Summary The spatial distribution of plant-available mineral nutrients in forest soils is often highly heterogeneous. To test the hypothesis that local nutrient enrichment of soil leads to increased root proliferation in the nutrient-rich soil zone, we studied the effects of nutrient enrichment on the growth and nutrient concentrations of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco), Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies (L.) Karst.) roots. Three-year-old seedlings were grown for 9 months in split-root containers filled with nutrient-poor forest mineral soil, with one side supplemented with additional mineral nutrients. Root dry weight and root length in Scots pine and Norway spruce were increased in the nutrient-supplemented soil compared with the nonsupple-mented side, whereas root growth in Douglas-fir was unaf-fected by nutrient enrichment. Of the three species examined, Norway spruce exhibited the highest root and shoot growth and the highest nutrient demand. Specific root length (m g−1) and the number of root tips per unit root length were not affected by local nutrient addition in any of the species. Despite in-creased root growth in Norway spruce and Scots pine in nutri-ent-supplemented soil, their root systems contained similar nutrient concentrations on both sides of the split-root container. Thus, coniferous trees may respond to local nutrient supply by increased root proliferation, but the response varies depending on the species, and may only occur when trees are nutrient deficient. As a response to local nutrient enrichment, increases in root dry matter or root length may be better indicators of pre-existing nutrient deficiencies in conifers than increases in root nutrient concentrations.
Keywords: Douglas-fir, local nutrient supply, Norway spruce, nutrient concentrations, root growth, root morphology, Scots pine.
Introduction
Plants may respond to a localized nutrient supply in soil by increasing root growth in the nutrient-rich soil zones. This response is advantageous to plants growing in soils with high spatial heterogeneity in nutrient distribution (Crick and Grime 1987, Jackson and Caldwell 1989), because roots in the
nutri-ent-rich soil zones can absorb more nutrients than roots grow-ing in the nutrient-poor soil zones. Thus, a high spatial plastic-ity of root growth may confer a competitive advantage on plant species in patchy soils (Jackson and Caldwell 1989).
On the other hand, a large, long-lived, and in terms of growth, relatively nonresponsive root system may be advanta-geous to plants on sites where nutrients become temporarily unavailable (e.g., during soil drying cycles) (Crick and Grime 1987, Campbell and Grime 1989). For example, the competi-tive ability of several arid-land grass species growing on natu-ral soils was unrelated to the spatial plasticity of root growth (Larigauderie and Richards 1994). Some species may possess a high physiological plasticity, i.e., an ability to increase ent uptake rates in existing roots during periods of high nutri-ent supply (Caldwell et al. 1992). In general, no consistnutri-ent predictions can be made about the advantages and disadvan-tages of high spatial plasticity of root growth in patchy soils (Hutchings and de Kroon 1994, Robinson 1994). Furthermore, many studies on root growth plasticity have been performed in the presence of extremely high concentrations of nutrients and on artificial substrates where root morphology differs from that of soil-grown plants.
The spatial distribution of nutrients in natural soils is more heterogeneous than in cultivated soils (Cattle et al. 1994). In forest soils, nutrient contents vary greatly over distances of a few meters (Lechowicz and Bell 1991, Koch and Matzner 1993) or even centimeters (Hildebrand 1994), for example, stem flow effects, gaps in the crown cover, old tree stumps, or soil fauna activity. Therefore, the response of forest trees to local nutrient supply is of particular interest. However, al-though there have been some studies of root growth plasticity in forest trees, most studies on root growth plasticity have been done with fast-growing grass species or agricultural crop spe-cies (Larigauderie and Richards 1994, Robinson 1994).
For coniferous tree species, detailed experiments using split-root systems with varying local nutrient concentrations have been described by Coutts and co-authors. Sitka spruce (Picea sitchensis (Bong.) Carr.) root growth in a peat/sand mix in-creased when supplemented with nutrients (Coutts and Philip-son 1976), and root growth in coarse sand increased when
Responses of
Picea
,
Pinus
and
Pseudotsuga
roots to heterogeneous
nutrient distribution in soil
ECKHARD GEORGE,
1BETTINA SEITH,
1CHRISTOPH SCHAEFFER
2and HORST
MARSCHNER
11
Institute of Plant Nutrition, Hohenheim University, 70593 Stuttgart, Germany
2 Physiological Ecology of Plants, Botanical Institute, University of Tübingen, 72076 Tübingen, Germany
Received November 27, 1995
supplemented with nitrogen (N) or phosphorus (P) (Philipson and Coutts 1977). In solution culture, root growth in lodgepole pine (Pinus contorta Dougl. ex Loud.) seedlings increased when supplemented with nutrients (Coutts and Philipson 1977). Local N addition to a peat /vermiculite mix also in-creased root growth of Douglas-fir (Pseudotsuga menziesii (Mirb.) Franco)) seedlings, particularly when the seedlings were N deficient (Friend et al. 1990). However, because these studies were done with artificial rooting substrates, root growth rates may have been higher than in natural soil.
