Genetic diversity and biocontrol activity of
Aureobasidium
pullulans
isolates against postharvest rots
Leonardo Schena
a, Antonio Ippolito
a,*, Tirtza Zahavi
b, Lea Cohen
b,
Franco Nigro
a, Samir Droby
baDipartimento di Protezione delle Piante dalle Malattie,Uni6ersita` degli Studi di Bari,Via Amendola165/A,70126Bari,Italy bDepartment of Posthar6est Science of Fresh Produce,Institute for Technology and Storage of Agricultural Products,ARO,
The Volcani Center,P.O.Box6,Bet Dagan50250,Israel
Received 10 February 1999; accepted 31 May 1999
Abstract
Aureobasidium pullulans, a cosmopolitan yeast-like fungus, colonises leaf surfaces and is a potential biocontrol agent for plant pathogens. Forty-one isolates of the fungus from the surface of several fruits and vegetables cultivated in Southern Italy were compared by molecular analysis and biocontrol activity. Characterisation of the isolates by using arbitrarily primed PCR (ap-PCR) confirmed the presence of a high genetic variability within the species. All the isolates were evaluated for their ability to control postharvest grey mold of apples, and two of them (SL250 and SL236), plus a proven antagonist (isolate L47), were able to control Penicillium digitatum on grapefruit, Botrytis cinerea,Rhizopus stoloniferandAspergillus nigeron table grape andB.cinereaandR.stoloniferon cherry tomato. Furthermore, preharvest application of isolate L47 on table grape resulted in a significant reduction of grey mold ranging from 27.1 to 49.5% compared to the untreated control. Random amplified polymorphic DNA technique (RAPD) was a useful method for the identification and evaluation of the survival rate of the applied antagonist. © 1999 Elsevier Science B.V. All rights reserved.
Keywords:Aureobasidium pullulans; Postharvest; Biological control; RAPD-PCR; ap-PCR
www.elsevier.com/locate/postharvbio
1. Introduction
Currently, fungicide treatments represent the primary method for the control of postharvest diseases of fruits and vegetables (Eckert and Ogawa, 1985). However, public concern about
fungicide residues, and development of fungicide-resistant isolates of postharvest pathogens have promoted the search for alternative means, less harmful to human health and to the environment. In recent years, considerable success has been achieved utilising microbial antagonists to control postharvest diseases and a large body of informa-tion on the subject is now available (Wilson and Wisniewski, 1994). Because the infection of fruits by postharvest pathogens often occurs in the field * Corresponding author. Tel.:+39-080-5443053; fax:+
39-080-5442911.
E-mail address:[email protected] (A. Ippolito)
prior to harvest (Biggs, 1995), it may be advanta-geous to apply antagonists before harvest. For this approach to be successful, putative biocontrol strains must be able to tolerate low nutrient availability, UV-B radiation, low temperatures, and climatic changes. The yeast-like fungus Au
-reobasidium pullulans (de Bary) Arnaud is one of the most widespread and well-adapted sapro-phytes, both in the phyllosphere (Blakeman and Fokkema, 1982) and in the carposphere.A.pullu
-lanshas a high tolerance to desiccation and irradi-ation (Elad et al., 1994; Kohl et al., 1995), and has been considered as a possible biocontrol agent of postharvest diseases (Bhatt and Vaughan, 1962; Leibinger et al., 1997).
A.pullulansis characterised by an extreme vari-ability and genetic instvari-ability (Cooke, 1959; Bulat and Mironenko, 1992; Yurlova et al., 1995). Mor-phological and cultural characteristics alone are not sufficient to assess interspecific variability and to differentiate closely related strains. Random amplified polymorphic DNA (RAPD) and arbi-trary primed polymerase chain reaction (ap-PCR) techniques can be valid means for characterising naturally occurring micro-organism populations and to obtain preliminary information about the genetic complexity of a natural epiphytic popula-tion (Welsh and McClelland, 1990; Williams et al., 1990).
Biological control agents differ fundamentally from chemical fungicides in that, in order to be effective, they must grow and proliferate on the plant surface. Therefore, effective antagonists must become established in crop ecosystems and remain active against target pathogens during pe-riods favourable to plant infection (Lo et al., 1998). The survival ability of biocontrol agents, in terms of population size, survival period, and distribution in crops, needs to be surveyed and compared with biocontrol effects. The most fre-quently used methods for the detection and quan-tification of microbes in ecosystems is the viable-cell count, typically reported as colony forming units (cfu) per unit area or sample weight (Li et al., 1996). However, the introduced strains are difficult to distinguish from indigenous strains. RAPD markers allow the unequivocal recognition of the selected antagonistic strain
af-ter its introduction into the phyllosphere (Gullino et al., 1995).
