O R I G I N A L A R T I C L E
Effects of washing with water on enzymatic saccharification
and
D-lactic acid production from steam-exploded sugarcane
bagasse
Chizuru Sasaki•Ryosuke Okumura•
Ai Asakawa•Chikako Asada•Yoshitoshi Nakamura
Received: 1 December 2011 / Accepted: 28 May 2012 / Published online: 27 June 2012
ÓSpringer 2012
Abstract This study investigated the production of
D-lactic acid from unutilized sugarcane bagasse using
steam explosion pretreatment. The optimal steam pressure for a steaming time of 5 min was determined. The steam-exploded sugarcane bagasse was hydrolyzed using cellu-lase (Meicecellu-lase) and then the hydrolyzate was subjected to fermentation substrate. By enzymatic saccharification using Meicelase, the highest recovery of glucose from raw bagasse, 73.7 %, was obtained at a steam pressure of 20 atm. For extracted residue with water after steam explosion, the glucose recovery increased up to 94.9 % at a steam pressure of 20 atm. These results showed that washing with water is effective in removing enzymatic reaction inhibitors. After steam pretreatment (steam pres-sure of 20 atm), D-lactic acid was produced by
Lactoba-cillus delbrueckii NBRC 3534 from the enzymatic hydrolyzate of steam-exploded bagasse and washed resi-due. The conversion rate ofD-lactic acid obtained from the
glucose concentration was 66.6 % for the hydrolyzate of steam-exploded bagasse without washing with water and 90.0 % for that derived from the extracted residue with water after steam explosion. These results also demon-strated that the hydrolyzate of steam-exploded bagasse (without washing with water) contains fermentation inhibitors and washing with water can remove them.
Keywords Sugarcane bagasse Steam explosion
Enzymatic saccharificationD-Lactic acid
Introduction
Sugarcane bagasse, a byproduct of the sugar industry, is an abundant source of lignocellulose. Although it is used as fuel for boilers, a large quantity of this material is accu-mulated in sugar processing plants, which leads to envi-ronmental problems. Advanced utilization of this material as a carbon resource holds promise for the bioproduction of useful chemicals.
The main components of cellulosic biomass, sugarcane bagasse, are cellulose, hemicellulose, and lignin. Because cellulose is surrounded by a hard network of lignin, it is necessary to decompose it using an environmentally friendly pretreatment method prior to its conversion into glucose using an enzymatic method.
Over the years, various pretreatment methods for the sugarcane bagasse have been developed, including physical [1], chemical [2], hydrothermal [3,4], hydrothermal-chem-ical [5], and hydrothermal-biological [6] methods. Steam explosion is a hydrothermal pretreatment method for plant biomass that uses high-pressure and high-temperature steam without the addition of any chemicals. And this method involves chemical effects such as auto hydrolysis, defibria-tion, and delignification. Compared to the generally used chemical treatments, it has several potential advantages: it eliminates the use of toxic substances, such as strong acids or alkalis, chemical tolerance equipment, waste solution processing, and preliminary feedstock size reduction. On the other hand, degradation products from sugarcane bagasse, which are inhibitors for enzymatic saccharification and fer-mentation, are generated. Therefore, the removal process with chemical reagents such as alkali is necessary [3].
Lactic acid is widely used in the food, pharmaceutical, and chemical industries. In particular, lactic acid has been used as a raw material for manufacturing poly(lactic acid),
C. Sasaki (&)R. OkumuraA. AsakawaC. Asada Y. Nakamura
Department of Life System, Institute of Technology and Science, The University of Tokushima, 2-1 Minamijosanjima-cho, Tokushima 770-8506, Japan
one of the most promising biodegradable polymers. For this reason, many researchers have studied L-lactic acid
production strategies using unutilized cellulosic plant material and starchy biomass, such as paper sludge, water wastes, agro wastes, and beer production byproducts [7–10]. Recently, it was discovered thatD-type andL-type
lactic acids form stable stereocomplexes that have thermal properties different from those of the original polylactic acid made from onlyL-type orD-type lactic acid [11–13].
However, few studies have focused on strategies for pro-ducingD-lactic acid from unutilized plant biomass [14,15].
