www.elsevier.com / locate / bres
Research report
Changes in sensitivity of cholinoceptors and adrenoceptors during
transhemispheric cortical reorganisation in rat SmI
a ,
*
c a,b aMojtaba Zarei
, Vladimir V. Raevsky , Gavin S. Dawe
, John D. Stephenson
a
Department of Neuroscience, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, UK b
Department of Psychology, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, UK c
Institute of Higher Nervous Activity and Neurophysiology, Russian Academy of Science, Moscow, Russia
Accepted 3 October 2000
Abstract
The reorganisation of primary somatosensory cortex that occurs after lesioning the corresponding cortex of the contralateral hemisphere in rat has been termed, ‘transhemispheric cortical reorganisation’. Cholinergic and noradrenergic innervations are hypothesized to be involved in cortical plasticity. The present study investigated the change in responses of somatosensory neurones in the hindpaw representation area to muscarinic cholinoceptor andb-adrenoceptor receptor stimulation, by iontophoretic application of acetylcholine, noradrenaline, propranolol and atropine, during the process of transhemispheric cortical reorganization at 3–4 days and at 20–21 days after lesioning the corresponding area in the contralateral hemisphere. Most neurones in control rats showed excitatory atropine-sensitive responses to acetylcholine, and inhibitory propranolol-sensitive responses to noradrenaline. A marked reduction in neurones exhibiting muscarinic responses (from 69% to 22%) andb-noradrenoceptor-mediated responses (from 62% to 24%) were seen in rats 3–4 days post lesion. The proportion of neurones responding had recovered by 3 weeks but the direction of the responses had changed with muscarinic response becoming predominantly inhibitory and b-noradrenoceptor responses predominantly excitatory. It is concluded that trans-hemispheric cortical reorganization involves both receptor types and that the reciprocal changes at different stages after injury maintain cortical plasticity. 2001 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Somatosensory cortex and thalamocortical relationship
Keywords: Reorganization; Plasticity; Acetylcholine; Noradrenaline; Brain damage
1. Introduction sphere [5]. The latter experiment showed that immediate
changes do not necessarily last for a long time, suggesting Several studies have described changes in receptive mediation by a different mechanism than that responsible fields in primary somatosensory cortex (SmI) after deacti- for the long-term reorganisation of cerebral cortex such as vation of the corresponding contralateral cortex. Reorgani- that which occurs after unilateral peripheral nerve damage. sation of receptive fields in the SmI of both flying foxes Recently it has been shown that a unilateral lesion of a and macaque monkeys occurred in contralateral as well as part of SmI results in the reorganisation of contralateral in ipsilateral hemisphere immediately after unilateral in- SmI in rat, a phenomenon termed ‘transhemispheric corti-jection of local anaesthetic into a digit [4]. Cooling of a cal reorganisation’ [28]. The net effect of this reorganisa-small region of the primary somatosensory cortex in the tion is to increase the area of cortex in the intact hemi-same species caused a reversible expansion of receptive sphere serving the body part previously served by the fields in the homologous area of the contralateral hemi- lesioned representation. This is accompanied by a marked increase in the proportion of neurones with bilateral receptive fields. The present paper describes the time
*Corresponding author. Present address: Rivermead Rehabilitation
course and neuromodulatory processes accompanying this
Centre, Abingdon Road, Oxford OX3 0LR, United Kingdom. Tel.:
phenomenon. This is important because transhemispheric 144-1865-240321; fax:144-1865-200185.
E-mail address: [email protected] (M. Zarei). cortical reorganization may contribute to recovery of
function after brain injury and the adjustments might be buprenorphine (Temgesic, 0.05 ml s.c.) and allowed to hastened or improved by appropriate pharmacological recover.
intervention.