The aims of this research were: (i) to obtain preliminary information about the genetic diver-sity of a natural epiphytic population of A.pullu
-lans, isolated in Southern Italy, by using ap-PCR; (ii) to evaluate the potential of several isolates of
A.pullulansas biocontrol agents; (iii) to determine the effectiveness of an isolate ofA.pullulans(L47) previously tested in Southern Italy (Lima et al., 1996, 1997; Nigro et al., 1997) in a new environ-ment (Israel) and on a different table grape vari-ety; (iv) to identify and to monitor the population of the antagonist L47 in the field using RAPD-PCR.
2. Materials and methods
2.1. Isolates
Isolates of A. pullulans were obtained from the surface of several untreated fruits and vegetables from Southern Italy as described by Wilson et al. (1993). For long-term storage, A. pullulans iso-lates were suspended in 15% glycerol and frozen at−80°C, or kept on PDA at 4°C for short-term storage. Pathogen isolates (Penicillium digitatum
Pers., Botrytis cinerea Pers., Aspergillus niger
Tiegh.,Rhizopus stoloniferEhrenb.) were obtained from decayed grape berries and kept on potato dextrose agar (PDA) at 5°C.
2.2. Preliminary screening of antagonists
All the isolated antagonists were tested on ap-ples (cv. Golden delicious) wounded with a pin at four sites around the stem-end to a uniform depth of 2 mm. Isolates were grown in 25 ml of Nutrient Yeast-extract Dextrose Broth (NYDB) in 100 ml Erlenmeyer flasks on a rotary shaker at 150 rpm for 48 h. Cells were harvested by centrifugation (1100×g for 15 min), rinsed twice in sterile deionized water, and resuspended in distilled wa-ter to the initial concentration of about 108 cells
/ ml. A water suspension (20 ml) of antagonist cells (107 cells/ml) was pipetted into each wound, and
20 ml of B. cinerea spore suspension (5×104 spores/ml).
The proven isolate L47 and two additional ones (LS236 and LS250) were selected for further stud-ies and tested for their biocontrol activity against decay of grapefruit, caused by P.digitatum, table grape, caused by B. cinerea, A. niger and R.
stolonifer, and cherry tomato, caused by B.
cinerea and R. stolonifer. Grapefruits were wounded to a uniform depth of 3 mm at three sites around the stem-end using a dissecting needle, while table grape berries and cherry toma-toes were wounded to a uniform depth of 2 mm at one site in the equatorial zone. Twenty microlitres of cell suspension of the antagonist at three differ-ent concdiffer-entrations (108, 107and 106cells
/ml) were pipetted into each wound, and 2 h later the wounds were inoculated with 20 ml of pathogens spore suspension (5×104spores/ml). The wounds
treated with water were used as controls.
In all the experiments, fruits were maintained at 20°C at high RH and the percentage of infected fruits was evaluated after 5 days. In table grape and cherry tomato tests, each treatment was repli-cated three times and each replicate consisted of ten fruits; in grapefruit and apples, five replica-tions of five fruits were used. Each test was re-peated at least twice.
2.3. In 6i6o assays with small table grape bunches
Isolates L47, SL236 and SL250 were also tested for their biocontrol efficacy on small table grape bunches with approximately ten berries each, against B. cinerea, A. niger and R. stolonifer. Small bunches were dipped in an antagonist sus-pension (107
cells/ml) obtained as described be-fore, or in water (control). Two hours later, bunches were sprayed with a spore suspension of the pathogens (105spores
/ml ofB.cinerea, 5×104
spores/ml of A. niger and R. stolonifer). Each treatment was replicated four times and each replicate consisted of eight small bunches. The treated bunches were stored at 20°C at high RH and the percentage of rotted berries was evaluated after 5 days.