In older, forest-grown trees, the response of root growth to local nutrient enrichment has been studied by means of minirhizotrons or ingrowth cores. When cores were supple-mented with additional nutrients, tree root growth increased (St. John et al. 1983) when the nutrient that was supplied was limiting in the forest ecosystem (Raich et al. 1994).
To study whether increased root growth in nutrient-rich soil patches occurs as a general response irrespective of plant species or plant nutrient status, we investigated the effects of local nutrient supply in soil on root growth and distribution of Douglas-fir, Scots pine, and Norway spruce. We also deter-mined the effects of local nutrient supply on root length, morphology, and nutrient concentrations.
Materials and methods
Experimental details
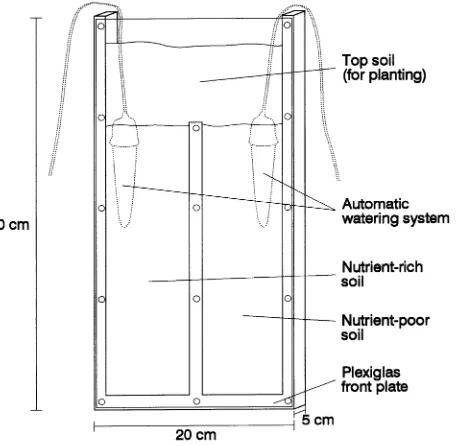
Three-year-old Douglas-fir (Pseudotsuga menziesii), Scots pine (Pinus sylvestris L.), and Norway spruce (Picea abies (L.) Karst.) seedlings were obtained from a commercial nursery. In December 1993, single plants were placed in split-root pots (Figure 1) with six replicates per plant species. The commer-cial growth substrate (peat) and approximately 50% of the original root mass were removed before planting. After
plant-ing, one Douglas-fir and one Scots pine seedling died, leaving five replicates each for these species.
Mineral soil (podsolic Ranker) from a Norway spruce forest at Ludwigsreuth (southern Germany) was used as substrate on both sides of the split-root container. The soil was taken from 5--30 cm soil depth, mixed, air-dried to 7.3% w/w water con-tent, and sieved (5 mm). Soil pH (H2O) was 4.5; other
proper-ties of the soil are described in Table 1. Each side of the container was filled with 1.25 kg of mineral soil, resulting in a bulk soil density of approximately 1.15 g cm−3. Before filling, the soil on one side of the split-root container was supple-mented with 100 mg N (NH4NO3), 20 mg P (Ca(H2PO4)2),
80 mg K (K2SO4), 40 mg Mg (MgSO4), and 53 mg Ca
(Ca(H2PO4)2 and CaSO4) per kg air-dry soil.
Seedlings were planted on top of the mineral soil (Figure 1), so that, at harvest, the mineral soil contained only roots pro-duced during the experiment. The upper soil layer consisted of material (sieved to 10 mm) taken from the humus horizon of the same site as the mineral soil. The upper layer was watered during the first four weeks of the experiment so that the trees could establish new roots after planting. Thereafter, only the mineral soil was watered to increase root growth in the mineral soil and decrease nutrient uptake from the upper soil layer. Watering of the mineral soil layer was done continuously with deionized water using a commercial clay cone system (Blu-mat, Telfs, Austria). The water content of the mineral soil was monitored with tensiometers and maintained between 6 and 10 kPa.
Trees were placed in a greenhouse with temperatures main-tained above 6 °C and with additional light (350 µmol m−2 s−1) supplied by HQL 400 W lamps from 1400 to 2200 h. The long-day conditions induced the onset of new shoot growth in February 1994, two months after the beginning of the experi-ment. At the end of April 1994, the pots were placed outdoors in the shade for the summer period. The soil was protected against rainwater by plastic covers, and the plants continued to be irrigated with deionized water.
Figure 1. Split-root container used in the experiment.
Table 1. Chemical properties of the soil used in the experiment.