The reorganisation of the barrel cortex that occurs in 2.1. Unit recording adult rats after chronic vibrissectomy of all but one
whisker, visualised with 2-deoxy glucose [13] was pre- 3–4 days or 20–21 days after lesioning, the rats were 21
vented by locus coeruleus lesions suggesting noradrenergic anaesthetised with urethane (1.0–1.5 g kg i.p.) and again involvement in cortical plasticity [22]. Transhemispheric placed in a stereotaxic frame. The skull overlying the left cortical reorganisation also depended on central norad- SmI was removed using a dental drill. The cortex was renergic activity because it was prevented by pretreatment exposed by a narrow slit in the dura and covered with 4% with DSP4, a neurotoxin destroying the noradrenergic agar in saline and core temperature maintained at 37618C innervation of the cortex from the locus coeruleus [28]. using a heating lamp. Five ml of 5% glucose solution in A role for acetylcholine (ACh) in cortical plasticity is 0.18% NaCl was administered s.c. to offset dehydration. also suggested by the observation that the reorganisation in Single units, isolated by amplitude discrimination, were the somatosensory cortex that would normally occur after recorded from a site contralateral to the centre of the digit removal or sciatic nerve transection was prevented by lesion, defined stereotaxically and confirmed electrophy-depleting cortical ACh with an ipsilateral basal forebrain siologically with cortical evoked potential recording as lesion [12,27]. Sciatic nerve transection also reduced the described previously [28,29], with the central saline-filled response of somatosensory cortical neurones to ion- barrel of a 6-barrelled microelectrode (impedance 1–3 MV tophoretic application of ACh suggesting the cortical at 10 KHz), digitised and stored for off-line analysis. reorganisation was associated with changes in cholinocep- Only spontaneously active neurones which responded to tor sensitivity [17]. The present study examined muscarinic iontophoretic application of ACh and NA with muscarinic and b-adrenoceptor responses of somatosensory neurones responses (i.e. blocked by atropine) and b -adrenoceptor-to ion-adrenoceptor-tophoretic application of ACh and noradrenaline mediated responses (i.e. blocked by propranolol), respec-(NA) 3–4 days and 20–21 days after a unilateral cortical tively, were analysed (see below). The depth of the lesion, i.e. at early and late stages of transhemispheric electrodes below the pial surface suggested that the
cortical reorganisation. neurones from which recordings were made were mostly
located in layers IV–V, and this was confirmed by the histological data.
2. Materials and methods 2.2. Microiontophoretic application of drugs
Male Sprague–Dawley albino rats (B and K Universal, Drugs were administered iontophoretically through the U.K.), weighing 200–250 g, were used. They were housed outer barrels of a 6-barrel glass microelectrode. These 4–6 per cage, with free access to food and water under an contained ACh chloride (0.2 M, pH 4.0), atropine sulphate alternating 12 h light (0800–2000) and dark cycle in a (0.2 M, pH 4.0), NA hydrochloride (0.1 M, pH 4.0) and controlled environment (23618C; 45– 60% relative propranolol hydrochloride (0.1 M, pH 4.0); recordings humidity). The rats were allowed to adapt to their home were made through the ACSF-filled central barrel. A 10 cage for at least 1 week before being used in any of the nA backing current was applied to each drug-containing
experimental procedures described below. barrel and compensated through a barrel filled with 2 M
Rats were studied either 3–4 days (n55) or 20–21 days NaCl. The agonists, ACh and NA, were each applied for (n55) after ablating the hindpaw region in the right SmI, 20 s with ejection currents of 30, 60, 100 and 150 nA. If as previously described [28]; a third group of rats served as either ACh or NA produced a significant effect on neuro-controls (n56). Briefly, the rats were anaesthetised with nal activity, then the appropriate antagonist was applied, halothane, 1.5–3% in oxygen, mounted in a stereotaxic by a current equal to the current that produced the response frame, and the skull overlying the right Sm1 was exposed under investigation, for 25 s commencing 5 s before the under sterile conditions. The hindpaw representation, agonist. The interval between application of each drug was located from recordings of short latency responses to a minimum of 3 min and the drugs were applied in the electrical stimulation of the contralateral hindpaw, was following sequence: ACh alone; Atropine1ACh; NA then ablated to the depth of the white matter using a glass alone; Propranolol1NA.
pipette connected to a vacuum source with pressure
control. After controlling bleeding, the dura was replaced 2.3. Histology and the scalp incision sutured and dusted with
electrode as described in details previously [28,29]. The centages of neurones responding to different drugs be-rats were then deeply anaesthetised and perfusion-fixed by tween experimental groups. All variables were tested for a transcardial infusion of 100 ml of saline followed by 300 two-tail hypothesis.
ml of 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Sequential vibratome sections (30mm) were cut
and stained with cresyl fast violet and examined with 3. Results reference to an atlas of rat cerebral cortex [30].