2.4. Field tests
The trials were conducted on seedless table grape cv. Thompson in a commercial vineyard located in Lachish (Israel) using L47. A cell suspension of the antagonist (107 cells/ml) was
sprayed four or five times before harvest at weekly intervals starting on July 15th. In the first trial (four sprayings), the last spraying was carried out one week before harvest; in the second trial (five sprayings) the last spraying was done 2 h before harvest. In addition to the untreated grape (control), four treatments with Rovral (iprodione 25% a.i.) at 0.15%, similar to the schedule used for the antagonist, were also included. For each treatment, four replications of three grapevines, arranged in a randomised block design, were considered. From each replication, 8 – 10 bunches were harvested, placed in commercial plastic boxes and covered with plastic sheeting.
The incidence of natural infections caused by
B. cinerea was evaluated after 30 days of storage at 0°C followed by 3 – 9 days of shelf-life at 20°C using the following empirical scale: 0, bunch without rots; 1, 0 – 10% of rotted berries; 2, 10 – 25% of rotted berries; 3, 25 – 50% of rotted berries; 4, 50 – 75% of rotted berries; 5, more than 75% of rotted berries. This empirical scale made it possible to calculate the McKinney index (McKinney, 1923), expressing the weighted average of the disease severity as actual percentage in terms of the max-imum disease severity. The index was cal-culated by the formula: Mi=[(d×f)/Tn
×D]100, where d is the degree of disease severity assessed on the bunch and f its frequency, Tn is the total number of the bunches
examined (healthy and diseased) and D the highest degree of disease intensity occurring on the empirical scale.
2.5. Population studies on the fruit surface in the field and during storage
To assess the survival of isolate L47 on the fruit surface, the antagonist was sprayed just once at a concentration of 107 cells/ml and epiphytic
popu-lation was evaluated on grape berries five times, at weekly intervals. Grape berries were picked the first time 2 h after the application of the antago-nist (July, 14th) and then after 7, 14 and 21 days. The epiphytic population was also evaluated at the beginning (August, 11th) and at the end of the storage period (September, 10th). Each replication consisted of five berries which were shaken in 100 ml of sterile distilled water on a rotatory shaker at 150 rpm for 30 min. The rinse water was diluted and plated on PDA plates (0.1 ml/plate). The plates were incubated at 25°C, and after 3 – 4 days the colonies showing the typical morphological characteristics of A. pullulans were recorded. Some of these colonies were randomly selected and genetically analysed, as described later, to assess if they belonged to the introduced antago-nist or to other natural occurring A. pullulans
strains.
2.6. Characterisation of epiphytic antagonists
To characterise the natural epiphytic popula-tion of A. pullulans the following primers derived from minisatellite or repeat sequences (ap-PCR), were used: GACAGGACAGGACAG (GACA-G)3, GACAGACAGACAGACA (GACA)4, and
CAGCAGCAGCAGCAG (CAG)5, (Freeman
and Shabi, 1996).
RAPD-PCR reaction, with the primer 5% -ACC-CGGTCAC-3% (OpD-20, from Operon Technolo-gies, Inc. USA), was used to identify the isolate L47 after field treatment and re-isolation. Single colonies showing the characteristic morphology of
A. pullulans were randomly selected from Petri dishes and analysed. A pure culture of isolate L47 was used as a control.
The primers used for ap-PCR and RAPD-PCR were preliminarily selected to be the best ones providing a greater number of polymorphisms.
Genomic DNA was extracted by the method of Hofman and Winston (1987) with some
modifica-tions. One loop of antagonists taken from a colony actively growing on a PDA plate, was suspended in Eppendorf tubes containing 100 ml of breaking buffer (2% Triton X-100, 1% SDS, 100 mM NaCl, 10 mM Tris pH 8, 1 mM EDTA). Acid-washed glass beads (0.3 g), phenol (50 ml), chloroform (48ml) and isoamyl alcohol (2ml) were added to the solution to make a final concentra-tion of 107– 108 cells/ml. Eppendorf tubes were
vortexed at high speed for 5 min, and then spun in a microfuge at room temperature for 5 min.
For ap-PCR, amplification reactions were per-formed in a total volume of 20 ml, containing 10 – 100 ng of genomic DNA, 50 mM KCl, 10 mM Tris – HCl, 0.2 mM each of dATP, dCTP, dGTP and dTTP, 1.5 mM MgCl2, 1 unit of Taq
polymerase (Promega, Madison, WI, USA) and 1 mM primer. The reactions were incubated in a programmable Thermal Controller (PTC-100tm,
Peltier-Effect Cycling, MJ Research, Inc, USA), starting with 5 min of denaturation at 94°C, followed by 30 cycles consisting of 30 s at 94°C, 30 s at either 60°C [for (CAG)5] or 48°C [for
(GACA)4, and (GACAC)3] and 1.5 min at 72°C
(Freeman and Shabi, 1996).