Nutrient mg kg−1
Mineral nitrogen1 NH4+ 2.6
NO3− 2.1
H2O-extractable nutrients2 Ca 3.2
K 3.1
Mg 0.7
P < 0.6
Exchangeable nutrients3 Ca 6.3
K 26.4
Mg 1.1
P 28.5
1 CaCl
2 extraction. 2 In water/soil (2/1) extract. 3 0.05 N NH
4Cl extraction (Ca, K, Mg) or CAL (calcium
Harvest
In September 1994 (after a 9-month growth period), all trees were harvested and their roots examined with the aid of a microscope. Ten to 70% of the lateral roots of all plants were ectomycorrhizal, as determined by visual observation. Scots pine mycorrhizae showed a typical dichotomous branching. The predominant ectomycorrhizal fungus in the mineral soil was Thelephora terrestris Ehrh.: Fr. and, in some cases, Ceno-coccum geophilum Fr. A detailed identification of all fungi associated with the roots was not done. Colonization rates were apparently not affected by the soil nutrient supply.
After harvest, shoot and roots were divided into current-year and older tissue. Roots in the humus top layer were treated as older roots, although approximately 5% of the root mass in this layer was newly formed roots. All roots in the mineral soil were newly formed. Soil was carefully washed from roots and a root subsample was used for element analyses. The remaining roots were used to determine root morphology. Total root length was estimated by the line intersect method (Tennant 1975), and the number of root tips per cm root length was counted in five representative subsamples. Dry weights of shoots and roots were determined after drying at 65 °C to constant weight.
Total N in needles was measured with a Macro N analyzer (Heraeus, Hanau, Germany), and total N in roots was measured with an automatic nitrogen analyzer (Model 1400, Carlo Erba, Mainz, Germany). For the determination of other elements, the dried and ground plant samples were dry ashed at 500 °C and dissolved in 100 mM HCl. After filtration, K and Ca concen-trations were measured by flame photometry, Mg, Mn, Fe, Cu, and Zn concentrations were analyzed by atomic absorption spectrometry, and P was determined colorimetrically as phos-phomolybdate.
Statistical analysis
Standard errors of the means were calculated. Differences between plant species were tested for statistical significance by analysis of variance, and differences in effects of soil nutrient supply on root growth and root nutrient concentrations within a plant species were tested by t-tests. When data were not normally distributed, analyses were carried out on transformed data. In all cases, results are reported as untransformed values.
Results
Plant growth
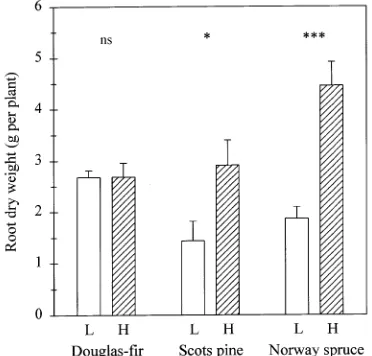
Distribution of newly formed roots in the two soil compart-ments varied with conifer species (Figure 2). Norway spruce showed twice the amount of root growth in the nutrient-en-riched soil than in the nutrient-poor soil. The growth response of Scots pine to the locally enriched nutrient supply was less marked than that of Norway spruce, and the Douglas-fir trees showed no difference in root dry weight between the two soil compartments.
Total dry weight of all current-year roots was not signifi-cantly different between plant species (P < 0.08). However, dry weight of new roots tended to be highest in Norway spruce,
followed by Douglas-fir and then Scots pine (Table 2). In Norway spruce, there was a larger increase in current-year shoot growth than in belowground growth (Table 2), whereas Scots pine and Douglas-fir produced similar amounts of new root and new shoot biomass during the experiment. Thus, the root/shoot dry weight ratio was approximately 0.7 in Norway spruce, and tended to be smaller than in the other two species (P < 0.16, Table 2).
Among species, total root length of all newly produced roots was greatest in Norway spruce (P < 0.001, Figure 3). Both Norway spruce and Scots pine formed greater root length in nutrient-rich soil than in nutrient-poor soil (Figure 3), but this effect was not observed in Douglas-fir.
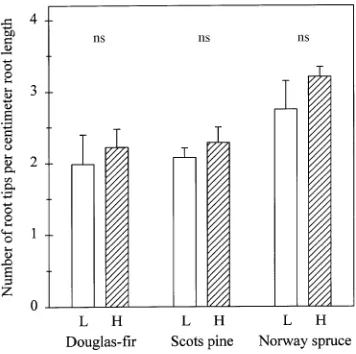
Specific root lengths (m g−1) were lower in Douglas-fir than in Norway spruce or Scots pine (P < 0.001; Figure 4), and did not vary significantly between nutrient-poor and nutrient-rich soil in any of the plant species. The number of root tips per centimeter of root length was not significantly affected by soil nutrient supply (Figure 5). Norway spruce roots had more root tips per centimeter of root length than roots of Scots pine and Douglas-fir (P < 0.05).