Responses of 61 neurones from control rats, 60 neurones
2.4. Statistical analysis from rats 3–4 days post lesion and 56 neurones from rats
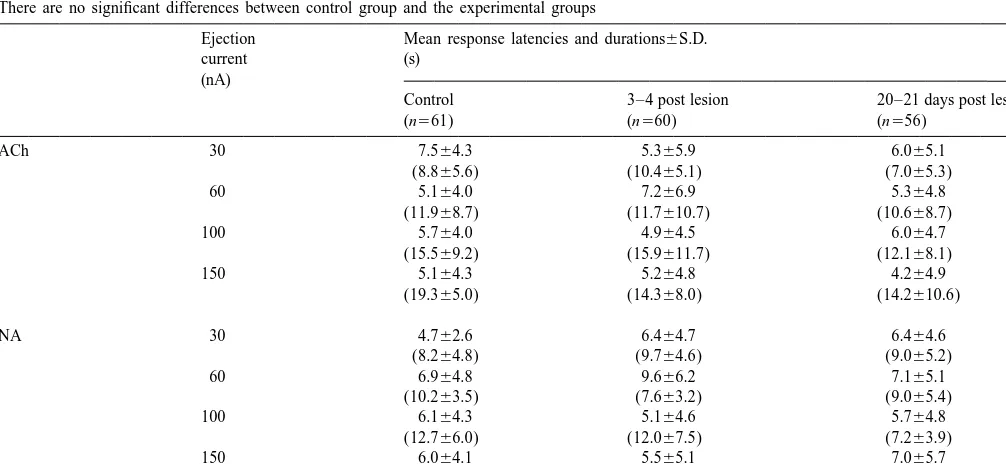
20–21 days post lesion to iontophoretic application of NA Responses of single neurones to iontophoretic applica- and ACh (each 30–150 nA) were studied. Background tion of drugs were determined from analysing the averages firing rates of the neurones in rats 3–4 days post lesion and of 3 histograms for each trial, each consisting of 3 rats 20–21 days post lesion (3.061.2 and 2.961.4 spikes
21
consecutive 20 s blocks (before, during and after drug s 6S.D., respectively) did not differ from those in the 21 administration). The mean firing rates before (20 s, back- age-matched control rats (3.161.2 spikes s 6S.D.). ground) and during iontophoretic drug application (20 s) There were no differences (P,0.05) in the mean latencies were noted and compared. Averages of 3 drug administra- and durations of neuronal responses to ACh and NA in the tions were used for statistical analysis of drug effects, different groups (Table 1).
significance of effects being calculated using Wilcoxon
tests for significant changes in neuronal activity (P,0.05) 3.1. Effects of ACh between the background activity and the activity during the
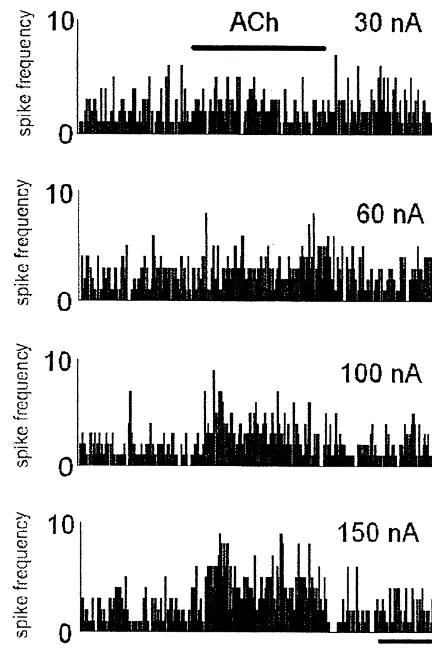
period of chemical stimulation. Latency and duration of About two-thirds (61–69%, depending on the ejection significant changes in neuronal firing after the beginning of current) of all somatosensory neurones recorded, respond-the stimulation was automatically detected and noted ed to iontophoretic application of ACh with an atropine-(Spike Neurosoft, Elprog Ltd., Moscow). The ratios of sensitive response in control rats (see Fig. 1 for an example excitatory to inhibitory responses (E /I ratio) were also of PSTH). The responses were largely excitatory and calculated. Firing rates are given as means6S.D. spikes dose-dependent i.e. the E /I ratio increased from 1.1:1 at 30
21
s unless otherwise stated. nA to 8.6:1 at 150 nA (Figs. 2 and 3a). This was mainly
To compare firing rates, latencies to onset of drug action due to neurones, which were inhibited by ACh at the lower and durations of drug action between control and ex- currents being excited at higher currents, because the total perimental groups an independent ANOVA were used. A number of responsive neurones was relatively unaffected
2
x cross-tabulation test was used to compare the per- (Fig. 4). About 10% of neurones showed a mixed
(bimod-Table 1
Latencies and, in parentheses, durations of neuronal responses to iontophoretic application of ACh or NA at each ejecting current (n5number of neurones). There are no significant differences between control group and the experimental groups
Ejection Mean response latencies and durations6S.D.
