Short communication
Ethanol vapour reduces leaf blackening in cut flower
Protea
‘Pink Ice’ stems
S.G. Crick, R. McConchie *
Department of Crop Sciences,John Woolley Blg,A20,Uni6ersity of Sydney,Sydney,NSW2006,Australia
Received 11 March 1999; accepted 21 July 1999
Abstract
The effect of ethanol vapour on postharvest leaf blackening ofProtea susannaeXcompacta‘Pink Ice’ stems stored in plastic bags under darkness at 20°C (91°C) was assessed over a 19 day period. Application of ethanol vapour to the stems significantly reduced leaf blackening. Stems exposed to 5.6 g ethanol kg−1 stem weight, had the least amount of leaf blackening with less than 20% of leaves blackened by day 14. In contrast, the control stems had 50% of leaves blackened by day 9, and 100% by day 15. The highest ethanol treatment at 11.2 g ethanol kg−1stem weight caused substantial blackening within the first 24 h of the treatment being applied. Ethanol vapour concentrations in the bag head space decreased rapidly in comparison with the bags with no stems, suggesting that ethanol was rapidly taken up by the stems. Only the highest ethanol treatment had detectable levels of ethanol in the bags after 17 days, and ethanol vapour had no effect on CO2concentration in the bag head space. Carbon dioxide concentrations ranged between 1.0 and 2.5%. The rate of leaf blackening on the bagged stems without ethanol was significantly less than on stems not in bags, suggesting that elevated CO2levels may have contributed to reduced blackening. © 1999 Elsevier Science B.V. All rights reserved.
Keywords:Polyphenol oxidase; Leaf browning; Cut flowers
www.elsevier.com/locate/postharvbio
1. Introduction
Leaf blackening in cut protea flower stems re-mains a serious postharvest problem, despite past
efforts to understand the physiological basis for the disorder (Reid et al., 1989; McConchie et al., 1991; Bieleski et al., 1992; McConchie and Lang, 1993; McConchie et al., 1994; Dai and Paull, 1995, also see Jones et al., 1995 for review). Leaf blackening is a particular problem in Protea neri
-ifolia, P. susannaeXcompacta ‘Pink Ice’,P.com
-pacta and P. eximia and usually occurs within
3 – 7 days after harvest. * Corresponding author. Tel./fax: +61-2-9351-4332.
E-mail address: [email protected] (R. Mc-Conchie)
The cause of postharvest leaf blackening in protea stems is not well understood, but it does appear to result from either enzymic (polyphe-nol oxidase (PPO)) or possibly non-enzymic oxi-dation of phenolic compounds (Whitehead and de Swardt, 1982; McConchie et al., 1994; Dai and Paull, 1997). In healthy plants, the phenols involved in the blackening reaction are located in the vacuolar compartment of the cell. There-fore, for leaf blackening to occur, the cell’s compartments must break down after harvest, releasing the phenolic substrate to react with ei-ther the chloroplast-bound PPO or O2.
It has been established that the blackening process is triggered by the rapid transport of carbohydrates from the leaves to the inflores-cence to supply respiratory substrate for the de-veloping inflorescence (McConchie et al., 1991; Bieleski et al., 1992; McConchie and Lang, 1993) and sugars for nectar production (Dai and Paull, 1995). However, the link between deple-tion of leaf carbohydrate and breakdown of the cells has not been explained, nor has it led to an effective treatment for the industry (Mc-Conchie et al., 1994; Dai and Paull, 1997).
Placing cut stems under light to enable the leaves to make their own carbohydrate inhibits leaf blackening to some extent (Reid et al., 1989; Jones and Clayton – Greene, 1992), how-ever, it is not a practical solution for the indus-try, particularly during transport. Addition of sugar to the vase solution doesn’t appear to solve the problem either, since high concentra-tions damage the leaves and low concentraconcentra-tions do not appear to be effective (McConchie and Lang, 1993).
Recently, it was discovered that ethanol va-pour successfully inhibits apple scald (Gha-hamani and Scott, 1998), a postharvest disorder which results in discolouration of the fruit skin similar to that of leaf blackening. Leaf blacken-ing in protea species is closely related to apple scald in so far as both involve oxidation of phe-nolic compounds. The aim of this experiment therefore, was to investigate whether ethanol va-pour had a similar inhibitory effect on phenolic blackening in protea leaves.