The main objective of this study is to evaluate an envi-ronmentally friendly method for the production ofD-lactic
acid from sugarcane bagasse using steam explosion. For this purpose, optimal steam explosion pretreatment conditions for sugarcane bagasse followed by enzymatic saccharifica-tion and microbial conversion of hydrolyzates derived from sugarcane bagasse to D-lactic acid by Lactobacillus
delbrueckii were investigated. In the process, the removal method of hydrolyzate and fermentation inhibitors with water extraction was investigated, and the final amount of production ofD-lactic acid was evaluated.
Materials and methods
Sugarcane bagasse
The raw sugarcane bagasse from Kyuyo Sugar Co. Ltd. (Okinawa, Japan) used in these experiments was cut into 1–3-cm-sized chips. The chemical composition (based on dry material) of bagasse was 45.1 % a-cellulose, 22.3 % Klason lignin, 27.9 % hemicellulose, and 4.7 % other. Steam explosion
Pretreatment of the bagasse was conducted in a steam explosion apparatus (Japan Chemical Engineering and Machinery Co. Ltd., Osaka, Japan). The reactor was charged with 100 g (dry matter) of feedstock per batch. Saturated steam from the boiler was then allowed to enter the reactor to heat the bagasse at a controlled pressure of 15, 20, 25, 30, or 35 atm. Each pressure was maintained for 5 min and then the reactor was suddenly depressurized. The exploded bagasse was recovered in a cyclone and cooled to room temperature.
Components analysis of bagasse and steam-exploded bagasse
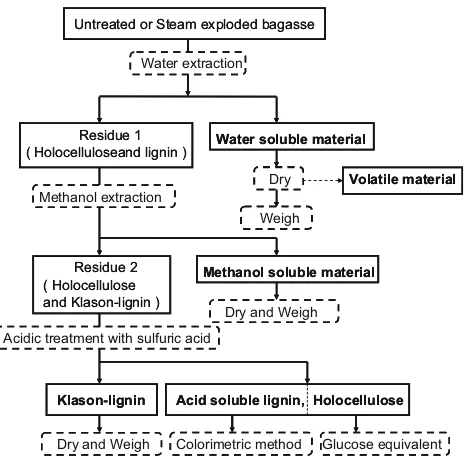
Components analysis of untreated bagasse and steam-exploded bagasse was conducted by Wayman’s method [16] using distilled water and methanol as extractive
solvents. The method is illustrated in Fig.1. The amount of components, i.e., water-soluble material (WSM), metha-nol-soluble material, acid-soluble lignin, holocellulose (cellulose and hemicellulose), and Klason lignin, and vol-atile materials were determined as follows.
Five grams of dry untreated or steam-exploded bagasse was added to 100 mL of distilled water and extracted for 24 h at room temperature. The solid (Residue 1) and liquid materials were separated by filtration, the filtrate was recovered from the liquid, then concentrated, dried, and weighed (WSM). Residue 1 was extracted at 80°C for
24 h in a Soxhlet extractor using 150 mL of methanol to dissolve the methanol-soluble material. After concentration and drying of the extract, the methanol-soluble material was weighed. Residue 2 from the methanol extraction consisted of holocellulose and Klason lignin, a high molecular weight lignin. This residue (1 g) was added to 15 mL of 72 % (w/v) sulfuric acid and kept at room tem-perature for 4 h. After that, the content was placed in a 100-mL conical flask, washed with 560 mL of distilled water, and then autoclaved for 1 h. After the insoluble material was washed with distilled water, it was heat-dried at 105°C to a constant weight and weighed (Klason
lignin). The weight of holocellulose was calculated by subtracting the weight of Klason lignin from 1 g of Residue 2. The volatile materials, i.e., degradation products from sugar [organic acids, i.e., acetic acid and formic acid, furfural, and 5-hydroxymethyl furfural (HMF)] analysis was carried out using the HPLC system with a refractive index detector with a Bio-Rad HPX-87H column at a
Residue 1 ( Holocelluloseand lignin )
Untreated or Steam exploded bagasse
Water soluble material
Residue 2 ( Holocellulose and Klason-lignin )
Methanol soluble material
Klason-lignin Acid soluble lignin, Holocellulose
Methanol extraction
Acidic treatment with sulfuric acid Water extraction
Dry Volatile material
Dry and Weigh Weigh
Dry and Weigh Colorimetric method Glucose equivalent Residue 1
( Holocelluloseand lignin )
Untreated or Steam exploded bagasse
Water soluble material
Residue 2 ( Holocellulose and Klason-lignin )
Methanol soluble material
Klason-lignin Acid soluble lignin, Holocellulose
Methanol extraction
Acidic treatment with sulfuric acid Water extraction
Dry
Dry Volatile material
Dry and Weigh Weigh Weigh
Dry and Weigh Colorimetric method Glucose equivalent
temperature of 50°C. The mobile phase was 5.0 mM
H2SO4, at a flow rate of 0.6 mL/min.