current (s)
(nA)
Control 3–4 post lesion 20–21 days post lesion
Fig. 1. A typical neurone in layer IV of SmI showing an excitatory response to iontophoretic application of ACh. The response is blocked by iontophoretic application of atropine commencing 5 s prior to application of ACh and ceasing at the same time as the application of ACh.
al) response but this percentage dropped to 2% at the highest current (Fig. 4).
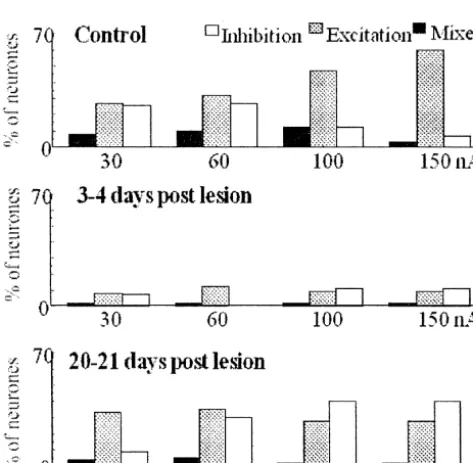
Three to 4 days after cortical ablation, the percentage of neurones responding to ACh decreased to between 14 and 22% of the proportion of spontaneously active neurones, depending on the current (P,0.05). The increase in the
E /I ratio described in control rats was not seen, there was
Fig. 2. Ratios of excitatory to inhibitory responses to iontophoretic
no dose–response effect and the proportions of excitatory
application of ACh (a) and NA (b) in hindpaw region of somatosensory
and inhibitory reactions were similar at most currents
cortex of control rats either 3–4 days or 20–21 days after ablation of the
except 60 nA (Figs. 3a and 4). Fewer neurones responded corresponding region of the contralateral cortex; negative values represent in a bimodal manner to ACh than in control rats, about inhibitory to excitatory ratios. ACh application usually excited neurones
10% cf. 2% or less (P,0.02). in control rats but in the rats 3–4 days post lesion, excitatory and inhibitory responses occurred in approximately equal proportions. In the
The total proportion of neurones responding to ACh had
rats 20–21 days post lesion, excitatory responses to ACh were only
returned to the control level by 3 weeks after the lesion
dominant at the lowest ejection current. NA usually evoked an inhibitory
and a limited dose–response effect was observed; 46% of response in control rats but an excitatory response in the rats 20–21 days neurones responded at 30 nA and 60% responded at 60 nA, post lesion. Intermediate effects were seen in the rats 3–4 days post
but further increase of current did not increase the propor- lesion. No. of neurones561 in control rats, 60 in rats 3–4 days post lesion and 56 in rats 20–21 days post lesion.
tion of responsive neurones. However, increasing current had little effect on the proportions of neurones excited by ACh but increased the proportion of inhibitory responses (Fig. 4) i.e. E /I ratio dropped considerably (P,0.05);
from 3.7:1 at 30 nA to 0.7:1 at 100 nA (Fig. 3a). The total 0.5:1 at higher currents and this was entirely due to an population of neurones exhibiting bimodal responses was increase in the proportion of neurones excited by NA
similar to that in control rats. because the proportion which were inhibited was
unaffect-ed (Fig. 3b). Depending on the ejection current, 4% to 9% of neurones showed a bimodal response.