Nutrient concentrations
Concentrations of most mineral elements did not differ mark-edly between roots grown in nutrient-poor and nutrient-rich soil (Table 3). However, root P and K concentrations in Douglas-fir and root Mg concentrations in Scots pine and Norway spruce were greater in nutrient-rich soil than in nutri-ent-poor soil (Table 3). Douglas-fir had the lowest root nutrient concentrations of all three species. Root Ca concentrations were low in all three species examined.
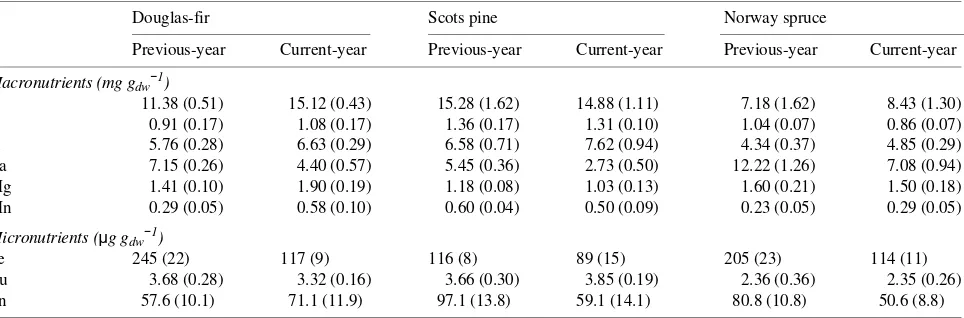
Neither current- nor previous-year needles showed severe deficiencies in any element (Table 4). In Norway spruce, con-centrations of N and P were suboptimal compared with stand-ard values for adequate nutrition (Hüttl 1990), but were not associated with any visible deficiency symptoms. Calcium concentrations in needles were high in Norway spruce com-pared with Scots pine and Douglas-fir.
Discussion
It is generally assumed that most plants respond to nutrient-rich patches in soil with enhanced root proliferation at these sites. This response has been shown for a range of crop species, and also, using artificial substrates, for Douglas-fir (Friend et al. 1990), lodgepole pine (Coutts and Philipson 1977), and Sitka spruce (Coutts and Philipson 1976). However, it is sur-prising that plant species well adapted to nutrient-poor soils should respond to locally enriched soil in the same way as
ruderal plant species respond similarly to locally enriched soil (Chapin 1980, Friend et al. 1990, Fitter 1994). The general assumption that all plant species respond with increased root growth in nutrient-rich soil zones may be erroneous, because studies in which no response was observed often do not appear in the literature (Caldwell 1994).
Root growth of Scots pine and particularly of Norway spruce, but not Douglas-fir, was increased in the nutrient-rich soil compartment (Figures 2 and 3). These varied responses may reflect genetically controlled differences among tree spe-cies with ‘‘exploitive’’ (Norway spruce, Scots pine) and ‘‘con-servative’’ (Douglas-fir) root growth habits (Griffin et al. 1995). Typically, tree species with finer roots (greater specific root length) have greater root extension rates, and are more responsive to root pruning than species with low specific root length, because they require smaller investments in biomass to extend the root system (Eissenstat 1991). Accordingly, among the tree species tested, Norway spruce had the greatest specific root length (Figure 4), and also showed the largest response to
Table 2. Shoot and root dry weights and root/shoot dry weight ratios of three coniferous tree species in a split-root system (n = 5--6; one SE in parentheses). Results were calculated separately for current-year and older tissue. For roots, dry weights of roots grown in nutrient-poor soil and of roots grown in nutrient-rich soil were added.
