were invaluable to me in preparing this revision:
Kathryn S. Asala University of Wisconsin–Whitewater
R. D. Braun University of Louisiana
Dana Chateilier University of Delaware
Beverly A. Clement Blinn College
Elzbieta Cook Louisiana State University
Nordulf W. G. Debye Towson University
Becky Gee Long Island University
Stephen Z. Goldberg Adelphi University
Robert Keil Moorpark College
Tracy Knowles Bluegrass Community and Technical College
Arthur A. Low Tarleton State University
Kristen L. Murphy University of Wisconsin–Milwaukee
Eric Potma University of California–Irvine
Bala Ramachandran Louisiana Tech University
James Schlegel Rutgers University
Mark W. Schraf West Virginia University
Lynn L. Thompson Butler County Community College
Paul J. Toscano State University of New York at Albany
Tim Zauche University of Wisconsin–Platteville
My thanks go to Michael Wood for his thorough review of the entire manuscript and his thoughtful comments.
As always, I have benefited much from discussions with my colleagues at Williams College and correspon-dence with many instructors here and abroad.
It is a pleasure to acknowledge the support given to me by the following members of McGraw-Hill’s College Division: Doug Dinardo, Tammy Ben, Marty Lange, Kent Peterson, and Kurt Strand. In particular, I would like to mention Gloria Schiesl for supervising the produc-tion, David Hash for the book design, Daryl Bruflodt and Judi David for the media, and Todd Turner, the marketing manager, for his suggestions and encouragement. My publisher Thomas Timp and my editor Tami Hodge provided advice and support whenever I needed them. Finally, my special thanks go to Shirley Oberbroeckling, the developmental editor, for her care and enthusiasm for the project, and supervision at every stage of the writing of this edition
xxvi
General chemistry is commonly perceived to be more difficult than most other subjects. There is some justifica-tion for this percepjustifica-tion. For one thing, chemistry has a very specialized vocabulary. At first, studying chemistry is like learning a new language. Furthermore, some of the concepts are abstract. Nevertheless, with diligence you can complete this course successfully, and you might even enjoy it. Here are some suggestions to help you form good study habits and master the material in this text.
• Attend classes regularly and take careful notes. • If possible, always review the topics discussed in
class the same day they are covered in class. Use this book to supplement your notes.
• Think critically. Ask yourself if you really under-stand the meaning of a term or the use of an equation. A good way to test your understanding is to explain a concept to a classmate or some other person. • Do not hesitate to ask your instructor or your
teach-ing assistant for help.
The fifth edition tools for General Chemistry are designed to enable you to do well in your general chem-istry course. The following guide explains how to take full advantage of the text, technology, and other tools.
• Before delving into the chapter, read the chapter
outlineand the chapter introductionto get a sense of the important topics. Use the outline to organize your notetaking in class.
• Use the Interactive Activity Iconas a guide to review challenging concepts in motion. The animations and interactives are valuable in presenting a concept and allowing the student to ma-nipulate or choose steps so full understanding can take place.
• At the end of each chapter you will find a summary of facts and concepts, key equations, and a list of key words, all of which will help you review for exams.
• Definitions of the key words can be studied in con-text on the pages cited in the end-of-chapter list or in the glossary at the back of the book.
• ARIS houses an extraordinary amount of resources. Go to www.mhhe.com/physsci/chemistry/chang and click on the appropriate cover to explore chapter quizzes, animations, interactivities, simulations, and more.
• Careful study of the worked-out examples in the body of each chapter will improve your ability to an-alyze problems and correctly carry out the calcula-tions needed to solve them. Also take the time to work through the practice exercise that follows each example to be sure you understand how to solve the type of problem illustrated in the example. The an-swers to the practice exercises appear at the end of the chapter, following the homework problems. For additional practice, you can turn to similar home-work problems referred to in the margin next to the example.
• The questions and problems at the end of the chapter are organized by section.
• For even more practice problems, use ChemSkill Builder. ChemSkill Builder is a problem-solving tutorial with hundreds of problems that include feedback.
• The back inside cover shows a list of important fig-ures and tables with page references. This index makes it convenient to quickly look up information when you are solving problems or studying related subjects in different chapters.
If you follow these suggestions and stay up-to-date with your assignments, you should find that chemistry is challenging, but less difficult and much more interesting than you expected.
Raymond Chang
A hydrogen-filled balloon exploding when heated with a flame. The hydrogen gas reacts with oxygen in air to form water. Chemistry is the study of the properties of matter and the changes it undergoes.