2. Materials and methods
2.1. Plant material
Floral stems of Protea susannae X compacta
‘Pink Ice’ were harvested from Harris Park Holdings, Robertson, NSW. The stems were packed and shipped under typical cut flower conditions, arriving approximately 6 h after harvest. The base of the stems (2 cm) were re-cut in air, then placed in distilled water and the number of leaves on the stem standardised to 20 per stem. Five stems were randomly assigned to one of five treatments, A to E, as follows: A, stem in bag, 0 ethanol; B, stem in bag, 2.8 g ethanol kg−1 fresh weight; C, stem in bag, 5.6 g ethanol kg−1 fresh weight; D, stem in bag, 11.2 g ethanol kg−1fresh weight; E, stem without bag, 0 ethanol. Treatment F had no stem, and the highest ethanol concentration applied to the bag. Each stem was weighed, and the appropriate amount of absolute ethanol for that stem was applied to filter paper and placed on a petri dish inside the 65mm thick, low density polyethylene bag. Each bag was inflated to a final volume of 4 L and placed in darkness for 24 h at 20°C (91°C).
2.2. Leaf blackening,ethanol 6apour and CO 2
measurement
Leaf blackening was recorded as the number of leaves (percentage) per stem with at least 10% area blackened over 19 days. The bag head-space was sampled every second day for ethanol vapour and CO2 concentrations. Ethanol gas concentrations were measured using a Hewlett – Packard 5710A gas chomatograph, with a Porapak T column (Alltech, Australia) and a flame ionisation detector. Nitrogen was the carrier gas at a flow rate of 25 ml min−1. Carbon dioxide concentrations were
measured using a Gow Mac 550P gas
2.3. Statistical analysis
The experiment was a completely randomized design with at least five stems used for each treatment and was repeated once. Percentage leaf blackening data were transformed by the formula arc siny to ensure homogeneity of variance (Ott, 1988). Each data set was analysed as a two way analysis of variance (time and ethanol).
3. Results and discussion
3.1. Effect of ethanol on leaf blackening
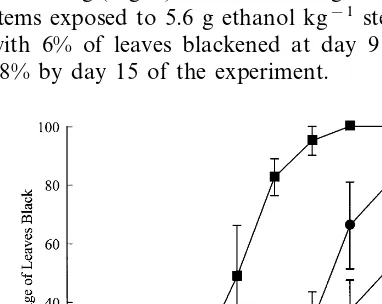
Leaf blackening increased rapidly in the control stems with no bag and no ethanol treatment, showing 50% of leaves blackened by day 9, and 100% blackened by day 15 (Fig. 1). These results are consistent with previous reports (McConchie et al., 1991; Bieleski et al., 1992). In contrast, application of ethanol vapour to the stems was significantly effective (pB0.001) in reducing leaf blackening (Fig. 1). Leaf blackening was lowest in stems exposed to 5.6 g ethanol kg−1
stem weight with 6% of leaves blackened at day 9 and only 18% by day 15 of the experiment.
Fig. 2. Relationship between percentage of leaves at least 10% blackened and amount of ethanol applied in the range 0 – 11.2 g ethanol kg−1stem weight over time. Day 1 (); Day 7 ( ); Day 13 (); Day 19 (").Vertical lines equal 1 SE (n=5).
3.2. Relationship between se6erity of leaf
blackening and ethanol 6apour applied
As ethanol concentration increased, the amount of leaf blackening decreased (pB0.001) for treat-ments up to 5.6 g ethanol kg−1
stem weight (Fig. 2). Leaves exposed to 11.2 g ethanol kg−1
stem weight resulted in enhanced blackening from day 1 onwards. Although leaf blackening increased over time for stems exposed to 5.6 g ethanol kg−1 stem weight, the extent of blackening was signifi-cantly less on day 19 than in the other ethanol treatments and in the control stems not in bags. The results suggest that the optimum amount of ethanol vapour required may lie between 5.6 and 11.2 g ethanol kg−1stem weight (Fig. 2). Ethanol vapour concentrations of between 2.8 and 5.6 g ethanol kg−1 fresh weight were most effective in inhibiting apple scald (Ghahamani and Scott, 1998) indicating that the optimum concentration for inhibiting blackening in proteas is within a similar range.
3.3. Ethanol and CO2 concentrations in the head
space of the bags
The maximum concentration of ethanol in the head space of all treatments was observed on Fig. 1. Percentage of leaves with at least 10% blackened leaf
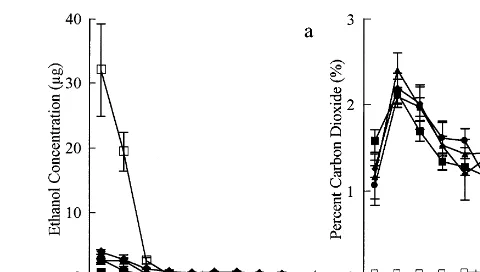
Fig. 3. (a) Mean ethanol vapour concentrations (mg) and (b) CO2concentrations (%) in the headspace (volume approximately 4 l) of sealed 65mm thick low density polyethylene bags without stems (); stem+bag+0 ethanol (); stem+bag+2.8 g ethanol
kg−1 stem weight ( ); stem+bag+5.6 g ethanol kg−1 stem weight (); stem+bag+11.2 g ethanol kg−1stem weight ("). Vertical lines equal 1 SE (n=5).
sample day 1, approximately 24 h after the bags were sealed (Fig. 3a). Uptake of ethanol by the stems was rapid as shown by the difference in concentrations measured between the bags with and without stems.
