Influence of climate and fertilization on net photosynthesis of
mature slash pine
R. O. TESKEY,1 H. L. GHOLZ2 and W. P. CROPPER, JR.2
1 Daniel B. Warnell School of Forest Resources, University of Georgia, Athens, GA 30602, USA 2 Department of Forestry, University of Florida, Gainesville, FL 32611, USA
Received February 9, 1994
Summary
Net photosynthesis was measured under field conditions in 23-year-old slash pine (Pinus elliottii
Engelm. var. elliottii) trees to determine how it was affected by fertilization and climate. There was only a small decrease in rates of net photosynthesis from late summer through winter demonstrating that appreciable carbon gain occurs throughout the year in slash pine. Although fertilization substantially increased leaf area and aboveground biomass, it only slightly increased the rate of net photosynthesis. Simultaneous measurements of gas exchange in fertilized and unfertilized (control) plots allowed the detection of a small, but statistically significant difference in average net photosynthesis of 0.14 µmol m−2 s−1. Irradiance, and to a lesser extent air temperature, were the environmental factors that exerted the most control on net photosynthesis. The highest rates of net photosynthesis occurred between air temperatures of 25 and 35 °C. Because air temperatures were within this range for 46% of all daylight hours during the year, air temperature was not often a significant limitation. Soil and atmospheric water deficits had less effect on photosynthesis than irradiance or air temperature. Although the depth to the water table changed during the year from 10 to 160 cm, predawn and midday xylem pressure potentials only changed slightly throughout the year. Predawn values ranged from −0.63 to −0.88 MPa in the control plot and from −0.51 to −0.87 MPa in the fertilized plot and were not correlated with water table depth. There was no correlation between xylem pressure potentials and net photosynthesis, presumably because water uptake was adequate. Although vapor pressure deficits reached 3.5 kPa during the summer, they had little effect on net photosynthesis. Over a vapor pressure deficit range from 1.0 to 3.0 kPa, net photosynthesis only decreased 21%. No differences in responses to these environmental factors could be attributed to fertilization.
Keywords: Pinus elliottii, gas exchange, irradiance, temperature, leaf area, water deficit, water table.
Introduction
tially increased photosynthetic rates (Brix 1972, 1983, Sheriff et al. 1986, Smolander and Oker-Blom 1989), whereas in others, it had little effect (Thompson and Wheeler 1992). Additionally, it is not clear how nutritional status modifies the response of foliage to other environmental factors. In some studies, increased nutrient concentra-tions in conifers increased water use efficiency (Sheriff et al. 1986), whereas other studies found no effect (Mitchell and Hinckley 1993). Similarly, the photosynthetic light response may (Brix 1971, Smolander and Oker-Blom 1989), or may not (Brix and Ebell 1969), be different after fertilization.
The purpose of this study was to characterize how fertilization and climate affect rates of net photosynthesis in slash pine (Pinus elliottii Engelm. var. elliottii) trees. At the outset of the study, little was known about how environment or soil nutrient availability affects rates of net photosynthesis in this species. It was expected that, because of the low soil nutrient availability of the site, fertilization would produce a large increase in growth. A primary objective of the study was to determine whether this growth response was related to an increase in rates of net photosynthesis.
We also determined the photosynthetic response to seasonal changes in air tem-perature and water availability. Because the climate of the region is mild in winter, with few frosts, we hypothesized that net photosynthesis would continue throughout the winter at appreciable rates. On the other hand, based on the photosynthetic temperature response of loblolly pine (P. taeda L.) (Drew and Ledig 1981), a species that has an overlapping range and similar growth characteristics, we hypothesized that the high air temperatures during the summer, which are commonly over 30 °C in the afternoon, would inhibit net photosynthesis.
Water deficits were also expected to reduce net photosynthesis, particularly in summer. The water table in slash pine forests in this region can fluctuate during the year from near the surface to depths of 2 meters as a result of the sandy texture of the soils, high potential evapotranspiration rates and the pattern of rainfall (Gaston et al. 1990). Because most fine roots of these trees are within the upper 10 cm of the soil (Gholz et al. 1986), a decrease in the depth of the water table below the main rooting zone was expected to produce water stress and reduce net photosynthesis.