Introduction
E
SSENTIALC
ONCEPTSThe Study of Chemistry Chemistry is the study of the properties of matter and the changes it undergoes. Elements and compounds are substances that take part in chemical transformation.
Physical and Chemical Properties To characterize a substance, we need to know its physical properties, which can be observed without changing its identity, and chemical properties, which can be demonstrated only by chemical changes.
Measurements and Units Chemistry is a quantitative science and requires measurements. The measured quantities (for exam-ple, mass, volume, density, and temperature) usually have units associated with them. The units used in chemistry are based on the international system (SI) of units.
Handling Numbers Scientific notation is used to express large and small numbers, and each number in a measurement must indicate the meaningful digits, called significant figures.
Doing Chemical Calculations A simple and effective way to perform chemical calculations is dimensional analysis. In this procedure, an equation is set up in such a way that all the units cancel except the ones for the final answer.
Activity Summary
1. Interactivity: Substances and Mixtures (1.3) 2. Interactivity: Elements (1.3)
3. Interactivity: SI Base Units (1.5) 4. Interactivity: Unit Prefixes (1.5)
5. Interactivity: Density (1.5)
6. Interactivity: Accuracy and Precision (1.6) 7. Interactivity: Dimensional Analysis Method (1.7)
C
HAPTERO
UTLINE1.1 The Study of Chemistry 2
How to Study Chemistry
1.2 The Scientific Method 2
1.3 Classifications of Matter 4
Substances and Mixtures •Elements and Compounds
1.4 Physical and Chemical Properties of Matter 7
1.5 Measurement 8
SI Units •Mass and Weight •Volume •Density •
Temperature Scales
1.6 Handling Numbers 13
Scientific Notation •Significant Figures •Accuracy and Precision
1.7 Dimensional Analysis in Solving Problems 18
A Note on Problem Solving
1.1
The Study of Chemistry
Whether or not this is your first course in chemistry, you undoubtedly have some preconceived ideas about the nature of this science and about what chemists do. Most likely, you think chemistry is practiced in a laboratory by someone in a white coat who studies things in test tubes. This description is fine, up to a point. Chem-istry is largely an experimental science, and a great deal of knowledge comes from laboratory research. In addition, however, today’s chemist may use a computer to study the microscopic structure and chemical properties of substances or employ sophisticated electronic equipment to analyze pollutants from auto emissions or toxic substances in the soil. Many frontiers in biology and medicine are currently being explored at the level of atoms and molecules—the structural units on which the study of chemistry is based. Chemists participate in the development of new drugs and in agricultural research. What’s more, they are seeking solutions to the problem of environmental pollution along with replacements for energy sources. And most industries, whatever their products, have a basis in chemistry. For exam-ple, chemists developed the polymers (very large molecules) that manufacturers use to make a wide variety of goods, including clothing, cooking utensils, artificial organs, and toys. Indeed, because of its diverse applications, chemistry is often called the “central science.”
How to Study Chemistry
Compared with other subjects, chemistry is commonly perceived to be more difficult, at least at the introductory level. There is some justification for this perception. For one thing, chemistry has a very specialized vocabulary. At first, studying chemistry is like learning a new language. Furthermore, some of the concepts are abstract. Nev-ertheless, with diligence you can complete this course successfully—and perhaps even pleasurably. Listed here are some suggestions to help you form good study habits and master the material:
• Attend classes regularly and take careful notes.
• If possible, always review the topics you learned in class the same day the top-ics are covered in class. Use this book to supplement your notes.
• Think critically. Ask yourself if you really understand the meaning of a term or the use of an equation. A good way to test your understanding is for you to explain a concept to a classmate or some other person.
• Do not hesitate to ask your instructor or your teaching assistant for help.
You will find that chemistry is much more than numbers, formulas, and abstract the-ories. It is a logical discipline brimming with interesting ideas and applications.
1.2
The Scientific Method
All sciences, including the social sciences, employ variations of what is called the
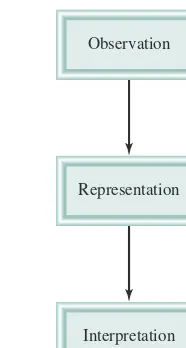
scientific method—a systematic approach to research. For example, a psychologist who wants to know how noise affects people’s ability to learn chemistry and a chemist interested in measuring the heat given off when hydrogen gas burns in air follow roughly the same procedure in carrying out their investigations. The first step is care-fully defining the problem. The next step includes performing experiments, making careful observations, and recording information, or data, about the system—the part
of the universe that is under investigation. (In these examples, the systems are the group of people the psychologist will study and a mixture of hydrogen and air.)