For RAPD-PCR, reactions were performed in a total volume of 25 ml, containing about 25 ng of genomic DNA, 50 mM KCl, 10 mM Tris – HCl, 100 mM each of dATP, dCTP, dGTP and dTTP, 2 mM MgCl2, 0.5 unit of Taq polymerase and 5
pmols of a single ten-base primer. The reactions were incubated in a programmable Thermal Con-troller starting with 5 min of denaturation at 94°C, followed by 45 cycles of 1 min at 94°C, 1 min at 36°C, and 2 min at 72°C. Negative con-trols (no template DNA) were used in every ex-periment to test for the presence of contamination in the reagents. The amplification products were analysed by electrophoresis in 2% agarose gels in TAE buffer at 70 V for 1.5 h, and detected by staining with ethidium bromide.
3. Results
3.1. Biocontrol acti6ity of A. pullulansisolates
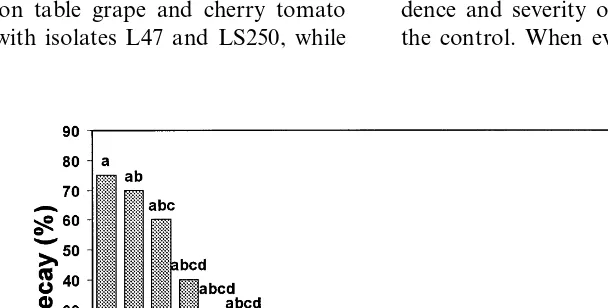
isolates varied in their biocontrol activity against
B. cinerea and some of them provided a
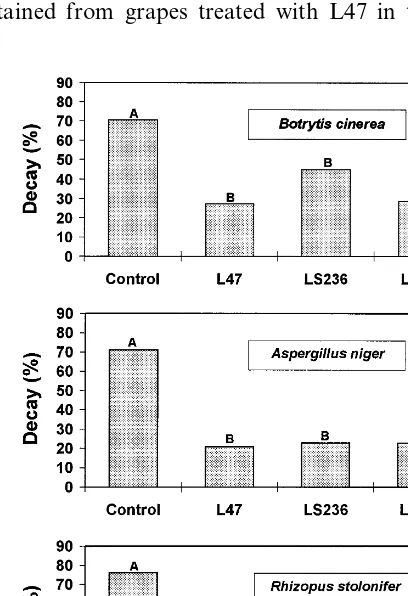
significant reduction of decay (P=0.05). In particular, a high level of control was achieved by the isolates L47 and 198 (93% less decay in comparison with the control) whereas complete inhibition of decay was obtained with the isolates 235, 240, LS236 and LS250 (Fig. 1). Among the most effective antagonists on apple, the isolates LS236 and LS250 were selected for further studies because they also showed a different ap-PCR pattern than L47; isolates 235 and 240 were discarded because they had the same ap-PCR patterns as L47. Isolate 198 was not differentiated from isolate LS236 (Fig. 6).
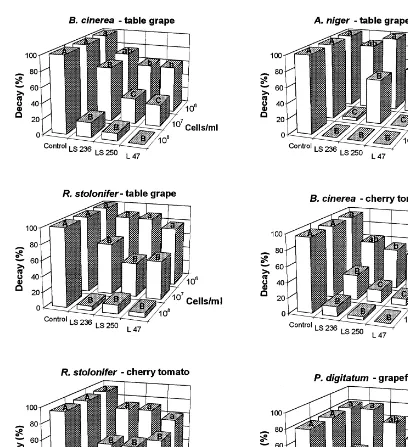
At high concentrations (108 and 107 cells /ml), isolates LS236, LS250 and L47 provided a significant (P=0.01) reduction of decay of grapefruit, cherry tomato and table grape (Fig. 2). In particular, on table grape and cherry tomato, complete inhibition of decay caused byB.cinerea
andA.nigerwas obtained with isolate L47, while complete inhibition of decay due to A. niger on table grape was achieved with isolates LS236 and LS250. At a lower concentration (106cells/ml), all
the antagonists showed less decay control. However, a significant reduction (P=0.05) of B.
cinerea decay on table grape and cherry tomato was obtained with isolates L47 and LS250, while
decay due to A. niger on table grape and P.
digitatum on grapefruit was reduced only by isolate L47.