Materials and methods
Study site
density of 1190 trees ha−1 and stands were not thinned, fertilized or otherwise manipulated until this study began. The mean tree height was 15.4 m, mean stem diameter at 1.3 m was 17.3 cm, and mean stand basal area was 25.9 m2 ha−1 at the beginning of the study (Gholz et al. 1991).
Two 50 × 50 m plots, one fertilized and one unfertilized, were used. To minimize leaching losses, fertilizer was applied every three months, beginning one year before the study and continuing throughout the study. Annual fertilization rates were 180 kg ha−1 urea N, 70 kg ha−1 triple superphosphate P, 50 kg ha−1 muriate of potash K, 46 kg ha−1 triple superphosphate Ca, 50 kg ha−1 dolomitic limestone Ca, 28 kg ha−1 dolomitic limestone Mg, 50 kg ha−1 elemental S and 2 kg ha−1 triple superphosphate S. Micronutrients (Cu, Bo, Fe, Mn, Zn and Mo) were added in the first year only. Foliage was analyzed for nutrient concentrations in February and August 1988. At both times, fully expanded, one-year-old needles from the upper one third of the canopy were sampled.
Measurements
Access to the canopies of six trees per plot was facilitated by scaffold-type towers. Net photosynthesis was estimated from diurnal measurements of carbon dioxide exchange with two portable gas analysis systems (LCA-2, Analytical Development Company, Hoddesdon, U.K.). Each system consisted of an air pump unit, a leaf cuvette (narrow leaf type) and a battery-operated infrared gas analyzer. Measure-ments were made approximately every three weeks from January to December 1988. All six study trees in each plot were measured each hour, with the mean representing the hourly value. From January to July, the first flush from the previous year was sampled. After that, the first flush from the current year, by then fully expanded, was measured. For all measurements, the cuvette was held horizontally. Shading from nearby foliage was intentionally minimized. Gas exchange data were calculated on a total surface area basis. Leaf area was determined from measurements of needle length and radius, knowing that the outside surface of the needle forms a segment of a cylinder, and the inner surfaces of the needle are flat and can be approximated by a rectangle.
The two gas exchange systems were calibrated together and, whenever possible, used simultaneously in the fertilized and control plots. This sampling procedure allowed paired comparisons to be made of the rates of net photosynthesis of fertilized and control trees under similar environmental conditions.
Photosynthetically active radiation (PAR), air temperature and relative humidity were measured with the sensors in the leaf cuvette. Photosynthetically active radia-tion was measured with a photodiode, air temperature with a thermistor and relative humidity with a capacitive sensor. In addition, air temperature was measured with a shielded thermistor at canopy height in each plot. These data were averaged each hour and recorded with an Omnidata EasyLogger data logger (Logan, Utah).
with hourly measurements of net photosynthesis. Water table depths were recorded continuously in both plots with a Type F recorder (Leupold & Stevens Inc., Beaver-ton, OR).
Statistical analyses were done with SigmaStat Ver. 1.1 (Jandel Scientific, San Rafael, CA). Tests performed were: t-tests for comparisons of mean rates of net photosynthesis and mean foliar nutrient concentrations, paired t-tests for compari-sons of net photosynthesis when measurements were made simultaneously in the fertilized and control plots, and simple and multiple linear regressions with net photosynthesis as the dependent variable and environmental factors as the inde-pendent variables.
Results
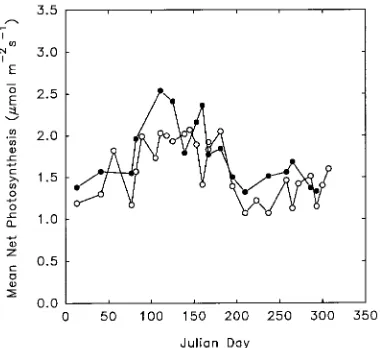
Mean midday rates of net photosynthesis exceeded 1.0 µmol m−2 s−1 on all sampling dates throughout the year (Figure 1). In April and May (Julian Dates 91 to 151), net photosynthesis (Pn) increased to about 2.0 µmol m−2 s−1 in the control trees. During these months, Pn appeared to be elevated in the fertilized trees; however, this trend was not consistent over time, and for the year, the mean rates of Pn in control (1.58 µmol m−2 s−1) and fertilized (1.76 µmol m−2 s−1) foliage were not statistically different (t-test, P = 0.068, n = 18).