3.2. Effects of NA Three to 4 days after lesion, the proportion of cortical
neurones which were sensitive to iontophoretic application In control rats, between 48% and 62% of neurones, of NA decreased significantly (P,0.05), to between 14% depending on the current applied, exhibited a propranolol- and 24% depending on the ejection current, and the dose– sensitive response to NA. The responses were predomi- response effect was abolished (Fig. 5). The lowest dose of nantly inhibitory, particularly at the lowest current when NA, which evoked the greatest proportion of inhibitory 17 out of 22 NA-sensitive neurones (77%), responded with responses in control rats, evoked the greatest proportion of inhibition and only 1 neurone was excited (Fig. 5). The excitatory responses in rats 3–4 days post lesion, so that
Fig. 5. Proportion of neurones in hindpaw region of SI responding to iontophoretic application of NA at ejection currents of 30, 60, 100 and Fig. 3. A typical does dependent response of a neurone in layer IV of rat 150 nA. The proportion of responding neurone at each ejection current
SmI. decreased markedly 3–4 days after lesioning the contralateral cortical
area but had recovered 3 weeks later. In reverse to changes in the effect of ACh in rats 20–21 days post lesion, NA which were usually inhibitory in control rats, showed a high proportion of excitatory responses to NA; indicating that unlike ACh, NA effect may reverse during cortical plasticity after the contralateral lesion. No. of neurones561 in control rats, 60 in rats 3–4 days post lesion and 56 in rats 20–21 days post lesion.
rats to 4.3:1 in the rats 3–4 days post lesion (Fig. 3b). The proportion of excitatory responses decreased at higher ejection currents. The proportion of neurones with bimodal responses was much lower than that in control rats.
Three weeks after the lesion, the percentage of NA-sensitive neurones had returned to the control level e.g. 52% at 30 nA compared with 48% in control rats at the same ejection current (Fig. 5). Increasing the ejection current to 60 nA, significantly increased the proportion of responsive neurones, evidence for the return of a dose– response effect. However, further increase of the current did not significantly alter the proportion of responding neurones (Fig. 5). Similarly, the E /I ratio of neuronal responses increased (P,0.05) from 1.9:1 at 30 nA to 3.9:1 at 60 nA but decreased to 1.0:1 after the current increased to 150 nA (Fig. 3b). This was due to an initial increase in
Fig. 4. The proportion of neurones in hindpaw region of control rats and
the proportion of neurones, which were excited by NA at
rats 3–4 days and 20–21 days after lesioning of the corresponding
the lower ejection current without affecting the proportion
contralateral cortex, responding with an inhibitory, excitatory or mixed
(biphasic) response to iontophoretic administration of ACh at 30, 60, 100, of neurones, which were inhibited; however the latter
150 nA for 20 s. The total proportion of neurones responding to increased at the higher ejection currents with a concomi-acetylcholine was reduced 3 days after the lesioning. Although this had tant decrease in the former (Fig. 5). The majority of recovered by 3 weeks, there was a shift from predominantly excitatory
neurones showed an excitatory response to NA at each of
responses in controls to a greater proportion of inhibitory responses. No.
the different ejection currents. It is noteworthy that
neuro-of neurones561 in control rats, 60 in rats 3–4 days post lesion and 56 in
reported previously [16]. The predominantly excitatory response is consistent with a previous study, which de-scribed their occurrence in all cortical layers, especially in laminae Vb and VIb [16], although the proportion of excitatory responses was higher in the present study. A modulatory role of ACh in SmI plasticity is suggested by the finding that it enhanced the strength of the neuronal responses to peripheral stimulation and glutamate for up to 1 h after iontophoretic application [20]. It has been proposed that the modulatory response to ACh enables cortical neurones to rapidly alter the magnitude of their responses to afferent stimulation [15]. These rapid changes in receptive field size may then be stabilised by longer lasting changes because ACh is also involved in the development of new receptive fields [27,12].