Each reducing sugar component in WSM and water-insoluble residue from steam-exploded bagasse was deter-mined using a sulfuric acid hydrolyzate of bagasse according to the Technical Association of the Pulp and Paper Industry Standard. Following that, each hydrolyzate was analyzed by the reducing sugar analysis system (Prominence series with post-column derivatization system using arginine, Shimadzu Co., Ltd.) with a fluorescence detector (RF-20Axs) [17]. All analytical determinations were performed in duplicate and the average results are shown.
Enzymatic hydrolysis
Steam-exploded bagasse was enzymatically hydrolyzed using Meicelase (produced by Trichoderma viride) pro-vided by Meiji Seika Co., Ltd. Enzymatic hydrolysis was performed using a 0.05-M sodium phosphoric acid buffer (pH 4.5) at 50°C on a rotary shaker at 140 rpm for 48 h.
The substrate, i.e., steam-exploded bagasse or water-extracted residue after steam explosion, concentration and enzyme loading were 100 g/L and 50 FPU/g substrate, respectively. The supernatant was centrifuged to remove solid waste and analyzed for glucose. All enzymatic hydrolysis experiments were performed in duplicate and the average results are shown.
Microorganism and inoculum cultivation
Lactobacillus delbrueckii NBRC 3534, a homofermenta-tive D-lactic acid producer, was used to produce D-lactic
acid from the hydrolyzate of steam-exploded bagasse. The microorganisms were subcultured every 4 weeks. The strain was precultured in 100 mL of medium in a 300-mL flask at 30°C for 24 h using a static incubator at 60 rpm.
The preculture media comprised 0.1 g/L (NH4)2SO4, 10 g/L glucose, 0.1 g/L KH2PO4, 0.1 g/L MgSO47H2O, and 1.0 g/L yeast extract. Later, the cells were harvested by centrifugation (2000g), rinsed thoroughly with sterile dis-tilled water, centrifuged again, and then resuspended in sterile distilled water.
D-Lactic acid fermentation using bagasse hydrolyzate
at optimal conditions
The nutrient medium for D-lactic acid fermentation
con-tained hydrolyzate derived from steam-exploded bagasse (the initial glucose concentration was adjusted at 20 g/L, the hydrolyzate was freeze-dried and then dissolved with water to adjust the concentration of the quantity of 20 g/L), yeast extract 0.1 g/L, ammonium sulfate 0.1 g/L, KH2PO4 0.1 g/L, MgSO47H2O 0.01 g/L, 0.05 M acetate buffer (pH
5.0), and 10 % (v/v) preculture solution. The nutrient medium and buffer were autoclaved at 121°C for 20 min.
The hydrolyzate was added after sterilizing with a 0.22-lm pore size filter. Cultivation was carried out in a 300-mL flask with 100 mL of the medium using a static incubator at 37°C. Aliquots of the samples were collected and assayed
for determining ethanol and residual glucose concentra-tions. All fermentative experiments were performed in duplicate and the average results are shown.
Determination of residual glucose and produced
D-lactic acid
Residual glucose and producedD-lactic acid concentrations in
the fermentation medium were determined by the mutarotase GOD method (Glucose C-II test, Wako Pure Chemicals Co., Ltd., Japan) and an enzymatic UV test withD-lactate
dehy-drogenase (D-LDH) (enzymatic fluid D-lactic acid,
R-Biop-harm GmbH, Darmstadt, Germany), respectively.
Results and discussion
Composition analysis of steam-exploded bagasse
To break down the lignin network that surrounds cellulose and increase the accessibility of the enzyme to cellulose, steam explosion was used to treat the bagasse. Table1
drying under 105°C, which was contained in the WSM.