In February, although an appreciable amount of fertilizer was added to the plot during the previous year, only Mg and Ca were higher in the fertilized foliage than in the unfertilized foliage (Table 1). In the spring, the stem wood growth rate was substantially higher in the fertilized trees than in the control trees, but by August, growth had slowed (Gholz et al. 1991). In August, foliar concentrations of four (N, P, K and Ca) of the five nutrients measured were higher in the fertilized trees than in
the control trees (Table 1). The foliar concentration of nitrogen increased in the fertilized trees from 0.93% in February to 1.16% in August and was significantly higher than in the control trees (0.75% N).
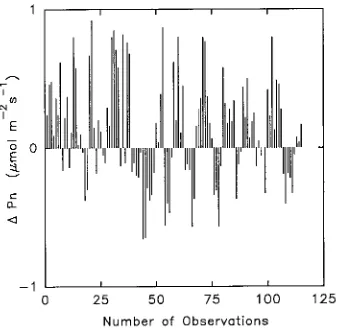
By using only the subset of measurements made simultaneously in the two plots, it was possible to detect an effect of fertilization on the rate of Pn (Figure 2). In this case, paired observations were examined to determine if there was any trend in rates of Pn when environmental differences such as irradiance, air temperature and vapor pressure deficit were minimized. Overall, there were more observations of higher Pn in the fertilized plot than in the control plot (Figure 2). The mean difference in Pn between fertilized and control trees was only 0.14 µmol m−2 s−1, but this difference was statistically significant (paired t-test, P = 0.001).
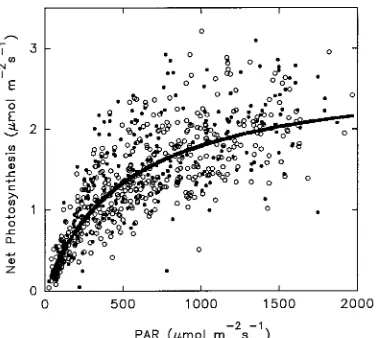
For the combined data set, a relationship between Pn and irradiance was evident
Figure 2. Comparison of measurements of net photosynthesis taken simultaneously in fertilized and control plots. Each observation represents the difference between the mean rate of net photosynthesis (∆ Pn) in each plot (n = 6), calculated as fertilized minus control, arranged chronologically from left to
right. A positive value indicates that rates of net photosynthesis in fertilized trees were higher than in control trees.
Table 1. Mean nutrient concentrations (%) for fully expanded one-year-old needles in the upper canopy of slash pine trees.
Date N P K Mg Ca
February
Fertilized 0.93 0.057 0.23 0.87a1 0.210a
Control 0.80 0.072 0.27 0.63 0.130
August
Fertilized 1.16a 0.097a 0.30a 0.84 0.285a
Control 0.75 0.053 0.13 0.89 0.217
(Figure 3). Based on nonlinear regression, a rectangular hyperbola was fitted to the data to describe the response of Pn to PAR, and to estimate the mean apparent quantum yield and mean maximum rate of net photosynthesis:
Pn = ΘPAR Pmax
ΘPAR + Pmax
, (1)
where Θ is the apparent quantum yield, and Pmax is the maximum rate of net photosynthesis at saturating irradiance. The best fit values for the parameters in Equation 1 were: Θ = 0.0043 and Pmax = 2.2432 µmol m−2 s−1. The 95% confidence intervals for the parameters were: Θ = 0.0039 to 0.0047 and Pmax = 2.0987 to 2.3877 µmol m−2 s−1. The relationships were not significantly different when fertilized and control foliage were analyzed separately.
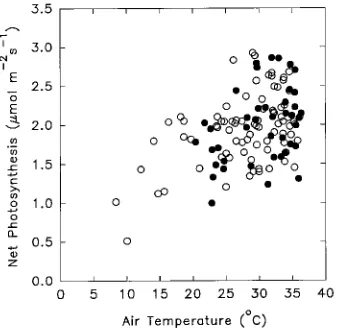
At high irradiances, Pn varied between about 1 and 3 µmol m−2 s−1. Part of this variation was caused by differences in air temperature during the year (Figure 4). Rates of net photosynthesis tended to increase with increasing air temperature, with the highest rates occurring at air temperatures between 25 and 35 °C. An examination of the air temperatures recorded hourly at the site indicated that in 46% of all daylight hours over the year, air temperatures were within the 25 to 35 °C range. Additionally, more than 73% of the daylight hours at high irradiance (PAR > 900 µmol m−2 s−1) were at air temperatures between 25 and 35 °C. There was no indication that high air temperatures, e.g., in excess of 30 °C, reduced the rate of Pn.