The involvement of the cholinergic system in cortical plasticity of adult rat somatosensory cortex was suggested by changes in cholinergic markers (choline acetyltrans-ferase, acetylcholine esterase, high-affinity choline uptake) in the hindlimb receptive fields of the rat SmI, 1 to 63 days after unilateral transection of sciatic nerve [25]. These studies showed that 1 day after sciatic nerve transection there was a 30% reduction in high-affinity choline uptake in layer V of the contralateral somatosensory cortex and, at 3 days, a 15% reduction in ChAT activity. In rat SmI, unilateral sciatic nerve transection altered binding of the
3
M1-selective ligand, [ H]pirenzepine, in bilateral hindlimb region of somatosensory cortex, indicating the involvement of muscarinic M1 receptors in somatosensory cortical plasticity [10]. Other studies suggested that the changes in
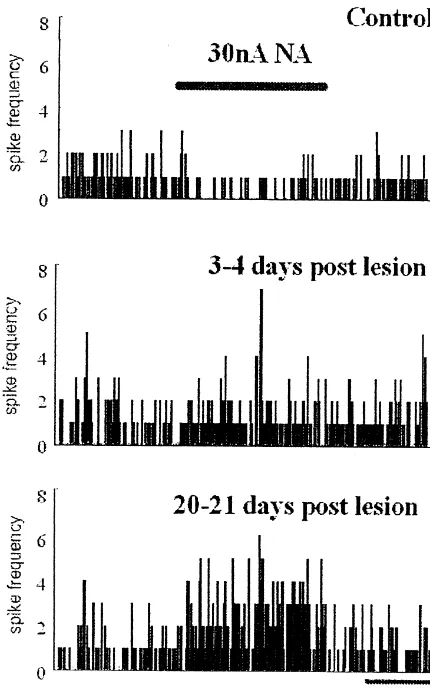
Fig. 6. An example of neuronal response to application of NA. Most
cholinergic markers in the rat barrel cortex after
vibrissec-neurones in SmI showed an inhibitory response to iontophoretic
applica-tomy were related to altered morphology and not to
tion of NA in control rats. However during transhemispheric cortical
reorganization, excitatory neuronal responses increased markedly. abnormal functioning of the barrel cortex as a result of the
reduced sensory input from the vibrissae [8].
Iontophoretic application of NA onto somatosensory over a period of 3 weeks after the lesion (see Fig. 6 for an neurones in urethane-anaesthetised rats has previously
example). been shown to produce inhibitory and, occasionally,
excitatory responses [1]. The nature of the neuronal responses, their latencies and the bell-shaped pattern of
4. Discussion PSTH constructed during application of NA, were
con-sistent with those described in this study. Several studies The present study examined the effects of lesioning the showed that NA promotes recovery of function after brain right hindpaw region on the responses of neurones in the injury [3] presumably by enhancing neural plasticity [2]. corresponding contralateral area to iontophoretic applica- There has been studies demonstrating changes in choliner-tion of ACh and NA, 3–4 days and 3 weeks later. The gic and noradrenergic activity following brain injury. For neurones studied all responded to stimulation of the example, 9 days after extirpation of the frontal cortex, NA contralateral hindpaw with short latency responses. Three concentrations in the locus coeruleus, measured by HPLC, to 4 days after lesioning, the proportion of neurones were increased [9]. Traumatic brain injury induced by fluid responding to ACh and NA decreased markedly but had percussion produced a temporary loss of ChAT-positive returned to normal at the later time interval. The responses neurones in basal forebrain nuclei [18] and subsequent to ACh showed a shift from predominantly excitatory to increase in density of cortical muscarinic receptors [11] 2 inhibitory, particularly at the earlier time interval (3–4 weeks after injury suggesting decrease in presynaptic days post lesion), whereas responses to NA showed a shift cholinergic activity and up-regulation of its post synaptic
in the opposite direction. receptors were observed. There is also evidence that
which could increase net presynaptic activity. It is pro- manipulation of cortical plasticity during recovery of posed that the plastic changes take place within 3–4 days function according to the time scale of the events. after lesion of the contralateral area and that some cortical
reorganisation had occurred by 3 weeks. Previous data [28]
showing an increase in the neuronal pool subserving the Acknowledgements lesioned representation area at 3 weeks suggests that many
neuronal connections have been made by this time, al- This work is supported by grants to M.Z. by the though the process may take much longer to complete. The Ministry of Health and Medical Education of Islamic fact that the E /I ratios for ACh and NA changed markedly Republic of Iran, to G.S.D. by the UK Medical Research after the lesion with ACh eliciting more inhibitory re- Council and to V.R. by British Royal Society.