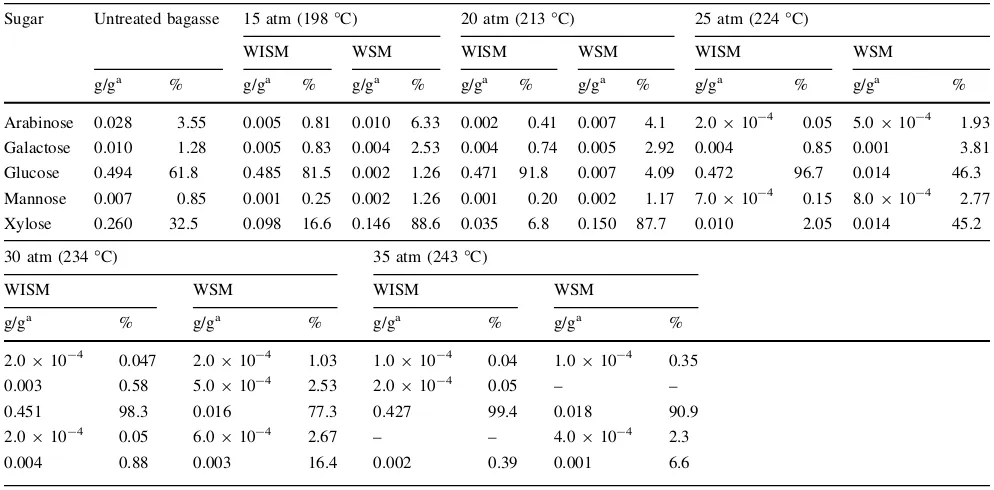
From the steam-exploded bagasse, furfural, HMF, acetic acid, and formic acid were generated. Acetic acid is liberated from acetyl groups in the hemicellulose fraction; furfural and HMF are products of pentose and hexose degradation, respectively. Moreover, formic acid is formed from furfural and HMF at elevated temperatures [18,24]. As can be observed, by increasing the pressure, i.e., tem-perature, the amount of these products was increased. Generally, these compounds are known to the inhibitors for fermentation [25]; therefore, it is necessary to remove these products.
Sugar composition analysis of steam-exploded bagasse It is necessary to analyze the sugar composition of pre-treated material to investigate the relationship between sugar and the conditions of steam explosion. Table2shows the sugar composition in WSM and water-insoluble
material (WISM) of steam-exploded bagasse at different steam pressures with a steaming time of 5 min. It was clear that untreated bagasse contained 0.494 and 0.26 g of fer-mentable sugars, i.e., glucose (converted into lactic acid or ethanol) and xylose (mainly converted into xylitol) in 1 g of dry bagasse, respectively. After steam explosion, the amounts of glucose in WISM were 0.485, 0.471, and 0.472 g in 1 g of dry untreated bagasse at steam pressures of 15, 20, and 25 atm, respectively. These correspond to 98.2, 95.3, and 95.5 % recovery of glucose (glucose in the untreated bagasse, i.e., 0.494 g of glucose in 1 g of dried untreated bagasse). At steam pressures of 30 and 35 atm, the amount of glucose gradually decreased. The xylose in WISM decreased dramatically with the increase in steam pressure. The amount was 0.098 g in 1 g of untreated bagasse at a steam pressure of 15 atm, which corresponds to a recovery rate of 37.7 % from the initial amount of xylose. In contrast, the amount of xylose in WSM increased (0.146 g in 1 g of dry bagasse). This result shows that the decomposition and solubilization of hemicellulose (mainly xylose) in water is caused by the increase of the treatment temperature (pressure). The amount of xylose in WSM was maximum (0.15 g in 1 g of dried untreated bagasse) at a steam pressure of 20 atm and then decreased with an increase in steam pressure. These results indicate that the decomposition of xylose started at around 210°C [26,27].