Water deficits did not significantly limit Pn. No statistical relationship was found between hourly xylem pressure potential measurements and Pn. During the year,
mean predawn XPP for trees in the control plot only varied from −0.63 to −0.88 MPa (Figure 5), and the range in mean predawn XPP in the fertilized plot was −0.51 to −0.87 MPa. Over the same time period, the water table depth fluctuated from 10 to 160 cm (Figure 5), but there was no correlation between water table depth and xylem pressure potentials measured at predawn or midday. Perhaps because the water supply was adequate, we only observed a weak negative correlation between vapor pressure deficit and Pn (Figure 6).
Multiple regression was used to assess the relative effect of the measured environ-mental factors on hourly mean net photosynthesis (Pn). Based on the entire data set, the best model was
Pn =−0.51 + 1.187(I)− 0.659(VPD)+ 0.022(T), (2)
where I is the mean response to irradiance (Equation 1 and Figure 3), VPD is the vapor pressure deficit, and T is the air temperature (R2 = 0.78). Irradiance was highly correlated with Pn, and it alone explained 69% of the variation in the data. Both VPD and T were weakly related to Pn, improving the fit of the model by 7 and 2%, respectively. Although numerous transformations of VPD and T were attempted, none improved the fit of the model by more than 1%, so the additional complexity they introduced into the model did not justify their use. Xylem pressure potential was not a significant variable in the model. Fertilization, introduced into the model as a dummy variable, was also not significant. The multiple regression was consistent with previous analyses of the separate environmental factors, i.e., it demonstrated that PAR was the most important factor influencing the rate of Pn, that VPD was negatively correlated with Pn, and that the other measured factors had little or no
Figure 6. Relationship between net photosynthesis (Pn) and vapor pressure deficit (VPD) for fertilized
(●) and control (s) foliage. Data shown in figure were collected in high irradiance (PAR > 900 µmol m−2 s−1) and air temperatures > 20 °C. The line though the data represents a linear regression of the form:
Pn = 2.68 − 0.35(VPD). The slope is significantly different from 0 (α = 0.05), R2 = 0.18.
effect.
Seasonal differences in cumulative net photosynthesis were estimated using Equa-tion 2 and mean hourly values of PAR, air temperature and VPD. On a relative basis, the highest seasonal net photosynthesis was in summer (0.35), followed by spring (0.26), autumn (0.20) and winter (0.19).
Discussion
Rates of net photosynthesis in slash pine changed with season, although the pattern was less pronounced than in pine species from cooler regions where net photosyn-thesis often falls to zero in winter (Troeng and Linder 1982, Jurik et al. 1988). The highest Pn rates were in April, May and June, with lower rates during the remainder of the year. High Pn rates may arise in the spring, because midday light intensity tends to increase as the solar azimuth decreases. High rates of net photosynthesis in the spring are also correlated with growth rate, and may be related to sink demands for carbohydrates as well as favorable environmental conditions (Maier and Teskey 1992). Although the midday rates of net photosynthesis were lower in summer than in spring, this could not be attributed to the higher air temperatures at this time of year. There was no appreciable decrease in rates of net photosynthesis from late summer and fall (July--November) through winter (December--February), demon-strating that appreciable carbon gain occurs throughout the year in slash pine.
Slash pine appeared to have lower rates of net photosynthesis than other pine species. For example, maximum rates of net photosynthesis on a total leaf area basis were about 5.4 µmol m−2 s−1 in P. sylvestris L. (Troeng and Linder 1982), 3.6--4.9 µmol m−2 s−1 in P. radiata D. Don (Sheriff et al. 1986, Thompson and Wheeler 1992), 4.1 µmol m −2 s−1 in P. strobus L. (Maier and Teskey 1992) and 6.0 µmol m−2 s−1 in
P. taeda (Fites and Teskey 1988). Low rates of Pn in slash pine seedlings in this study were similar to those reported by Doley (1988) and van den Driessche (1972).