Shoot dry weight (g per plant) Root dry weight (g per plant) Root/shoot dry weight ratio
Older Current-year Older Current-year Older Current-year
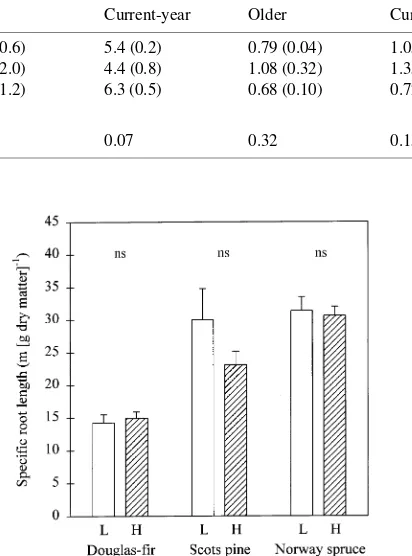
Douglas-fir 8.9 (0.5) 5.4 (0.7) 7.0 (0.6) 5.4 (0.2) 0.79 (0.04) 1.05 (0.11)
Scots pine 10.0 (1.2) 4.9 (1.6) 9.9 (2.0) 4.4 (0.8) 1.08 (0.32) 1.35 (0.37)
Norway spruce 18.8 (2.6) 9.4 (1.4) 11.6 (1.2) 6.3 (0.5) 0.68 (0.10) 0.72 (0.08)
F-test
P value < 0.01 0.05 0.10 0.07 0.32 0.15
Figure 3. Total length of newly grown roots of three coniferous tree species in a split-root system. Roots of the same plant were grown either in poor soil (L, low nutrient availability) or in nutrient-rich soil (H, high nutrient availability). Values are means, vertical bars indicate one standard error of the mean (n = 5--6). Effects of soil nutrient supply on root growth within a species are: ns, not significant; *, significant at P < 0.05; or ***, significant at P < 0.001.
nutrient enrichment in the soil (Figure 2). Such a response to local nutrient supply may indicate that Norway spruce is a competitive species that is adapted to relatively nutrient-rich soil (Grime 1994, Hutchings and de Kroon 1994).
Beside possible genetic differences in root growth among tree species, the effect of enhanced nutrient supply on root growth may depend on the nutrient demand of the plant (Jack-son and Caldwell 1989). Under our experimental conditions, Norway spruce produced more shoot dry matter than Scots pine and Douglas-fir. Also, the root/shoot ratio was lowest in Norway spruce (Table 2), indicating that more nutrients and water were provided for shoot growth per unit root dry weight in Norway spruce than in the other species. The low concen-trations of N, P and Mn (Table 4) in needles indicate nutrient deficiency, and thus, high nutrient demand, especially in Nor-way spruce. High demand may have driven NorNor-way spruce to proliferate roots in the nutrient-enriched soil. Similar evidence suggesting a higher root growth plasticity in nutrient-deficient plants than in nutrient-sufficient plants has been obtained for
trees (Friend et al. 1990), and annual plants (Drew et al. 1973), but not for perennial grasses (Larigauderie and Richards 1994).
Within a plant, roots growing in the nutrient-rich soil com-partment may have higher nutrient concentrations than roots growing in the nutrient-poor soil compartment. For example, differences in nutrient concentrations among roots of the same plant have been observed shortly after large local additions of P to the soil (Jackson and Caldwell 1992). However, within a plant, many nutrients (including N, P, and K, but not Ca) can be translocated in the phloem to sites of high nutrient demand, including roots (Marschner 1995). Also, increased root dry matter formation in nutrient-rich soil requires additional nutri-ents for tissue formation, because of a dilution effect. For both reasons, a long-term increase in nutrient concentrations in roots growing in nutrient-rich soil is unlikely, at least for elements that can be retranslocated within the plant.
Accordingly, when tree seedlings responded to increased local nutrient supply by increased root growth, concentrations of the growth-limiting nutrients (N and P in Norway spruce and Scots pine) did not increase in roots in the nutrient-rich soil compared with concentrations in roots in the nutrient-poor soil (Table 3). Conversely, when a nutrient was not growth limiting (e.g., Mg), or when a species did not respond to increased local nutrient supply with increased growth (e.g., Douglas-fir), nu-trient concentrations did increase in roots growing in nunu-trient- nutrient-rich soil (Table 3). Thus, in long-term field experiments with local nutrient addition to soil, increased root growth is a better indicator of previous nutrient deficiency than increased root nutrient concentrations. These results also suggest that, be-cause of the high mobility of most nutrients in plants, and because of nutrient cycling between shoot and roots, root growth in the nutrient-poor soil in the split-root experiment was not limited, in the long-term, by nutrient deficiency. Rather, allocation of photoassimilates to the root zones may have differed, leading to greater root growth in nutrient-rich soil than in nutrient-poor soil. Greater carbohydrate allocation may have been the result of a faster decrease in the cytosolic sugar pool in metabolically active root cells (Minchin et al. 1994). With enhanced unloading of phloem solutes, growth-stimulating phytohormones are also unloaded in increased amounts (Sattelmacher and Thoms 1995).
Figure 5. Number of root tips per centimeter of root length of newly grown roots of three coniferous tree species in a split-root system. Roots of the same plant were growing either in nutrient-poor soil (L, low nutrient availability) or nutrient-rich soil (H, high nutrient avail-ability). Values are means, vertical bars indicate one standard error of the mean (n = 5--6). Effects of soil nutrient supply on root growth within a species are not significant (ns).