The data obtained in a research study may be both qualitative,consisting of gen-eral observations about the system, and quantitative, comprising numbers obtained by various measurements of the system.Chemists generally use standardized symbols and equations in recording their measurements and observations. This form of repre-sentation not only simplifies the process of keeping records, but also provides a com-mon basis for communications with other chemists. Figure 1.1 summarizes the main steps of the research process.
When the experiments have been completed and the data have been recorded, the next step in the scientific method is interpretation, meaning that the scientist attempts to explain the observed phenomenon. Based on the data that were gathered, the researcher formulates a hypothesis,or tentative explanation for a set of observations.
Further experiments are devised to test the validity of the hypothesis in as many ways as possible, and the process begins anew.
After a large amount of data has been collected, it is often desirable to summa-rize the information in a concise way, as a law. In science, a lawis a concise verbal or mathematical statement of a relationship between phenomena that is always the same under the same conditions. For example, Sir Isaac Newton’s second law of motion, which you may remember from high school science, says that force equals mass times acceleration (F ⫽ ma). What this law means is that an increase in the mass or in the acceleration of an object always increases the object’s force propor-tionally, and a decrease in mass or acceleration always decreases the force.
Hypotheses that survive many experimental tests of their validity may evolve into theories. A theory is a unifying principle that explains a body of facts and/or those laws that are based on them.Theories, too, are constantly being tested. If a theory is disproved by experiment, then it must be discarded or modified so that it becomes consistent with experimental observations. Proving or disproving a theory can take years, even centuries, in part because the necessary technology is not available. Atomic theory, which we will study in Chapter 2, is a case in point. It took more than 2000 years to work out this fundamental principle of chemistry proposed by Democritus, an ancient Greek philosopher.
Scientific progress is seldom, if ever, made in a rigid, step-by-step fashion. Some-times a law precedes a theory; someSome-times it is the other way around. Two scientists may start working on a project with exactly the same objective, but may take drasti-cally different approaches. They may be led in vastly different directions. Scientists are, after all, human beings, and their modes of thinking and working are very much influenced by their backgrounds, training, and personalities.
The development of science has been irregular and sometimes even illogical. Great discoveries are usually the result of the cumulative contributions and experi-ence of many workers, even though the credit for formulating a theory or a law is usually given to only one individual. There is, of course, an element of luck involved in scientific discoveries, but it has been said that “chance favors the prepared mind.” It takes an alert and well-trained person to recognize the significance of an acciden-tal discovery and to take full advantage of it. More often than not, the public learns only of spectacular scientific breakthroughs. For every success story, however, there are hundreds of cases in which scientists spent years working on projects that ul-timately led to a dead end. Many positive achievements came only after many wrong turns and at such a slow pace that they went unheralded. Yet even the dead ends contribute something to the continually growing body of knowledge about the physical universe. It is the love of the search that keeps many scientists in the laboratory.
1.2 The Scientific Method 3
Representation Observation
Interpretation
Figure 1.1
1.3
Classifications of Matter
Matteris anything that occupies space and has mass, and chemistryis the study of matter and the changes it undergoes.All matter, at least in principle, can exist in three states: solid, liquid, and gas. Solids are rigid objects with definite shapes. Liquids are less rigid than solids and are fluid—they are able to flow and assume the shape of their containers. Like liquids, gases are fluid, but unlike liquids, they can expand indefinitely.
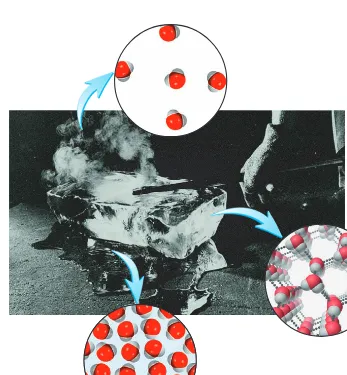
The three states of matter can be interconverted without changing the composi-tion of the substance. Upon heating, a solid (for example, ice) will melt to form a liq-uid (water). (The temperature at which this transition occurs is called the melting point.) Further heating will convert the liquid into a gas. (This conversion takes place at the boiling point of the liquid.) On the other hand, cooling a gas will cause it to condense into a liquid. When the liquid is cooled further, it will freeze into the solid form. Figure 1.2 shows the three states of water. Note that the properties of water are unique among common substances in that the molecules in the liquid state are more closely packed than those in the solid state.