Enzymatic saccharification of steam-exploded bagasse The effect of steam explosion conditions on the enzymatic saccharification of bagasse was studied using a substrate concentration of 100 g/L and the results are presented in Fig.3a. Steam pressures of 15, 20, 25, 30, and 35 atm for a steaming time of 5 min were investigated. The maximum amount of glucose produced was observed at a steam pressure of 20 atm and steaming time of 5 min: 364 mg/g dry steam-exploded bagasse, which corresponds to a 73.7 % glucose yield from untreated bagasse. For steam pressures of 25, 30, and 35 atm, the glucose yield decreased more than that for a steam pressure of 15 atm. Figure3b shows the enzymatic saccharification of washed
Table 1 Chemical composition (%) of untreated and steam-exploded bagasse at different steam pressures (steaming time 5 min) Components Untreated bagasse Steam pressure (atm) [steaming temperature]
15 [198°C] 20 [213°C] 25 [224°C] 30 [234°C] 35 [243°C]
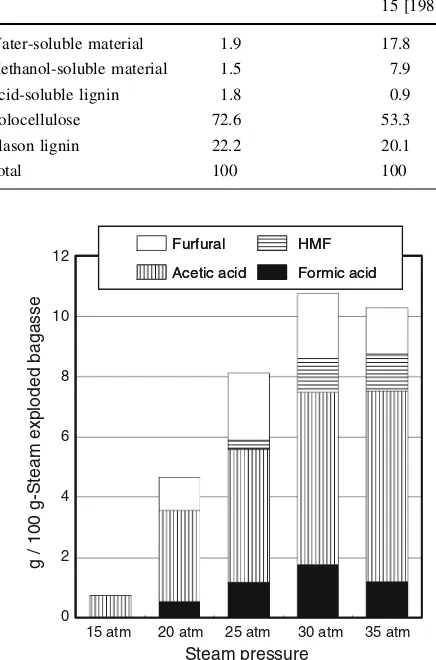
Water-soluble material 1.9 17.8 13.6 11.2 9.7 10.0
Methanol-soluble material 1.5 7.9 22.0 22.8 23.7 22.6
Acid-soluble lignin 1.8 0.9 0.4 0.4 0.4 0.4
Holocellulose 72.6 53.3 46.2 44.6 41.7 39.0
Klason lignin 22.2 20.1 17.8 21.0 24.5 28.0
Total 100 100 100 100 100 100
Steam pressure
12
10
8
6
4
2
0
Furfural HMF
Acetic acid Formic acid
g / 100 g-Steam exploded bagasse
15 atm 20 atm 25 atm 30 atm 35 atm Furfural HMF
Acetic acid Formic acid Furfural HMF
Acetic acid Formic acid
steam-exploded bagasse, i.e., WISM, after steam explosion as a substrate (substrate concentration is 50 g/L). Under all conditions, the amount of glucose produced increased. Similarly, higher glucose concentration was achieved at steam pressures of 20 and 25 atm. The value was 469 mg in 1 g of steam-exploded dry bagasse, corresponding to a 94.9 % glucose yield from untreated bagasse. These results indicated that, if water-soluble inhibitors for saccharifica-tion were washed with water, the enzyme could access cellulose in the treated bagasse. Dunlop [20], Ulbricht et al. [28], Bardet and Robert [29], and Lapierre et al. [30] reported that, during high-temperature or acid pretreatment of wood, water-soluble inhibitors such as sugar- and lignin-derived materials, organic acids, fran compounds, and phenol compounds, are formed. Delgenes et al. [31] and
Palmqvist et al. [32] reported that organic acids, i.e., acetic acid and fran derivatives, are well-known inhibitors for yeasts and bacteria, but have little effect on cellulases. Kim et al. [33] also demonstrated that 13 g/L acetic acid and furfurals at 4 g/L, which is larger than of our concentration, were not inhibitory for enzymatic saccharification using cellulase. On the other hand, simple phenolic compounds, such as vanillin, caffeine acid, and catechol [34], were reported as cellulase inhibitors. Other researchers reported that polyphenolics also inhibit cellulase reaction [35, 36]. Consequently, the removal of phenolic compounds with water resulted in an increase of glucose recovery. From the viewpoint of low energy input, this study indicated that the optimal conditions for the steam explosion of bagasse was a steam pressure of 20 atm for a steaming time of 5 min.