Table 3. Nutrient concentrations in newly grown roots of three coniferous tree species in a split-root system. Roots of the same plant were grown either in nutrient-poor soil (L, low nutrient availability) or in nutrient-rich soil (H, high nutrient availability). Effects of soil nutrient supply on nutrient concentrations within one species are ns, not significant; *, significant at P < 0.05; or **, significant at P < 0.01 (n = 5--6; one SE in parentheses).
Nutrient Douglas-fir (mg gdw−1) Scots pine (mg gdw−1) Norway spruce (mg gdw−1)
L H L H L H
N 4.96 (0.10) 6.34 (0.65)ns 9.91 (1.14) 7.93 (1.14)ns 7.50 (0.53) 7.60 (0.49)ns
P 0.79 (0.03) 1.02 (0.09)* 1.40 (0.14) 1.21 (0.16)ns 1.25 (0.09) 1.30 (0.05)ns
K 3.66 (0.19) 5.09 (0.43)* 6.06 (0.62) 7.28 (0.55)ns 4.21 (0.18) 3.86 (0.25)ns
Ca 0.33 (0.01) 0.28 (0.01)** 0.48 (0.04) 0.37 (0.02)ns 0.48 (0.03) 0.43 (0.02)ns
An extensive root system would be advantageous for uptake of nutrients with low mobility in soil such as P (Theodorou and Bowen 1993). Plants may respond to nutrient deficiency by forming a more exploratory root system, with finer roots or more branches or both. However, in some studies, a localized nutrient supply had the opposite effect, and roots in nutrient-rich soil were thinner (Jackson and Caldwell 1989) or more branched (Larigauderie and Richards 1994, see also Fitter 1994) than roots in nutrient-poor soil. We found that neither specific root length (Figure 4) nor the number of root tips per centimeter of root length (Figure 5) was affected by local nutrient supply. Similarly, Friend et al. (1990) found that specific root length of Douglas-fir was not affected by local N supply (Friend et al. 1990).
Effects of local nutrient additions on root morphology may be transient and appear only shortly after the nutrient addition, because of initiation of a large number of young roots. How-ever, in the long term, effects of nutrient additions on root longevity (Pregitzer et al. 1995) are important for overall root morphology. In soil-grown plants, factors such as root penetra-tion resistance and soil oxygen supply have large formative effects on root growth, and this may be more important for root morphology than local nutrient supply (Caldwell 1994).
The supply of a particular nutrient may have different effects on root growth (Wikström and Ericsson 1995) and morphology than simultaneous supply of several nutrients as in this experi-ment. Generalization about the formative effects of local nutri-ent supply on root morphology is more difficult for trees than for annual plants, because many tree root systems are com-prised of both ‘‘pioneer,’’ ‘‘extension’’ or ‘‘long’’ roots, and ‘‘fibrous,’’ ‘‘lateral’’ or ‘‘fine roots’’ (Atkinson 1983, Eissenstat 1991, Marschner et al. 1991, George and Marschner 1995). In ‘‘pioneer’’ roots, individual roots with larger diameter may have high growth rates, whereas in ‘‘fibrous roots,’’ a high specific root length may favor fast root extension (Eissenstat 1991). Thus, morphological effects of local nutrient supply on tree root systems will also depend on the balance between ‘‘pioneer’’ roots and ‘‘fibrous’’ roots. This balance is determined
by a range of conditions including soil factors, plant age, and mycorrhizal colonization of the root system.
Few data are available to quantify nutrient uptake of the different parts of the tree root system (George and Marschner 1995). Physiological as well as morphological changes in the root system in response to nutrient availability, for example, increased nutrient uptake rates of roots in nutrient-rich soil, may be important in the adaptation of plants to soils that are heterogeneous in nutrient availability (Caldwell et al. 1992, Hutchings and de Kroon 1994).
In our experiment, colonization of roots with ectomycorrhi-zal fungi appeared unaffected by the soil nutrient supply. This may have been because root colonization was dominated by fungal species adapted to nursery conditions, i.e., both a rela-tively high supply of nutrients in inorganic form and a dis-turbed soil. In forest-grown plants, extraradical hyphae of mycorrhizal fungi may be more important than tree roots in nutrient absorption from the soil, particularly when the nutri-ent supply is in organic form (George and Marschner 1995).
We have demonstrated that roots of coniferous tree species can respond to an increase in local nutrient supply in soil by increasing root dry matter growth without changing specific root length. The effect varied with plant species (Norway spruce > Scots pine > Douglas-fir) and was correlated with nutrient demand of the plant (high demand > low demand). Changes in root dry weight were a better indicator of nutrient limitations in the system than changes in root nutrient concen-trations.