4 CHAPTER 1 Introduction
Figure 1.2
The three states of matter. A hot poker changes ice into water and steam.
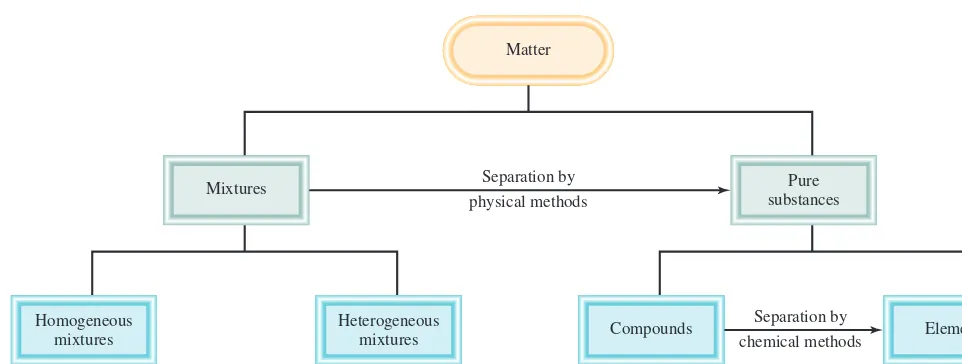
Substances and Mixtures
A substanceis matter that has a definite or constant composition and distinct prop-erties. Examples are water, silver, ethanol, table salt (sodium chloride), and carbon dioxide. Substances differ from one another in composition and can be identified by their appearance, smell, taste, and other properties. At present, over 20 million sub-stances are known, and the list is growing rapidly.
A mixture is a combination of two or more substances in which the substances retain their distinct identities.Some examples are air, soft drinks, milk, and cement. Mixtures do not have constant composition. Therefore, samples of air collected in dif-ferent cities would probably differ in composition because of differences in altitude, pollution, and so on.
Mixtures are either homogeneous or heterogeneous. When a spoonful of sugar dissolves in water, the composition of the mixture,after sufficient stirring, is the same throughout the solution. This solution is a homogeneous mixture. If sand is mixed with iron filings, however, the sand grains and the iron filings remain visible and sep-arate (Figure 1.3). This type of mixture, in which the composition is not uniform, is called a heterogeneous mixture.Adding oil to water creates another heterogeneous mixture because the liquid does not have a constant composition.
Any mixture, whether homogeneous or heterogeneous, can be created and then separated by physical means into pure components without changing the identities of the components. Thus, sugar can be recovered from a water solution by heating the solution and evaporating it to dryness. Condensing the water vapor will give us back the water component. To separate the iron-sand mixture, we can use a magnet to remove the iron filings from the sand, because sand is not attracted to the magnet (see Figure 1.3b). After separation, the components of the mixture will have the same com-position and properties as they did to start with.
Elements and Compounds
A substance can be either an element or a compound. An element is a substance that cannot be separated into simpler substances by chemical means.At present, 114 elements have been positively identified. (See the list inside the front cover of this book.)
1.3 Classifications of Matter 5
Interactivity:
Substances and Mixtures ARIS, Interactives
Interactivity:
Elements ARIS, Interactives
Figure 1.3
(a) The mixture contains iron filings and sand. (b) A magnet separates the iron filings from the mixture. The same technique is used on a larger scale to separate iron and steel from nonmagnetic objects such as aluminum, glass, and plastics.
Chemists use alphabetical symbols to represent the names of the elements. The first letter of the symbol for an element is alwayscapitalized, but the second letter is
nevercapitalized. For example, Co is the symbol for the element cobalt, whereas CO is the formula for carbon monoxide, which is made up of the elements carbon and oxygen. Table 1.1 shows some of the more common elements. The symbols for some elements are derived from their Latin names—for example, Au from aurum (gold), Fe from ferrum(iron), and Na from natrium (sodium)—although most of them are abbreviated forms of their English names.
Figure 1.4 shows the most abundant elements in Earth’s crust and in the human body. As you can see, only five elements (oxygen, silicon, aluminum, iron, and cal-cium) comprise over 90 percent of Earth’s crust. Of these five elements, only oxygen is among the most abundant elements in living systems.
