Parasite risk factors for stunting in grade 5 students
in a community of extreme poverty in Peru
Martin Casapı´a
a, Serene A. Joseph
b, Carmen Nu´n˜ez
a, Elham Rahme
b,c, Theresa W. Gyorkos
b,c,*
aAsociacio´n Civil Selva Amazo´nica, Iquitos, Peru
bDivision of Clinical Epidemiology, Research Institute of the McGill University Health Centre, Montre´al, Que´., Canada cDepartment of Epidemiology, Biostatistics and Occupational Health, McGill University, Montre´al, Que´., Canada
Received 6 December 2005; received in revised form 6 March 2006; accepted 21 March 2006
Abstract
Malnutrition in school-age children is common in developing countries and includes both stunting and underweight. Stunting, which represents a chronic state of nutritional stress, leads to adverse health, educational and cognitive effects. Although much research is focused on preschool-age children, recent studies show both the high prevalence of stunting and the effectiveness of interventions in school-age children. The objectives of the current study were to determine the risk factors for stunting only, and stunting and underweight. A survey was conducted in 1074 grade 5 children (mean age 10 years) from 17 schools in Belen, Peru, a community of extreme poverty. Prevalence of underweight and stunting were 10.5 and 34.5%, respectively, co-prevalence was 9.3%. Based on multivariable logistic regression analyses, significant independent risk factors (odds ratio: OR) for stunting and underweight were: age (per 1 year increment) (ORZ1.55; 95% confidence interval (CI): 1.33, 1.81); diarrhoea in the
last week (ORZ1.96; 95% CI: 1.17, 3.29) and hookworm infection (ORZ1.74; 95% CI: 1.05, 2.86). Significant independent risk factors for
stunting only were: age (per 1 year increment) (ORZ1.51; 95% CI: 1.35, 1.70); anaemia (ORZ1.98; 95% CI: 1.26, 3.11); and moderate and
heavyTrichurisandAscarisco-infection (ORZ1.95; 95% CI: 1.35, 2.82). Our results indicate a high prevalence of stunting, in addition to other
adverse health indicators, in the study population. Due to the interrelation between many of these health and nutrition problems, interventions at both the school and community levels, including de-worming, feeding programs and health and hygiene education, are needed to reduce malnutrition in this and other similar populations living in conditions of extreme poverty.
q2006 Australian Society for Parasitology Inc. Published by Elsevier Ltd. All rights reserved.
Keywords:Stunting;Ascaris;Trichuris; Hookworm; School-age children; Poverty
1. Introduction
Malnutrition in school-age children is common in develop-ing countries. Both stuntdevelop-ing (low height-for-age (HAZ)) and underweight (low weight-for-age (WAZ)), in comparison with healthy reference populations, can be used as indicators of malnutrition. Stunting, in particular, is thought to be a good indicator of malnutrition (WHO, 1986) and often represents a state of chronic nutritional stress. It can lead to both short-and long-term adverse events in childhood and adulthood, including effects on health, cognition and educational out-comes (Santos, 1994; Mendez and Adair, 1999; Partnership for
Child Development, 1999; Kossmann et al., 2000; Crompton and Nesheim, 2002). In addition, children of stunted stature are more likely to become stunted adults. For women, this can lead to low birth weight babies who will themselves be more likely to suffer from stunting. Therefore, the effects of malnutrition can be multigenerational.
Although the predictors of stunting are multifactorial, parasitic infections, notably helminth infections (e.g. Ascaris and Trichuris), can impair growth (Stephenson et al., 1993, 2000; Hadju et al., 1997; Stoltzfus et al., 1997; Beach et al., 1999; Hagel et al., 1999; O’Lorcain and Holland, 2000). This is especially relevant as both Ascaris and Trichuris infections have peak prevalences and intensities in children of school-age (Bundy et al., 1987a,b). While hookworm infection usually peaks in young adulthood, it may also be a factor in stunting in school-age children. Mechanisms for the effect of parasitic infections such as hookworm,AscarisandTrichurisin stunted www.elsevier.com/locate/ijpara
0020-7519/$30.00q2006 Australian Society for Parasitology Inc. Published by Elsevier Ltd. All rights reserved. doi:10.1016/j.ijpara.2006.03.004
* Corresponding author. Address: Division of Clinical Epidemiology, Montreal General Hospital, 1650 Cedar Avenue, Montre´al, Que´., Canada H3G 1A4. Tel.:C1 514 934 1934x44721; fax:C1 514 934 8293.
children include: (i) decreased appetite due to infection and (ii) decreased food intake (Stephenson et al., 1993). This can lead to a decreased growth rate, as well as decreased activity, fitness, and school performance. Intestinal parasites are also thought to deplete micronutrients (Levav et al., 1995). It has been suggested that nutrient malabsorption, micronutrient losses and increased metabolic requirements can result from chronic infections such as Ascaris (Stephenson, 1999). The blood loss that is associated withTrichurisinfection can lead to chronic dysentery, iron deficiency, iron deficiency anaemia and poor growth rate (Stephenson et al., 2000). Well nourished children who have better nutrient reserves are thought to be less vulnerable to the adverse effects of parasitic infections.