Acknowledgment
We thank Sabine Kircher, Carsten Marohn, Angela Schmeisser, and Julia Ströbele for their excellent help. This work was financed by Projekt Europäisches Forschungszentrum für Luftreinhaltung (PEF) in Karlsruhe, Germany.
Table 4. Concentrations of the macronutrients N, P, K, Mg, Ca, and Mn, and the micronutrients Fe, Cu, and Zn in previous-year and current-year needles of three coniferous tree species in a split-root system (n = 5--6; one SE in parentheses).
Douglas-fir Scots pine Norway spruce
Previous-year Current-year Previous-year Current-year Previous-year Current-year
Macronutrients (mg gdw−1)
N 11.38 (0.51) 15.12 (0.43) 15.28 (1.62) 14.88 (1.11) 7.18 (1.62) 8.43 (1.30)
P 0.91 (0.17) 1.08 (0.17) 1.36 (0.17) 1.31 (0.10) 1.04 (0.07) 0.86 (0.07)
K 5.76 (0.28) 6.63 (0.29) 6.58 (0.71) 7.62 (0.94) 4.34 (0.37) 4.85 (0.29)
Ca 7.15 (0.26) 4.40 (0.57) 5.45 (0.36) 2.73 (0.50) 12.22 (1.26) 7.08 (0.94)
Mg 1.41 (0.10) 1.90 (0.19) 1.18 (0.08) 1.03 (0.13) 1.60 (0.21) 1.50 (0.18)
Mn 0.29 (0.05) 0.58 (0.10) 0.60 (0.04) 0.50 (0.09) 0.23 (0.05) 0.29 (0.05)
Micronutrients (µg gdw−1)
Fe 245 (22) 117 (9) 116 (8) 89 (15) 205 (23) 114 (11)
Cu 3.68 (0.28) 3.32 (0.16) 3.66 (0.30) 3.85 (0.19) 2.36 (0.36) 2.35 (0.26)
References
Atkinson, D. 1983. The growth, activity and distribution of the fruit tree root system. Plant Soil 71:23--35.
Caldwell, M.M. 1994. Exploiting nutrients in fertile soil microsites. In
Exploitation of Environmental Heterogeneity by Plants: Ecophysi-ological Processes Above- and Belowground. Eds. M.M. Caldwell and R.W. Pearcy. Academic Press, San Diego, pp 325--347. Caldwell, M.M., L.M. Dudley and B. Lilieholm. 1992. Soil solution
phosphate, root uptake kinetics and nutrient acquisition: implica-tions for a patchy soil environment. Oecologia 89:305--309. Campbell, B.D. and J.P. Grime. 1989. A comparative study of plant
responsiveness to the duration of episodes of mineral nutrient en-richment. New Phytol. 112:261--267.
Cattle, S.R., A.J. Koppi and A.B. McBratney. 1994. The effect of cultivation on the properties of a Rhodoxeralf from the wheat/sheep belt of New South Wales. Geoderma 63:215--225.
Chapin III, F.S. 1980. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 11:233--260.
Coutts, M.P. and J.J. Philipson. 1976. The influence of mineral nutri-tion on the root development of trees. I. The growth of Sitka spruce with divided root systems. J. Exp. Bot. 27:1102--1111.
Coutts, M.P. and J.J. Philipson. 1977. The influence of mineral nutri-tion on the root development of trees. III. Plasticity of root growth in response to changes in the nutrient environment. J. Exp. Bot. 28:1071--1075.
Crick, J.C. and J.P. Grime. 1987. Morphological plasticity and mineral nutrient capture in two herbaceous species of contrasted ecology. New Phytol. 107:403--414.
Drew, M.C., L.R. Saker and T.W. Ashley. 1973. Nutrient supply and the growth of the seminal root system in barley. I. The effect of nitrate concentration on the growth of axes and laterals. J. Exp. Bot. 24:1189--1202.
Eissenstat, D.M. 1991. On the relationship between specific root length and the rate of root proliferation: a field study using citrus rootstocks. New Phytol. 118:63--68.
Fitter, A.H. 1994. Architecture and biomass allocation as components of the plastic response of root systems to soil heterogeneity. In
Exploitation of Environmental Heterogeneity by Plants: Ecophysio-logical Processes Above- and Belowground. Eds. M.M. Caldwell and R.W. Pearcy. Academic Press, San Diego, pp 305--323. Friend, A.L., M.R. Eide and T.M. Hinckley. 1990. Nitrogen stress
alters root proliferation in Douglas-fir seedlings. Can. J. For. Res. 20:1524--1529.