Most elements can interact with one or more other elements to form compounds. We define a compoundas a substance composed of two or more elements chemically united in fixed proportions.Hydrogen gas, for example, burns in oxygen gas to form water, a compound whose properties are distinctly different from those of the start-ing materials. Water is made up of two parts of hydrogen and one part of oxygen. This composition does not change, regardless of whether the water comes from a faucet in the United States, the Yangtze River in China, or the ice caps on Mars. Unlike mixtures, compounds can be separated only by chemical means into their pure components.
6 CHAPTER 1 Introduction
Name Symbol Name Symbol Name Symbol
Aluminum Al Fluorine F Oxygen O
Arsenic As Gold Au Phosphorus P
Barium Ba Hydrogen H Platinum Pt
Bromine Br Iodine I Potassium K
Calcium Ca Iron Fe Silicon Si
Carbon C Lead Pb Silver Ag
Chlorine Cl Magnesium Mg Sodium Na
Chromium Cr Mercury Hg Sulfur S
Cobalt Co Nickel Ni Tin Sn
Copper Cu Nitrogen N Zinc Zn
TABLE 1.1 Some Common Elements and Their Symbols
Magnesium 2.8%
Oxygen
45.5% Oxygen
65%
Silicon
27.2% Carbon Hydrogen 10%
18% Calcium 4.7%
All others 5.3%
All others 1.2% Phosphorus 1.2% Calcium 1.6% Nitrogen 3% Iron 6.2%
Aluminum 8.3%
(a) (b)
Figure 1.4
The relationships among elements, compounds, and other categories of matter are summarized in Figure 1.5.
1.4
Physical and Chemical Properties of Matter
Substances are identified by their properties as well as by their composition. Color, melting point, boiling point, and density are physical properties. A physical property can be measured and observed without changing the composition or identity of a sub-stance.For example, we can measure the melting point of ice by heating a block of ice and recording the temperature at which the ice is converted to water. Water dif-fers from ice only in appearance and not in composition, so this is a physical change; we can freeze the water to recover the original ice. Therefore, the melting point of a substance is a physical property. Similarly, when we say that helium gas is lighter than air, we are referring to a physical property.
On the other hand, the statement “Hydrogen gas burns in oxygen gas to form water” describes a chemical property of hydrogen because to observe this property we must carry out a chemical change,in this case burning. After the change, the orig-inal substances, hydrogen and oxygen gas, will have vanished and a chemically dif-ferent substance—water—will have taken their place. We cannot recover hydrogen and oxygen from water by a physical change such as boiling or freezing.
Every time we hard-boil an egg, we bring about a chemical change. When subjected to a temperature of about 100⬚C, the yolk and the egg white undergo reactions that alter
not only their physical appearance but their chemical makeup as well. When eaten, the egg is changed again, by substances in the body called enzymes.This digestive action is another example of a chemical change. What happens during such a process depends on the chemical properties of the specific enzymes and of the food involved.
All measurable properties of matter fall into two categories: extensive properties and intensive properties. The measured value of an extensive property depends on how much matter is being considered.Mass, length, and volume are extensive prop-erties. More matter means more mass. Values of the same extensive property can be added together. For example, two copper pennies have a combined mass that is the sum of the masses of each penny, and the total volume occupied by the water in two beakers is the sum of the volumes of the water in each of the beakers.
1.4 Physical and Chemical Properties of Matter 7
Homogeneous mixtures
Mixtures
Separation by chemical methods Separation by
physical methods Matter
Pure substances
Heterogeneous
mixtures Compounds Elements
Figure 1.5
Classification of matter.
The measured value of an intensive propertydoes not depend on the amount of mat-ter being considered. Temperature is an intensive property. Suppose that we have two beakers of water at the same temperature. If we combine them to make a single quan-tity of water in a larger beaker, the temperature of the larger amount of water will be the same as it was in two separate beakers. Unlike mass and volume, temperature and other intensive properties such as melting point, boiling point, and density are not additive.
1.5
Measurement
The study of chemistry depends heavily on measurement. For instance, chemists use measurements to compare the properties of different substances and to assess changes resulting from an experiment. A number of common devices enable us to make sim-ple measurements of a substance’s properties: The meterstick measures length; the buret, the pipet, the graduated cylinder, and the volumetric flask measure volume (Figure 1.6); the balance measures mass; the thermometer measures temperature. These instruments provide measurements of macroscopic properties, which can be determined directly.Microscopic properties,on the atomic or molecular scale, must be determined by an indirect method,as we will see in Chapter 2.