3.2. In 6i6o assays with small table grape bunches
Isolates L47, LS236 and LS250 also were very effective in reducing the incidence of decay on small grape bunches. For instance, isolate L47 reduced the incidence of decay caused by R.
stolonifer, A. niger and B. cinerea by 54.8, 70.7 and 61.3%, respectively, as compared to the con-trol (Fig. 3). No significant differences were found between isolates LS236, LS250 and L47 in con-trolling decay caused by the three pathogens tested.
3.3. Biocontrol acti6ity under field conditions
L47 was further tested to control grey mold on table grape under field conditions. The results shown in Fig. 4 indicate that the chemical treat-ment (iprodione) was the most effective treattreat-ment in reducing grey mold after 30 days of storage at 0°C followed by 3 or 9 days of shelf-life. Prehar-vest application of L47 (four and five sprayings) resulted in a significant reduction of both inci-dence and severity of the decay, as compared to the control. When evaluated at 3 or 9 days, grey
Fig. 2. Biocontrol activity of isolates L47, LS236, and LS250 on injured fruits againstA.nigeron table grape, P.digitatumon grapefruit,B.cinereaandR.stoloniferon table grape and cherry tomato. Antagonists were applied at three different concentrations (108, 107and 106cells/ml). For each concentration, columns with different letters are statistically different according to Duncan’s multiple range test atP=0.05, small letters, andP=0.01, capital letters.
mold decay on grape sprayed four times, was reduced by 33.5 and 27.1%, respectively; when the antagonist was applied five times before harvest, a greater reduction (P=0.01) in disease severity was achieved (49.5 and 30.1%, respectively).
Populations of isolate L47 rapidly increased on
grape berries under field condition. During the period starting from the first application until harvest, the number of cfu/berry increased from 1×103 to 1.6×104. During cold storage, the
3.4. Characterisation of epiphytic antagonists
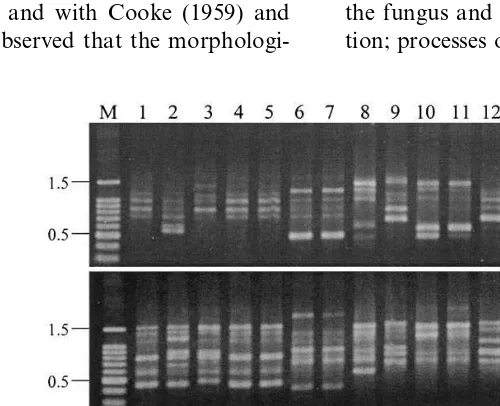
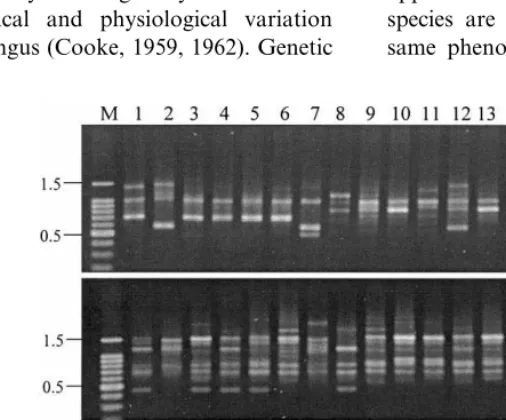
The genetic characterisation of the antagonists by ap-PCR showed an extremely high variability in the natural epiphytic population ofA.pullulans
isolated in Southern Italy (Figs. 6 – 8). Only a few isolates showed the same band patterns with the three primers tested. Particularly, isolates 235 and 240 showed the same patterns as L47, isolate 198 showed the same patterns as LS236 and isolate 379 showed the same patterns as 380. Isolates showing the same ap-PCR patterns, showed a similar biocontrol activity (Fig. 1). All the other isolates showed different ap-PCR patterns with at least one of the primers used. The isolates ob-tained from grapes treated with L47 in the field
Fig. 4. Incidence of gray mold on table grape treated in the field with isolate L47, Rovral (iprodione) or untreated (con-trol). The grape was sprayed four (A) or five (B) times at weekly intervals and the rots were evaluated after 4 weeks of storage at 0°C followed by 3 and 9 days of shelf-life. For each assessment columns with different letters are statistically dif-ferent according to Duncan’s multiple range test atP=0.05, small letters, and P=0.01, capital letters.