Table 2 Sugar composition of water soluble material (WSM) and water insoluble material (WISM) of steam exploded bagasse at different steam pressures
Sugar Untreated bagasse 15 atm (198°C) 20 atm (213°C) 25 atm (224°C)
WISM WSM WISM WSM WISM WSM
g/ga % g/ga % g/ga % g/ga % g/ga % g/ga % g/ga %
Arabinose 0.028 3.55 0.005 0.81 0.010 6.33 0.002 0.41 0.007 4.1 2.0910-4 0.05 5.0
910-4 1.93
Galactose 0.010 1.28 0.005 0.83 0.004 2.53 0.004 0.74 0.005 2.92 0.004 0.85 0.001 3.81
Glucose 0.494 61.8 0.485 81.5 0.002 1.26 0.471 91.8 0.007 4.09 0.472 96.7 0.014 46.3
Mannose 0.007 0.85 0.001 0.25 0.002 1.26 0.001 0.20 0.002 1.17 7.0910-4 0.15 8.0910-4 2.77
Xylose 0.260 32.5 0.098 16.6 0.146 88.6 0.035 6.8 0.150 87.7 0.010 2.05 0.014 45.2
30 atm (234°C) 35 atm (243°C)
WISM WSM WISM WSM
g/ga % g/ga % g/ga % g/ga %
2.0910-4 0.047 2.0910-4 1.03 1.0910-4 0.04 1.0910-4 0.35
0.003 0.58 5.0910-4 2.53 2.0910-4 0.05 – –
0.451 98.3 0.016 77.3 0.427 99.4 0.018 90.9
2.0910-4 0.05 6.0
910-4 2.67 – – 4.0
910-4 2.3
0.004 0.88 0.003 16.4 0.002 0.39 0.001 6.6
a Based on untreated bagasse
B A
mg-glucose/g-dry steam exploded bagasse
0
10 20 30 40 10 20 30 40 50
500
0 50
400
300
200
100
0
500
400
300
200
100
0
Time (h) Time (h) mg-glucose/g-dry steam exploded bagasse
Fig. 3 Enzymatic saccharification of steam-exploded bagasse (a) and water-insoluble residue after steam explosion (b).Open circles
steam pressure 15 atm,filled squares20 atm,open squares
D-Lactic acid production from bagasse
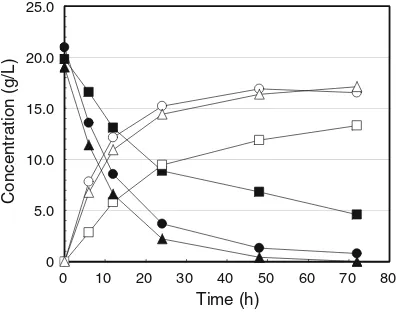
Figure4 shows the time courses of glucose consumption andD-lactic acid production byLactobacillus delbrueckii
NBRC 3534 using available glucose, steam-exploded bagasse (steam pressure of 20 atm, steaming time 5 min), and water-insoluble residue after steam explosion (20 atm, 5 min) as substrates. When water-insoluble residue after steam explosion was used as the substrate, D-lactic acid
production behavior similar to the results obtained using commercially available glucose was observed. In this case, the maximum amounts of D-lactic acid produced were
16.5 g/L (available glucose) and 17.1 g/L (water-insoluble residue), at a 72-h cultivation time, which corresponds to 81.7 and 90.0 % of the conversion rate of the initial glucose concentration, respectively. On the other hand, when steam-exploded bagasse was used as the substrate, i.e., without washing with water after steam explosion
treatment, the amount ofD-lactic acid produced decreased
(conversion rate was 66.5 %) and no dramatic glucose consumption was observed. This phenomenon also showed the presence of fermentation inhibition materials in the steam-exploded bagasse, similar to the case of enzymatic saccharification. This study found that a simple method with low environmental loading, such as washing only with water, could remove the inhibitors for D-lactic acid
pro-ducers and cellulase from steam-exploded bagasse. As a result, the final amounts of D-lactic acid production were
compared between the method without water extraction and with water extraction after steam explosion of bagasse (Fig.5). Only 24.2 g of D-lactic acid was produced from
100 g of bagasse using the process without water extrac-tion; however, 42.2 g ofD-lactic acid can be produced from
100 g of bagasse using the process with water extraction after steam explosion.(steam pressure of 20 atm and steaming time 5 min).
Conclusions
This study showed that high amounts of glucose could be produced from the enzymatic hydrolyzate of steam-explo-ded bagasse. In particular, washing the steam-explosteam-explo-ded sample with water effectively removes the inhibitors that interrupt the reaction between cellulose and the enzyme (cellulase). Furthermore, it was found that a large amount of D-lactic acid could be produced from a hydrolyzate of
water-extracted residue of bagasse after steam explosion usingLactobacillus delbrueckiiNBRC 3534, while, only a small amount of D-lactic acid was produced from a
hydrolyzate of steam-exploded bagasse. Thus, washing with water is a simple and environmentally friendly method for removing inhibitors from the pretreated residue. This study will contribute to improving large-scale indus-trial methods for the production of D-lactic acid from
unutilized plant materials.