George, E. and H. Marschner. 1995. Nutrient and water uptake by roots of forest trees. Z. Pflanzenernähr. Bodenkd. 159:11--21. Griffin, K.L., W.E. Winner and B.R. Strain. 1995. Growth and dry
matter partitioning in loblolly and ponderosa pine seedlings in response to carbon and nitrogen availability. New Phytol. 129:547--556.
Grime, J.P. 1994. The role of plasticity in exploiting environmental heterogeneity. In Exploitation of Environmental Heterogeneity by Plants: Ecophysiological Processes Above- and Belowground. Eds. M.M. Caldwell and R.W. Pearcy. Academic Press, San Diego, pp 1--19.
Hildebrand, E.E. 1994. The heterogeneous distribution of mobile ions in the rhizosphere of acid forest soils: facts, causes and conse-quences. J. Environ. Sci. Health, Part A 29:1973--1992.
Hutchings, M.J. and H. de Kroon. 1994. Foraging in plants: the role of morphological plasticity in resource acquisition. In Advances in Ecological Research, Volume 25. Eds. M. Begon and A.H. Fitter. Academic Press, London, pp 159--238.
Hüttl, R.F. 1990. Nutrient supply and fertilizer experiments in view of N saturation. Plant Soil 128:45--48.
Jackson, R.B. and M.M. Caldwell. 1989. The timing and degree of root proliferation in fertile-soil microsites for three cold-desert perennials. Oecologia 81:149--153.
Jackson, R.B. and M.M. Caldwell. 1992. Shading and the capture of localized soil nutrients: nutrient contents, carbohydrates, and root uptake kinetics of a perennial tussock grass. Oecologia 91:457--462. Koch, A.S. and E. Matzner. 1993. Heterogeneity of soil and soil solution chemistry under Norway spruce (Picea abies Karst.) and European beech (Fagus silvatica L.) as influenced by distance from the stem basis. Plant Soil 151:227--237.
Larigauderie, A. and J.H. Richards. 1994. Root proliferation charac-teristics of seven perennial arid-land grasses in nutrient-enriched microsites. Oecologia 99:102--111.
Lechowicz, M.J. and G. Bell. 1991. The ecology and genetics of fitness in forest plants. 2. Microspatial heterogeneity of the edaphic environment. J. Ecol. 79:687--696.
Marschner, H. 1995. Mineral nutrition of higher plants, 2nd Edn. Academic Press, London, 889 p.
Marschner, H., M. Häussling and E. George. 1991. Ammonium and nitrate uptake rates and rhizosphere pH in non-mycorrhizal roots of Norway spruce (Picea abies (L.) Karst.). Trees 5:14--21.
Minchin, P.E.H., J.F. Farrar and M.R. Thorpe. 1994. Partitioning of carbon in split root systems of barley: effect of temperature of the root. J. Exp. Bot. 45:1103--1109.
Philipson, J.J. and M.P. Coutts. 1977. The influence of mineral nutri-tion on the root development of trees. 2. The effect of specific nutrient elements on the growth of individual roots of Sitka spruce. J. Exp. Bot. 28:864--871.
Pregitzer, K.S., D.R. Zak, P.S. Curtis, M.E. Kubiske, J.A. Teeri and C.S. Vogel. 1995. Atmospheric CO2, soil nitrogen and turnover of
fine roots. New Phytol. 129:579--585.
Raich, J.W., R.H. Riley and P.M. Vitousek. 1994. Use of root-ingrowth cores to assess nutrient limitations in forest ecosystems. Can. J. For. Res. 24:2135--2138.
Robinson, D. 1994. The responses of plants to non-uniform supplies of nutrients. New Phytol. 127:635--674.
Sattelmacher, B. and K. Thoms. 1995. Morphology and physiology of the seminal root system of young maize (Zea mays L.) plants as influenced by a locally restricted nitrate supply. Z. Pflanzenernähr. Bodenkd. 158:493--497.
St. John, T.V., D.C. Coleman and C.P.P. Reid. 1983. Growth and spatial distribution of nutrient-absorbing organs: selective exploita-tion of soil heterogeneity. Plant Soil 71:487--493.
Tennant, D. 1975. A test of a modified line intersect method of estimating root length. J. Ecol. 63:995--1001.
Theodorou, C. and G.D. Bowen. 1993. Root morphology, growth and uptake of phosphorus and nitrogen of Pinus radiata families in different soils. For. Ecol. Manage. 56:43--56.