Fig. 3. Biocontrol activity of isolates L47, LS236 and LS250 on small table grape bunches againstB.cinerea,A.nigerand R. stolonifer. Columns with different letters are statistically different according to Duncan’s multiple range test at P=
0.01.
had RAPD-PCR patterns characteristic of the L47 isolate (Fig. 9).
4. Discussion
The yeast like-fungusA.pullulans, is one of the most widespread and well adapted saprophytes in the phyllosphere (Blakeman and Fokkema, 1982). It is one of the most frequent micro-organisms found on fruits such as grapes (Davenport, 1976), strawberries (Buhagiar and Barnett, 1971) and on various vegetables (Webb and Mundt, 1978). A.
pullulans is an effective nutrient competitor in the phyllosphere (Fokkema, 1973) and has been con-sidered as a possible biocontrol agent against B.
cinerea on strawberries (Bhatt and Vaughan, 1962), P. expansum,B. cinerea and Pezicula mal
-icorticis (H. Jacks.) Nannf. on apples (Leibinger et al., 1997), B.cinerea and R. stoloniferon table grape, kiwifruit and strawberries (Lima et al., 1996, 1997). In addition, A. pullulans has been used in combination with UV-C light to control
Fig. 5. Population dynamics of isolate L47 on grape berry surface in the field and during storage at 0°C.
The results of this study demonstrated the very high genetic variability of 41 isolates ofA. pullu
-lansobtained from several fruits and vegetables in Southern Italy. These results are in agreement with the work reported by Yurlova et al. (1995) in which universal primers (UP-PCR) were used to demonstrate a high degree of heterogeneity among A. pullulans, and with Cooke (1959) and Cooke (1962) who observed that the
morphologi-cal characteristics of A. pullulans are subject to considerable variation from strain to strain. The fungus A. pullulans has been known for several years under a wide variety of names and having a wide variety of habitats (Cooke 1959; Her-manides-Nijhof, 1977). The wide range of species names is partially due to the extreme instability of the fungus and to its highly heterocaryotic condi-tion; processes of mitotic gene exchange known to
Fig. 7. Gels stained with ethidium bromide showing amplification products generated fromA.pullulansisolates with primers A (GACAG)3, B (GACA)4and C (CAG)5. Lanes from 1 to 12, show amplification products from isolates 371, 372, 373, 374, 376, 377, 378, 379, 380, 382, 383 and 384. The first lane on the left contains DNA markers (M) as described in Fig. 6.
occur in heterocaryotic fungi may contribute to the morphological and physiological variation found in this fungus (Cooke, 1959, 1962). Genetic
approaches to characterise different isolates of a species are important, because isolates with the same phenotype may differ significantly in their
Fig. 9. DNA amplification products with primer OpD20 ofA. pullulans, isolate L47. Lane number 1 contains the amplifica-tion products generated from a pure culture of isolate L47. Lanes from 2 to 10 contain amplification products generated from different single colonies ofA.pullulans reisolated from table grape berries sprayed in the field with isolate L47. The first lane on the left and the last one on the right contain DNA markers (M) consisting of 14 discrete fragments of 3, 2, 1.5, 1.2, 1.0, 0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2 and 0.1 kb (100 bp DNA ladder plus, M-Medical, Genenco).
and during the storage. Moreover, it is worth noting that isolate L47 was able to survive and significantly increase its population in a new envi-ronment (in Israel) characterised by high tempera-ture and low RH. These results suggest that L47 is able to survive and control gray mold in differ-ent environmdiffer-ental conditions.
The methods traditionally used to monitor the survival of a biocontrol agent under field condi-tions only distinguished between different species, but were ineffective in identifying a specific isolate of the same species. The integration of RAPD-PCR analysis with conventional dilution plating made it possible to track isolate L47 introduced into the field. This technique allowed us to distin-guish it from other natural micro-organisms oc-curring on grape berries. Thus, we were able to confirm that the isolate of A. pullulans when introduced, successfully colonised table grape berry surfaces and persisted on the fruit surface. The ability to identify a specific isolate is impor-tant, especially once a biocontrol agent is deliv-ered in the field and there is a need to monitor its population dynamics (Gullino et al., 1995; Lo et al., 1998).