Ethanol treatment had no significant effect on CO2 concentration in the bag (Fig. 3b). Carbon dioxide concentrations in the head space for all treatments increased from ambient (approxi-mately 0.035%) to 2.5% on day 3 and then slowly decreased to between 1.0 and 1.5% for the remain-ing postharvest period. Interestremain-ingly, the rate of blackening in the bagged stems without ethanol was significantly less than in stems not in bags (Fig. 1). The ‘bag’ effect may be due to the high CO2 concentrations slowing down the respiration rate in the stems and consequently, the use of carbohydrates. The role of high CO2 or low O2 concentrations in slowing the rate of blackening warrants further investigation.
In tomatoes (Ritenour et al., 1997), peach (Lurie and Pesis, 1992) and avocado (Pesis et al., 1998), ethanol inhibits ripening or softening via acetaldehyde. It is suggested that acetaldehyde, in turn, inhibits the ethylene production that would normally trigger ripening in these fruits. Since ethylene production is not associated with protea leaf blackening (McConchie and Lang, 1993), it is difficult to draw parallels between these processes. However, Pesis et al. (1998) report that avocado pulp, which is rich in PPO and turns brown on
exposure to air, was prevented from turning brown by application of acetaldehyde. They sug-gested this might result from acetaldehyde inhibit-ing the oxidizinhibit-ing enzymes.
Postharvest application of ethanol vapour to the protea leaves effectively kept leaf blackening below 20% for two weeks which represents a significant improvement in control of this postharvest disorder. Although these results are preliminary, and do not yet explain the physiolog-ical process in leaf blackening, they do have the potential to assist in solving the leaf blackening problem.
References
Bieleski, R.L., Ripperda, J., Newman, J.P., Reid, M.S., 1992. Carbohydrate changes and leaf blackening in cut flower stems of Protea eximia. J. Amer. Soc. Hort. Sci. 117, 124 – 127.
Dai, J., Paull, E.R., 1995. Source-sink relationship andProtea postharvest leaf blackening. J. Amer. Soc. Hort. Sci. 120, 475 – 480.
Dai, J., Paull, E.R., 1997. Comparison of leaf susceptibility to enzymatic blackening inProtea nerrifoliaR.Br. andLeu -cospermum‘Rachel’. Postharvest Biol. Technol. 11, 101 – 106.
Ghahamani, F., Scott, K.J., 1998. The action of ethanol in controlling superficial scald of apples. Aust. J. Agric. Res. 49, 199 – 205.
Jones, R.B., McConchie, R., van Doorn, W.G., Reid, M.S., 1995. Leaf blackening in cut Protea flowers. Hort. Rev. 17, 173 – 201.
Lurie, S., Pesis, E., 1992. Effect of acetaldehyde and anaerobio-sis as postharvest treatments on the quality of peaches and nectarines. Postharvest Biol. Technol. 1, 317 – 326. McConchie, R., Lang, S.N., Gross, K.C., 1991. Carbohydrate
depletion and leaf blackening inProtea neriifolia. J. Amer. Soc. Hort. Sci. 116, 1019 – 1024.
McConchie, R., Lang, S.N., 1993. Carbohydrate metabolism and possible mechanisms of leaf blackening in Protea neriifoliaunder dark postharvest conditions. J. Amer. Soc. Hort. Sci. 118, 355 – 361.
McConchie, R., Lang, S.N., Lax, A.R., Lang, G.A., 1994. Re – examining polyphenol oxidase, peroxidase and leaf
blackening activity inProtea. J. Amer. Soc. Hort. Sci 119, 1248 – 1254.
Ott, L., 1988. An Introduction to Statistical Methods and Data Analysis. PWS-Kent Publishing Company, Boston, p. 420. Pesis, E., Faiman, D., Dori, S., 1998. Postharvest effects of acetaldehyde vapour on ripening-related enzyme activity in avocado fruit. Postharvest Biol. Technol. 13, 245 – 253. Reid, M.S., van Doorn, W., Newman, J.P., 1989. Leaf
blacken-ing in proteas. Acta Hort. 261, 81 – 84.
Ritenour, M.A., Mangrich, M.E., Beaulieu, J.C., Rab, A., Saltveit, M.E., 1997. Ethanol effects on the ripening of climacteric fruit. Postharvest Biol. Technol. 12, 35 – 42. Whitehead, C.S., de Swardt, G.H., 1982. Extraction and activity
of polyphenoloxidase and peroxidase from senescing leaves ofProtea neriifolia. S. Afr. J. Bot. 1, 127 – 130.