A measured quantity is usually written as a number with an appropriate unit. To say that the distance between New York and San Francisco by car along a certain route is 5166 is meaningless. We must specify that the distance is 5166 kilometers. In science, units are essential to stating measurements correctly.
SI Units
For many years scientists recorded measurements in metric units, which are related decimally, that is, by powers of 10. In 1960, however, the General Conference of Weights and Measures, the international authority on units, proposed a revised metric
8 CHAPTER 1 Introduction
Graduated cylinder Volumetric flask Pipet
Buret
mL 100
90
80
70
60
50
40
30
20
10 mL
0
1
2
3
4
15
16
17
18
20 19
25 mL
1 liter Figure 1.6
Some common measuring devices found in a chemistry laboratory. These devices are not drawn to scale relative to one another. We will discuss the uses of these measuring devices in Chapter 4.
Interactivity:
SI Base Units ARIS, Interactives
Interactivity:
system called the International System of Units (abbreviated SI, from the French
System International d’Unites). Table 1.2 shows the seven SI base units. All other SI units of measurement can be derived from these base units. Like metric units, SI units are modified in decimal fashion by a series of prefixes, as shown in Table 1.3. We use both metric and SI units in this book.
Measurements that we will utilize frequently in our study of chemistry include time, mass, volume, density, and temperature.
Mass and Weight
Mass is a measure of the quantity of matter in an object. The terms “mass” and “weight” are often used interchangeably, although, strictly speaking, they refer to dif-ferent quantities. In scientific terms, weight is the force that gravity exerts on an object. An apple that falls from a tree is pulled downward by Earth’s gravity. The mass of the apple is constant and does not depend on its location, but its weight does. For example, on the surface of the moon the apple would weigh only one-sixth what it does on Earth, because of the smaller mass of the moon. This is why astronauts
1.5 Measurement 9
Base Quantity Name of Unit Symbol
Length meter m
Mass kilogram kg
Time second s
Electrical current ampere A
Temperature kelvin K
Amount of substance mole mol
Luminous intensity candela cd
TABLE 1.2 SI Base Units
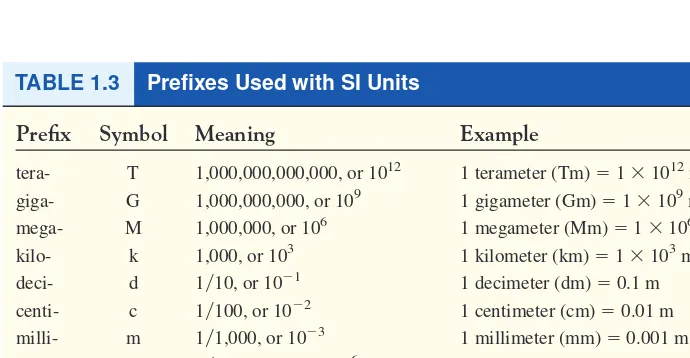
Prefix Symbol Meaning Example
tera- T 1,000,000,000,000, or 1012 1 terameter (Tm) ⫽1 ⫻1012m
giga- G 1,000,000,000, or 109 1 gigameter (Gm) ⫽1 ⫻109m mega- M 1,000,000, or 106 1 megameter (Mm) ⫽1 ⫻106m
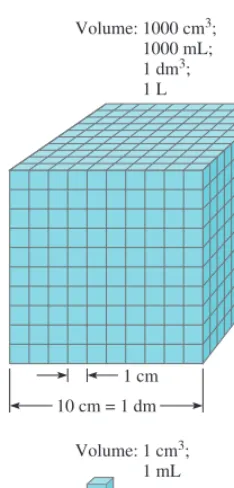
kilo- k 1,000, or 103 1 kilometer (km) ⫽1 ⫻103m deci- d 1兾10, or 10⫺1 1 decimeter (dm) ⫽0.1 m
centi- c 1兾100, or 10⫺2 1 centimeter (cm) ⫽0.01 m
milli- m 1兾1,000, or 10⫺3 1 millimeter (mm) ⫽0.001 m
micro- 1兾1,000,000, or 10⫺6 1 micrometer (m) ⫽1 ⫻10⫺6m
nano- n 1兾1,000,000,000, or 10⫺9 1 nanometer (nm)