Considering its high biocontrol efficacy, its abil-ity to survive in different field conditions and during cold storage, and that its mode of action appears to be due to competition for nutrient and space (Lima et al., 1996; Ippolito et al., 1997), the use of isolate L47 could be a valid way to control the rots of grape and other fruits, especially where the use of chemical products is restricted. Further-more, considering the resistance of this isolate to some commonly used fungicides (Lima et al., 1997) and the promising results achieved on strawberry in combination with procymidone (Ip-polito et al., 1997a), an integrated application of the antagonist with chemical fungicides to control postharvest rots could prove to be a successful strategy.
References
Bhatt, D.D., Vaughan, E.K., 1962. Preliminary investigation on biological control of grey mold (Botrytis cinerea) of strawberries. Plant Dis. Rep. 46, 342 – 345.
genotype and in their biocontrol activity (Leibinger et al., 1997; Droby et al., 1998). The relationship among isolates having the same ap-PCR patterns is not yet clear; however isolates of
A. pullulans with similar band patterns do not seem to have a different biocontrol activity. Con-sequently, the ap-PCR technique may be a valid method for preliminary screening of antagonists.
Isolates L47, LS236 and LS250 showed differ-ent ap-PCR patterns with all the primers used and were also effective against several postharvest pathogens at low concentration. In our experi-ments, isolates LS236 and LS250 were similar to L47 in their ability to significantly reduce decay caused by four postharvest pathogens that cannot be controlled with the same chemical fungicide.
Isolate L47 reduced gray mold when applied five times in the field with no significant differ-ences results from the heavy chemical control schedule. These results are in agreement with pre-vious trials carried out on table grape (cv. Italia) in Italy where isolate L47 showed a strong and long-lasting activity againstB.cinerea both in the field and in cold storage (Lima et al., 1996).
Biggs, A.R., 1995. Detection of latent infections in apple fruit with paraquat. Plant Dis. 79, 1062 – 1067.
Blakeman, J.P., Fokkema, N.J., 1982. Potential for biological control of plant diseases on the phylloplane. Annu. Rev. Phytopathol. 20, 167 – 192.
Buhagiar, R.W.M., Barnett, J.A., 1971. The yeasts of straw-berries. J. Appl. Bact. 34, 727 – 739.
Bulat, S.A., Mironenko, N.V., 1992. Polymorphism of the yeastlike fungus Aureobasidium pullulans (De Bary) de-tected with universally primed-polymerase reaction: species divergence. Genetics 28, 19 – 30.
Cooke, W.B., 1959. An ecological life history ofAureobasid -ium pullulans(de Bary) Arnaud. Mycopath. Mycol. Appl. 12, 1 – 45.
Cooke, W.B., 1962. A taxonomic study in the ‘black yeasts’. Mycopath. Mycol. Appl. 17, 1 – 43.
Davenport, R.R., 1976. Distribution of yeasts and yeasts-like organisms on aerial surface of developing apples and grapes. In: Dickinson, C.H., Preece, T.F. (Eds.), Microbi-ology of Aerial Plant Surfaces. Academic Press, New York, pp. 325 – 359.
Droby, S., Zahavi, T., Schena, L., Cohen, L., Wiess, B., Daus, A., Benarie, R., Chalutz, E., Ippolito A., 1998. Biocontrol of pre- and postharvest bunch decay of wine and table grapes. Proceedings of the Seventh International Congress on Plant Protection, Edinburgh, Scotland, 9 – 16 August, 5.2.67 (Abstract).
Elad, Y., Kohl, J., Fokkema, N.J., 1994. Control of infection and sporulation ofBotrytis cinereaon bean and tomato by saprophytic bacteria and fungi. Eur. J. Plant Pathol. 100, 315 – 336.
Eckert, J.W., Ogawa, J.M., 1985. The chemical control of postharvest diseases: subtropical and tropical fruits. Ann. Rev. Phytopathol. 23, 421 – 454.
Kohl, J., Molhoek, W.M.L., van der Plas, C.H., Fokkema, N.J., 1995. Effect ofUlocladium atrumand other antago-nists on sporulation ofBotrytis cinereaon dead lily leaves exposed to field conditions. Phytopathology 85, 393 – 401. Fokkema, N.J., 1973. The role of saprophytic fungi in
antago-nism againstDrechslera sorokiniana(Helminthosporium sa -ti6um) on agar plates and on rye leaves with pollen. Physiol. Plant Pathol. 3, 195 – 205.