Although fertilization had little effect on net photosynthesis of slash pine trees, it has a large effect on growth. Gholz et al. (1991) reported that, by August, as a result of the growth of new foliage in two flushes, the fertilized plot had a leaf area index of 6.8, which was 41% higher than that of the control plot. The annual stem wood biomass increment was also higher, increasing from 4 Mg ha−1 in the control plot to 5.7 Mg ha−1 in the fertilized plot. The initial increase in growth in response to fertilization was too large to be accounted for by the small increase in net photosyn-thesis, and suggests that the carbohydrates needed for the initial increase in growth came from the utilization of stored carbohydrates (Cropper and Gholz 1993). Later, the higher growth rates in the fertilized plot could be sustained by the higher leaf area. It is also possible that a shift in carbon allocation from root to shoot occurred after fertilization, as has been demonstrated in seedlings. Brix and Ebell (1969) also noted that fertilization had no effect on net photosynthesis in Pseudotsuga menziesii
fertilized trees was leaf area. This observation is consistent with the high correlation between leaf area and aboveground growth after fertilization in P. taeda stands (Vose and Allen 1988).
Evans (1989) compared published data from a variety of C3 species and found good agreement between rates of net photosynthesis and leaf nitrogen content. Compared with other C3 species, conifers had smaller increases in net photosynthesis per unit increase in nitrogen concentration. A positive correlation between leaf nitrogen content and net photosynthesis has been demonstrated for several trees including deciduous species (e.g., Prunus persica (L.) Batsch., DeJong et al. 1989; Acer saccharum Marsh., Reich et al. 1991), broad-leaved evergreen species (e.g., Arbutus menziesii Pursh. and Umbellularia californica (Hook & Arn.) Nutt., Field et al. 1983;
Eucalyptus globulus Labill., Sheriff and Nambiar 1991) and evergreen conifers (e.g.,
Pinus ponderosa Dougl., DeLucia et al. 1989; P. radiata, Thompson and Wheeler 1992; P. sylvestris, Smolander and Oker-Blom 1989; Pseudotsuga menziesii, Mitchell and Hinckley 1993). However, there are exceptions to this general response. Van den Driessche (1972) reported that nitrogen fertilization decreased rates of net photosynthesis in slash pine seedlings. Sheriff et al. (1986) found that nitrogen fertilization had no effect on net photosynthesis in the foliage of P. radiata trees, even though the nitrogen concentration was significantly higher in fertilized trees than in unfertilized trees; however, the higher rates of net photosynthesis were associated with an increased phosphorus content. Similarly, Reid et al. (1983) reported that the net photosynthetic rate of P. contorta Dougl. was correlated with the concentration of phosphorus in the foliage, but not with nitrogen. Phosphorus concentrations in the foliage have also been correlated with rates of net photosynthesis in P. radiata trees (Attiwill and Cromer 1982) and seedlings (Conroy et al. 1988). We found that, by August, the foliar concentrations of both phosphorus and nitrogen had increased as a result of fertilization (Table 1), but these increases had little effect on net photosyn-thesis.
In general, seedlings show a larger photosynthetic response to fertilization than large trees (Linder and Rook 1984). It is only when relatively large differences in nutrient concentrations in the foliage are produced by fertilization that detectable differences in net photosynthesis can be found in older trees. For example, in 14-year-old P. radiata, a difference of 1.0% N resulted in a 20% increase in net photosynthesis (Thompson and Wheeler 1992). However, to produce the high nitro-gen content (1.9%) on the low-fertility site, large amounts of fertilizer were applied over a long period.
might have been possible to detect a more sensitive VPD response. Maier and Teskey (1992) found that P. strobus had no detectable VPD response until predawn xylem pressure potentials were less than −1 MPa. Similar observations have been made for other conifers including Picea sitchensis (Bong.) Carr. (Beadle et al. 1981) and