Fig. 4 Residual glucose concentration andD-lactic acid production profiles by Lactobacillus delbrueckii NBRC3534. Filled circles
glucose with commercial glucose, open circles D-lactic acid with commercial glucose, filled squares glucose with steam-exploded bagasse, open squaresD-lactic acid with steam-exploded bagasse,
filled triangles glucose with water-insoluble residue after steam explosion, and open triangles D-lactic acid with water-insoluble residue after steam explosion
Conversion rate*1
(100 g) Conversion rate* 1 Conversion rate*1
( 36.4 %)
Glucose
(36.4 g) Conversion rate* 2 Conversion rate*2
( 66.6 %)
D-lactic acid (24.2 g) Without water extraction after steam explosion of bagasse
With water extraction after steam explosion of bagasse
Steam exploded bagasse
*1: = Produced glucose (mg) / 1 g of dry steam exploded bagasse 100
*2: = Produced D-lactic acid (g/l) / Initial glucose concentration (g/l) 100
Acknowledgments A part of this study was funded by the Shiseido Female Researcher Science Grant and a Grant-in-Aid for Young Scientists (B) (no. 21750159) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
1. da Silva AS, Inoue H, Endo T, Yano S, Bon EPS (2010) Milling pretreatment of sugarcane bagasse and straw for enzymatic hydro-lysis and ethanol fermentation. Bioresour Technol 101:7402–7409 2. Zhao X, Peng F, Cheng K, Liu D (2009) Enhancement of the
enzymatic digestibility of sugarcane bagasse by alkali-peracetic acid pretreatment. Enzyme Microb Technol 44(1):17–23 3. Rocha GJM, Gonc¸alves AR, Oliveira BR, Olivares EG, Rossell
CEV (2012) Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production. Ind Crops Prod 35:274–279
4. Soares IB, Travassos JA, Baudel HM, Benachour M, Abreu CAM (2011) Effects of washing, milling and loading enzymes on the enzymatic hydrolysis of a steam pretreated sugarcane bagasse. Ind Crops Prod 33:670–675
5. Binod P, Satyanagalakshmi K, Sindhu R, Janu KU, Sukumaran RK, Pandey A (2012) Short duration microwave assisted pre-treatment enhances the enzymatic saccharification and ferment-able sugar yield from sugarcane bagasse. Renew Energy 37(1): 109–116
6. Sasaki C, Takada R, Watanabe T, Honda Y, Karita S, Nakamura Y, Watanabe T (2011) Surface carbohydrate analysis and bio-ethanol production of sugarcane bagasse pretreated with the white rot fungus,Ceriporiopsis subvermisporaand microwave hydro-thermolysis. Bioresour Technol 102:9942–9946
7. Marques S, Santos JAL, Gı´rio FM, Roseiro JC (2008) Lactic acid production from recycled paper sludge by simultaneous sac-charification and fermentation. Biochem Eng J 41:210–216 8. Nakasaki K, Adachi T (2003) Effects of intermittent addition of
cellulase for production ofL-lactic acid from wastewater sludge by simultaneous saccharification and fermentation. Biotechnol Bioeng 82:263–270
9. John RP, Nampoothiri KM, Pandey A (2006) Solid-state fer-mentation for L-lactic acid production from agro wastes using
Lactobacillus delbrueckii. Process Biochem 41:759–763 10. Shindo S, Tachibana T (2004) Production ofL-lactic acid from
spent grain, a by-product of beer production. J Inst Brew 110: 347–351
11. Ikeda Y, Jamshidi K, Tsuji H, Hyon SH (1987) Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 20:904–906
12. Tsuji H, Horii F, Hyon SH, Ikada Y (1991) Stereocomplex for-mation between enantiomeric poly(lactic acid)s. 2. Stereocomplex formation in concentrated solutions. Macromolecules 24:2719–2724 13. Slivniak R, Domb AJ (2002) Stereocomplexes of enantiomeric lactic acid and sebacic acid ester-anhydride triblock copolymers. Biomacromolecules 3:754–760
14. Fukushima K, Sogo K, Miura S, Kimura Y (2004) Production of D-lactic acid by bacterial fermentation of rice starch. Macromol Biosci 4:1021–1027