Freeman, S., Shabi, E., 1996. Cross-infection of subtropical and temperate fruits by Colletotrichumspecies from vari-ous hosts. Physiol. Mol. Plant Pathol. 49, 395 – 404. Gullino, M.L., Migheli, Q., Mezzalama, M., 1995. Risk
analy-sis in the release of biological control agents. Plant Dis. 79, 1193 – 1201.
Hermanides-Nijhof, E.J., 1977. The black yeasts and allied Hyphomycetes. Studies in Mycol. 15, 141 – 177.
Hofman, C.S., Winston, F., 1987. A ten-minute DNA prepa-ration from yeast efficiently releases autonomous plasmids for transformation ofEscherichia coli. Gene 57, 267 – 272. Ippolito, A., El Ghaouth, A., Wilson, C., 1997. Indagini su alcuni possibili meccanismi di azione dell’isolato L47 di Aureobasidium pullulans, attivo contro marciumi postrac-colta di ortofrutticoli. Atti V Convegno annuale S.I.Pa.V.
Agripolis-Legnaro (PD) Italy, September 18 – 19, (Abstract).
Ippolito, A., Nigro, F., Romanazzi, G., Campanella, V., 1997a. Field application ofAureobasidium pullulansagainst Botrytis storage rot of strawberries. Proceedings of the Cost 914 – 915 ‘Non conventional methods for the control of postharvest disease and microbiological spoilage’, Bologna, Italy, October 9 – 11, 127 – 133.
Leibinger, W., Breuker, B., Hahn, M., Mendgen, K., 1997. Control of postharvest pathogens and colonization of the apples surface by antagonistic micro-organisms in the field. Phytopathology 87, 1103 – 1110.
Li, S., Cullen, D., Hjort, M., Spear, R., Andrews, J.H., 1996. Development of an oligonucleotide probe forAureobasid -ium pullulans based on the small-subunit rRNA gene. Appl. Environ. Microbiol. 62, 1514 – 1518.
Lima, G., Ippolito, A., Nigro, F., Romanazzi, G., Schena, L., Gatto, M.A., Salerno, M., 1996. Lotta biologica contro marciumi post-raccolta conAureobasidium pullulanseCan -dida oleophila. Inf. tore Agrario 45, 79 – 85.
Lima, G., Ippolito, A., Nigro, F., Salerno, M., 1997. Effective-ness of Aureobasidium pullulans and Candida oleophila against postharvest strawberry rots. Postharvest Biol. Technol. 10, 169 – 178.
Lo, C.T., Nelson, E.B., Hayes, C.K., Harman, G.E., 1998. Ecological studies of transformed Trichoderma harzianum strain 1295-22 in the rhizosphere and on the phylloplane of creeping Bentgrass. Phytopathology 88, 129 – 136. McKinney, H.H., 1923. Influence of soil temperature and
moisture on infection of wheat seedlings byHelminthospo -rium sati6um. J. Agric. Res. 26, 195 – 218.
Nigro, F., Ippolito, A., Romanazzi, G., Salerno, M., 1997. Prove di integrazione tra UV-C, curing e Aureobasidium pullulansnella lotta contro la muffa grigia dell’actinidia in post-raccolta. Primi risultati. Atti V Convegno annuale S.I.Pa.V. Agripolis-Legnaro (PD), September 18 – 19 (Abstract).
Webb, T.A., Mundt, J.O., 1978. Mold on vegetables at the time of harvest. Appl. Environ. Microbiol. 4, 655 – 658. Welsh, J., McClelland, M., 1990. Fingerprinting genomes
us-ing PCR with arbitrary primers. Nucl. Acids Res. 18, 7213 – 7218.
Williams, J.G.K., Kubelik, A.R., Livak, K.J., Rafalski, J.A., Tingey, S.V., 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl. Acid Res. 18, 6531 – 6535.
Wilson, C.L., Wisniewski, M.E., Droby, S., Chalutz, E., 1993. A selection strategy for microbial antagonists to control postharvest diseases of fruits and vegetables. Sci. Hortic. 53, 183 – 189.
Wilson, C.L., Wisniewski, M.E., 1994. Biological Control of Postharvest Diseases — Theory and Practice. CRC Press, Boca Raton, Florida, 182 pp.
Yurlova, N.A., Mokrousov, I.V., de Hoog, G.S., 1995. In-traspecific variability and exopolysaccaride production in Aureobasidium pullulans. Antonie van Leeuwenhoek 68, 57 – 63.