sponses and NA more excitatory responses suggests that there might be a balance between the cholinergic and
noradrenergic innervations to SmI which was disrupted References temporarily during the first few days. It is hypothesized
that muscarinic actions of the cholinergic system play an [1] M. Armstrong-James, K. Fox, Effects of ionophoresed noradrenaline
early prominent role in cortical plasticity changes, perhaps on the spontaneous activity of neurones in rat primary somato-sensory cortex, J. Physiol. Lond. 335 (1983) 427–447.
rapidly adjusting neural systems to a cortical lesion, and
[2] P. Bach-y-Rita, Brain plasticity as a basis for recovery of function in
that b-adrenoceptor mediated effects of the noradrenergic
humans, Neuropsychologia 28 (1990) 547–554.
system come in to prominence later and perhaps regulate [3] M.G. Boyeson, D.M. Feeney, Intraventricular norepinephrine facili-the development of neuronal connections. Furfacili-ther studies tates motor recovery following sensorimotor cortex injury,
Phar-are required to investigate the suggested phasic contribu- macol. Biochem. Behav. 35 (1990) 497–501.
[4] M.B. Calford, R. Tweedale, Interhemispheric transfer of plasticity in
tions of the cholinergic and noradrenergic systems to the
the cerebral cortex, Science 249 (1990) 805–807.
plasticity changes.
[5] J.C. Clarey, R. Tweedale, M.B. Calford, Interhemispheric
modula-In vitro studies have shown that GABA receptors are tion of somatosensory receptive fields: evidence for plasticity in associated with both the noradrenergic nerve terminals and primary somatosensory cortex, Cerebral Cortex 6 (1996) 196–200.
post-synaptic neurones receiving a noradrenergic input [6] J.M. Conner, B. Fass-Holmes, S. Varon, Changes in nerve growth factor immunoreactivity following entorhinal cortex lesions:
pos-[26]. However, the decreased sensitivity during the first
sible molecular mechanism regulating cholinergic sprouting, J.
few days was not thought to be due to increased
GABAer-Comp. Neurol. 345 (1994) 409–418.
gic activity because the spontaneous firing rate of the [7] M.E. Diamond, W. Huang, F.F. Ebner, Laminar comparison of neurones was unchanged after the lesion. Also the pro- somatosensory cortical plasticity, Science 265 (1994) 1885–1888.
portions of inhibitory responses in both experimental [8] S. Glazewski, M. Kossut, E. Siucinska, J. Skangiel-Kramska, Cholinergic markers in the plasticity of murine barrel field, Acta
groups were similar.
Neurobiol. Exp. (Warsz) 50 (1990) 163–172.
Changes in cortical organisation during transhemispheric
[9] T.V. Grekhova, V.S. Kudrin, I.I. Miroshnichenko, G.A. Romanova,
cortical plasticity occur primarily in the superficial layers, Role of monoamines in the recovery of the conditioned reflex e.g. pruning of dendrites [14] located in superficial cortical activity of rats following extirpation of the frontal cortex, Biull.
layers [7] which have the greatest noradrenergic innerva- Eksp. Biol. Med. 103 (1987) 12–14.
[10] U.K. Hanisch, T. Rothe, K. Krohn, R.W. Dykes, Muscarinic
tion from the locus coeruleus [19]. After nucleus basalis
cholinergic receptor binding in rat hindlimb somatosensory cortex
lesions, the greatest changes in the response of hindpaw
following partial deafferentation by sciatic nerve transection,
Neuro-cortical neurones to iontophoretic application of ACh chem. Int. 21 (1992) 313–327.
occurred in the granular layer, arguing for a role of [11] J.Y. Jiang, B.G. Lyeth, T.M. Delahunty, L.L. Phillips, R.J. Hamm,
cholinergic terminals in the superficial layers in cortical Muscarinic cholinergic receptor binding in rat brain at 15 days following traumatic brain injury, Brain Res. 651 (1994) 123–128.
plasticity [17]. The present study supports the involvement
[12] S.L. Juliano, W. Ma, D. Eslin, Cholinergic depletion prevents
of both cholinergic and noradrenergic terminals in cortical
expansion of topographic maps in somatosensory cortex, Proc. Natl.
plasticity. The most likely explanation for the acute Acad. Sci. USA 88 (1991) 780–784.