P. sylvestris (Ng and Jarvis 1978).
The high predawn xylem pressure potentials measured throughout the year and the lack of correlation between water table depth and XPP indicated that the small number of roots that extend deeply through the soil profile were sufficient to supply the trees with water (cf. Teskey et al. 1985). However, it has been reported that under dry soil conditions, all of the root system of a loblolly pine tree is needed to maintain water uptake (Carlson et al. 1988). Apparently, in slash pine, as long as some of the roots are in contact with the water table, the transpirational needs of the tree are met. In summary, net photosynthetic rates of slash pine trees growing under field conditions were limited primarily by irradiance. The second most important limita-tion was air temperature. The highest rates of net photosynthesis occurred at air temperatures between 25 and 35 °C. Over the course of the year, in 73% of the hours when irradiance was high (> 900 µmol m−2 s−1), air temperatures were within this range. Net photosynthesis was not constrained by air temperatures in excess of 30 °C. Similarly, water table depth and vapor pressure deficits had little effect on the rate of
Pn. Finally, although the rates of Pn were low compared with other conifer species, as were the foliar concentrations of nutrients, two years of fertilization had only a slight effect on Pn. The lack of a fertilizer effect on Pn was consistent with other reports from field studies in older conifer stands. When more nutrients were made available through fertilization, slash pine trees increased carbon gain almost exclu-sively by expanded leaf area rather than by increased rates of net photosynthesis.
Acknowledgments
We thank Sheryll Vogel, Denise Guerin, Kevin McKelvy and Steven Smitherman for collecting the field data, and Amy Horne and Erika Mavity for assistance with data analysis. This project was funded by NSF Grant BSR 8106678. The stands for this research were used with permission of the Jefferson-Smerfit Company. Electrical power to the site was provided by the Florida Electric Power Coordinating Group.
References
Attiwill, P.M. and R.N. Cromer. 1982. Photosynthesis and transpiration of Pinus radiata D. Don under plantation conditions in southern Australia. I. Response to irrigation with waste water. Aust. J. Plant Physiol. 9:749--760.
Beadle C.L., R.E. Nelson, P.G. Jarvis and H. Talbot. 1981. Photosynthesis as related to xylem water potential and carbon dioxide concentration in Sitka spruce. Physiol. Plant. 52:391--400.
Bongarten, B.C. and R.O. Teskey. 1986. Water relations of loblolly pine seedlings from diverse geo-graphic origins. Tree Physiol. 1:265--276.
Brix, H. 1971. Effects of nitrogen fertilization on photosynthesis and respiration in Douglas-fir. For. Sci. 17:407--414.
Brix, H. 1972. Nitrogen fertilization and water effects on photosynthesis and earlywood-latewood production in Douglas-fir. Can. J. For. Res. 2:467--478.
Brix, H. and L.F. Ebell. 1969. Effects of nitrogen fertilization on growth, leaf area and photosynthesis rate in Douglas-fir. For. Sci. 15:189--196.
Carlson, W.C., C.A. Harrington, P. Farnum and S.W. Hallgren. 1988. Effects of root severing treatments on loblolly pine. Can. J. For. Res. 18:1376--1385.
Conroy, J.P., M. Küppers, B. Küppers, J. Virgona and E.W.R. Barlow. 1988. The influence of CO2
enrichment, phosphorus deficiency and water use of Pinus radiata D. Don. Plant Cell Environ. 11:91--98.
Cropper, W.P., Jr. and H.L. Gholz. 1993. Simulation of the carbon dynamics of a Florida slash pine plantation. Ecol. Model. 66:231--249.
DeJong, T.M., K.R. Day and R.S. Johnson. 1989. Partitioning of leaf nitrogen with respect to within canopy light exposure and nitrogen availability in peach (Prunus persica). Trees 3:89--95.
DeLucia, E.H., W.H. Schlesinger and W.D. Billings. 1989. Edaphic limitations to growth and photosyn-thesis in Sierran and Great Basin vegetation. Oecologia 78:184--190.
Doley, D. 1988. Fluoride-induced enhancement and inhibition of photosynthesis in four taxa of Pinus. New Phytol. 110:21--31.
Drew, A.P. and F.T. Ledig. 1981. Seasonal patterns of CO2 exchange in the shoot and root of loblolly pine
seedlings. Bot. Gaz. 142:200--205.
Evans, J.R. 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78:9--19.
Field, C., J. Merino and H.A. Mooney. 1983. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia 60:384--389.
Fites, J.A. and R.O. Teskey. 1988. CO2 and water vapor exchange in Pinus taeda in relation to stomatal
behavior: test of an optimization hypothesis. Can. J. For. Res. 18:150--157.
Gaston, L., P. Nkedi-Kizza, G. Sawka and P.S.C. Rao. 1990. Spatial variability of morphological properties at a Florida flatwoods site. Soil Sci. Soc. Am. J. 54:527--533.