15. Lu Z, Lu M, He F, Yu L (2009) An economical approach for D-lactic acid production utilizing unpolished rice from aging paddy as major nutrient source. Bioresour Technol 100:2026–2031 16. Chua MGS, Wayman M (1979) Characterization of
autohydro-lysis aspen (P. tremuloides) lignins. Part 1. Composition and molecular weight distribution of extracted autohydrolysis linin. Can J Chem 57:1141–1149
17. Mikami H, Ishida Y (1983) Post-column fluorometric detection of reducing sugars in high performance liquid chromatography using arginine (in Japanese). Bunseki Kagaku 32:E207–E210 18. Palmqvist E, Hahn-Ha¨gerdal B (2000) Fermentation of
ligno-cellulosic hydrolysates. II: inhibitors and mechanisms of inhibi-tion. Bioresour Technol 74:25–33
19. Ando H, Sakaki T, Kokusho T, Shibata M, Uemura Y, Hatate Y (2000) Decomposition behavior of plant biomass in hot-com-pressed water. Ind Eng Chem Res 39:3688–3693
20. Dunlop AP (1948) Furfural formation and behavior. Ind Eng Chem 40:204–209
21. Nakamura Y, Sawada T, Inoue E (2001) Enhanced ethanol pro-duction from enzymatically treated steam-exploded rice straw using extractive fermentation. J Chem Technol Biotechnol 76: 879–884
22. Kobayashi F, Take H, Asada C, Nakamura Y (2004) Methane production from steam-exploded bamboo. J Biosci Bioeng 97: 426–428
23. Asada C, Nakamura Y, Kobayashi F (2005) Chemical charac-teristics and ethanol fermentation of the cellulose component in autohydrolyzed bagasse. Biotechnol Bioprocess Eng 10:346–352 24. Larsson S, Palmqvist E, Hahn-Ha¨gerdal B, Tengborg C, Stenberg K, Zacchi G, Nilvebrant N-O (1999) The generation of fermen-tation inhibitors during dilute acid hydrolysis of softwood. Enzyme Microb Technol 24:151–159
25. Helle S, Cameron D, Lam J, White B, Duff S (2003) Effect of inhibitory compounds found in biomass hydrolysates on growth and xylose fermentation by a genetically engineered strain of
S. cerevisiae. Enzyme Microb Technol 33:786–792
26. Ruiz E, Cara C, Manzanares P, Ballesteros M, Castro E (2008) Evaluation of steam explosion pre-treatment for enzymatic hydrolysis of sunflower stalks. Enzyme Microb Technol 42: 160–166
27. Caparro´s S, Ariza J, Lo´pez F, Nacimiento JA, Garrote G, Jime´nez L (2008) Hydrothermal treatment and ethanol pulping of sun-flower stalks. Bioresour Technol 99:1368–1372
28. Ulbricht RJ, Northup SJ, Thomas JA (1984) A review of 5-hydroxymethylfurfural (HMF) in parenteral solutions. Fundam Appl Toxicol 4:843–853
29. Bardet M, Robert DR (1985) On the reactions and degradation of the lignin during steam hydrolysis of aspen wood. Svensk Papperstidning 6:61–67
30. Lapierre C, Rolando C, Monties B (1983) Characterization of poplar lignins acidolysis products: capillary gas–liquid and liquid–liquid chromatography of monomeric compounds. Holzf-orschung 37:189–198
31. Delgenes JP, Moletta R, Navarro JM (1996) Effects of ligno-cellulose degradation products on ethanol fermentations of glu-cose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, andCandida shehatae. Enzyme Microb Technol 19:220–225
32. Palmqvist E, Almeida JS, Hahn-Ha¨gerdal B (1999) Influence of furfural on anaerobic glycolytic kinetics of Saccharomyces cerevisiaein batch culture. Biotechnol Bioeng 62:447–454 33. Kim Y, Ximenes E, Mosier NS, Ladisch MR (2011) Soluble
inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzyme Microb Technol 48:408–415
34. Highley TL (1975) Inhibition of cellulases of wood-decay fungi. USDA Forest Service Research Paper FPL 247:1–8
35. Mcallister TA, Bae HD, Yanke LJ, Cheng KJ, Muir A (1994) Effect of condensed tannins from birdsfoot trefoil on endoglu-canase activity and the digestion of cellulose filter paper by ru-minal fungi. Can J Microbiol 40:298–305