reductions in b-adrenoceptor-and muscarinic receptor-me- [13] M. Kossut, S. Glazewski, E. Siucinska, J. Skangiel-Kramska,
diated responses is decrease receptor sensitivity in re- Functional plasticity and neurotransmitter receptor binding in the vibrissal barrel cortex, Acta Neurobiol. Exp. (Warsz) 53 (1993)
sponse to sustained increases in cholinergic and
norad-161–173.
renergic activity. Clearly an understanding of the
mecha-[14] D.A. Kozlowski, T.A. Jones, T. Schallert, Pruning of dendrites and
nisms underlying the acute desensitisation is crucial to restoration of function after brain-damage; Role of the NMDA understanding of later recovery, i.e. whether it is a receptor, Restorative Neurol. Neurosci. 7 (1994) 19–126.
consequence of a temporary process or a persistent change [15] K. Krnjevic, Central cholinergic mechanisms and functions, Prog. Brain Res. 98 (1993) 285–292.
which is later compensated for by, for example, increased
[16] Y. Lamour, P. Dutar, A. Jobert, R.W. Dykes, An iontophoretic study
densities of b-adrenoceptors and muscarinic
cholinocep-of single somatosensory neurones in rt granular cortex serving the
tors, as occurs in the somatosensory cortex 2 weeks after limbs: a laminar analysis of glutamate and acetylcholine effects on peripheral deafferentation [13]. Findings in the present receptive-field properties, J. Neurophysiol. 60 (1988) 725–750.
deaf-ferented rat hindlimb granular cortex subsequent to transection of [25] T. Rothe, U.K. Hanisch, K. Krohn, R. Schliebs, W. Hartig, H.H. the sciatic nerve: effects of glutamate and acetylcholine, Brain Res. Webster, D. Biesold, Changes in choline acetyltransferase activity 449 (1988) 18–33. and high-affinity choline uptake, but not in acetylcholinesterase [18] J.R. Leonard, D.O. Maris, M.S. Grady, Fluid percussion injury activity and muscarinic cholinergic receptors, in rat somatosensory causes loss of forebrain choline acetyltransferase and nerve growth cortex after sciatic nerve injury, Somatosens. Motor Res. 7 (1990) factor receptor immunoreactive cells in the rat, J. Neurotrauma 11 435–446.
(1994) 379–392. [26] P.D. Suzdak, G. Gianutsos, GABA-noradrenergic interaction; evi-[19] P. Levitt, R.Y. Moore, Noradrenaline neuron innervation of the dence for differential sites of action for GABA-A and GABA-B
neocortex in the rat, Brain Res. 139 (1978) 219–231. receptors, J. Neural Transm. 64 (1985) 163–172.
[20] R. Metherate, N. Tremblay, R.W. Dykes, The effects of acetyl- [27] H.H. Webster, U.K. Hanisch, R.W. Dykes, D. Biesold, Basal choline on response properties of cat somatosensory cortical neu- forebrain lesions with or without reserpine injection inhibit cortical rons, J. Neurophysiol. 59 (1988) 1231–1252. reorganisation in rat hindpaw primary somatosensory cortex follow-[21] S. Nakamura, T. Sakaguchi, F. Aoki, Electrophysiological evidence ing sciatic nerve section, Somatosens. Motor Res. 8 (1991) 327–
for terminal sprouting of locus coeruleus neurons following repeated 346.
mild stress, Neurosci. Lett. 100 (1989) 147–152. [28] M. Zarei, J.D. Stephenson, Transhemispheric cortical reorganisation [22] M.C. Osterheld-Haas, H. Van der Loos, J.P. Hornung, Monoaminer- in rat SmI and involvement of central noradrenergic system, Brain
gic afferents to cortex modulate structural plasticity in the barrel Res. 870 (2000) 142–149.
field of the mouse, Brain Res. Dev. Brain Res. 77 (1994) 189–202. [29] M. Zarei, J.D. Stephenson, Ipsilateral and bilateral receptive fields in [23] G.M. Peterson, Sprouting of central noradrenergic fibers in the rat primary somatosensory cortex, NeuroReport 7 (1996) 647–651. dentate gyrus following combined lesions of its entorhinal and septal [30] K. Zilles, The Cortex of the Rat, A Stereotaxic Atlas, Springer-afferents, Hippocampus 4 (1994) 635–648. Verlag, 1985.