Gholz, H.L., L.C. Hendry and W.P. Cropper, Jr. 1986. Organic matter dynamics of fine roots in plantations of slash pine (Pinus elliottii) in north Florida. Can. J. For. Res. 16:529--538.
Gholz, H.L., S.A. Vogel, W.P. Cropper, Jr., K. McKelvey, K.C. Ewel, R.O. Teskey and P.J. Curran. 1991. Dynamics of canopy structure and light interception in Pinus elliottii stands, north Florida. Ecol. Monogr. 61:33--51.
Jokela, E.J. and S.C. Stearns-Smith. 1993. Fertilization of established southern pine stands: effects of single and split nitrogen applications. Southern J. Appl. For. 17:135--138.
Jurik, T.W., G.B. Briggs and D.M. Gates. 1988. Springtime recovery of photosynthetic activity of white pine in Michigan. Can. J. Bot. 66:138--141.
Linder, S. and D.A. Rook. 1984. Effects of mineral nutrition on carbon dioxide exchange and partitioning of carbon in trees. In Nutrition of Plantation Forests. Eds. G.D. Bowen and E.K.S. Nambiar. Academic Press, London, pp 211--236.
Linder, S., M.L. Benson, B.J. Meyers and R.J. Raison. 1987. Canopy dynamics and growth of Pinus radiata. I. Effects of irrigation and fertilization during a drought. Can. J. For. Res. 17:1157--1165. Maier, C.A. and R.O. Teskey. 1992. Internal and external control of net photosynthesis and stomatal
conductance of mature eastern white pine (Pinus strobus L.). Can. J. For. Res. 22:1387--1394. Miller, H.G. 1984. Dynamics of nitrogen cycling in plantation ecosystems. In Nutrition of Plantation
Forests. Eds. G.D. Bowen and E.K.S. Nambiar. Academic Press, London, pp 53--78.
Mitchell, A.K. and T.M. Hinckley. 1993. Effects of foliar nitrogen concentration on photosynthesis and water use efficiency in Douglas-fir. Tree Physiol. 12:403--410.
Nambiar, E.K.S. 1990. Management of forests under nutrient and water stress. Water Air Soil Pollut. 54:209--230.
Ng, P.A.P. and P.G. Jarvis. 1980. Hysteresis in the response of stomatal conductance in Pinus sylvestris L. needles to light: observations and a hypothesis. Plant Cell Environ. 3:207--216.
NOAA. 1989. Climate data----Florida. National Climate Data Center, NOAA, Asheville, NC, pp 17--26. Reich, P.B., M.B. Walters and D.S. Ellsworth. 1991. Leaf age and season influence the relationships between leaf nitrogen, leaf mass per unit area, and photosynthesis in maple and oak trees. Plant Cell Environ. 14:251--259.
Sandford, A.P. and P.G. Jarvis. 1986. Stomatal responses to humidity in selected conifers. Tree Physiol. 2:89--104.
Sheriff, D.W. and E.K.S. Nambiar. 1991. Nitrogen nutrition, growth and gas exchange in Eucalyptus globulus Labill. seedlings. Aust. J. Plant. Physiol. 18:37--52.
Sheriff, D.W., E.K.S. Nambiar and D.N. Fife. 1986. Relationship between nutrient status, carbon assimilation and water use in Pinus radiata (D. Don) needles. Tree Physiol. 2:73--88.
Smolander, H. and P. Oker-Blom. 1989. The effect of nitrogen content on the photosynthesis of Scots pine needles and shoots. Ann. Sci. For. 46(suppl.):473s--475s.
Teskey, R.O., C.C. Grier and T.M. Hinckley. 1985. Relation between root system and water inflow capacity of Abies amabilis growing in a subalpine forest. Can. J. For. Res. 15:669--672.
Thompson, W.A. and A.M. Wheeler. 1992. Photosynthesis by mature needles of field-grown Pinus radiata. For. Ecol. Manage. 52:225--242.
Troeng, E. and S. Linder. 1982. Gas exchange in a 20-year-old stand of Scots pine. II. Variation in net photosynthesis and transpiration within and between trees. Physiol. Plant. 54:15--23.
van den Driessche, R. 1972. Different effects of nitrate and ammonium forms of nitrogen on growth and photosynthesis of slash pine seedlings. Aust. For. 36:125--137.