Previous studies have found parasitic infections, older age, gender, lower socio-economic status and other family characteristics to be associated with stunting or low HAZ in school-age children (Table 1). However, there have been some methodological limitations in previous research (e.g. small sample sizes and selection bias). Independent risk factors for stunting have not been examined in depth and very rarely have potential confounding variables been taken into account. Many studies have examined children of a wide age-range and have been unable to distinguish different risk factors for stunting for
specific age groups. Therefore, research into significant independent risk factors for stunting in endemic areas is needed in school-age children. The objective of this study was to evaluate the importance of helminth infections on stunting in a population of grade 5 students living in a community of extreme poverty in an endemic area of Peru.
2. Materials and methods
2.1. Study population
Belen is located in the rural and peri-urban surroundings of Iquitos, the capital city of the region of Loreto, in the Peruvian Amazon. Because Belen is situated on the banks of the Rio Itaya, whose seasonal floods can change the water level by as much as 30 feet, its simple wooden houses are built on stilts. The availability of potable water is erratic and it has no sewer system. Human waste contaminates the river water directly from floating latrines or is left exposed from primitive land-based latrines to be removed by natural elements. Belen is considered to be a community of extreme poverty.
A list of all primary schools in Belen was obtained from the Ministerio de Educacio´n in Iquitos, Peru for the 2004 school
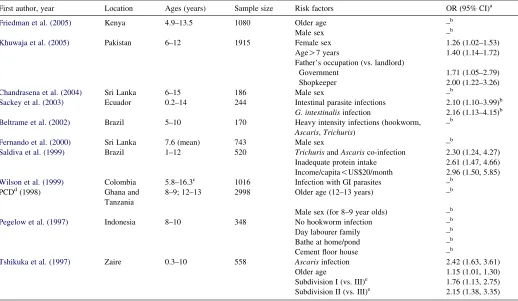
Table 1
Previous cross-sectional studies in developing countries on risk factors for stunting in school-age children
First author, year Location Ages (years) Sample size Risk factors OR (95% CI)a
Friedman et al. (2005) Kenya 4.9–13.5 1080 Older age –b
Male sex –b
Khuwaja et al. (2005) Pakistan 6–12 1915 Female sex 1.26 (1.02–1.53)
AgeO7 years 1.40 (1.14–1.72)
Father’s occupation (vs. landlord)
Government 1.71 (1.05–2.79)
Shopkeeper 2.00 (1.22–3.26)
Chandrasena et al. (2004) Sri Lanka 6–15 186 Male sex –b
Sackey et al. (2003) Ecuador 0.2–14 244 Intestinal parasite infections 2.10 (1.10–3.99)b G. intestinalisinfection 2.16 (1.13–4.15)b Beltrame et al. (2002) Brazil 5–10 170 Heavy intensity infections (hookworm,
Ascaris,Trichuris)
–b
Fernando et al. (2000) Sri Lanka 7.6 (mean) 743 Male sex –b
Saldiva et al. (1999) Brazil 1–12 520 TrichurisandAscarisco-infection 2.30 (1.24, 4.27) Inadequate protein intake 2.61 (1.47, 4.66) Income/capita!US$20/month 2.96 (1.50, 5.85) Wilson et al. (1999) Colombia 5.8–16.3c 1016 Infection with GI parasites –b
PCDd(1998) Ghana and
Tanzania
8–9; 12–13 2998 Older age (12–13 years) –b
Male sex (for 8–9 year olds) –b Pegelow et al. (1997) Indonesia 8–10 348 No hookworm infection –b
Day labourer family –b
Bathe at home/pond –b
Cement floor house –b
Tshikuka et al. (1997) Zaire 0.3–10 558 Ascarisinfection 2.42 (1.63, 3.61)
Older age 1.15 (1.01, 1.30)
Subdivision I (vs. III)e 1.76 (1.13, 2.75) Subdivision II (vs. III)e 2.15 (1.38, 3.35)
a Reported adjusted odds ratio (OR) (Khuwaja et al., 2005; Tshikuka et al., 1997) controlled for the variables reported as significant in the final model of each
study, respectively,Saldiva et al. (1999)controlled for the variables reported as significant in the final model of the study, as well as caloric intake,Giardia lamblia infection, and father’s characteristics (employment, schooling, harvested land and livestock). CI-confidence interval.
b Variables reported as significant (P!0.05) but ORs either not reported or univariate. c Study conducted only in male children.
d PCD, Partnership for Child Development.
year. Schools with an enrolment of at least 10 girls and 10 boys in grade 5 in the 2004 school year were eligible to participate. Information on the study was provided to the principal of each eligible school, who then provided consent for the school. Inclusion criteria for children were: (i) enrolment in grade 5 in an eligible school, (ii) parental informed consent and (iii) child assent. The study was conducted between April and June 2005.
2.2. Information sessions
One information session at each school was coordinated with help from the principal and/or representative to inform parents of grade 5 students about the study and to obtain parental informed consent. On the day of the information session, the project coordinator explained the purpose and procedures of the project and answered any parent or teacher questions. Parents were then asked to sign an informed consent form. Parents who were unable to attend the information session were contacted by the school and informed about the project to obtain informed consent. In larger schools or schools with a high number of absent parents at the first session, a second session was arranged.
2.3. Questionnaires
A questionnaire for collecting information on each school’s characteristics was adapted from the School Form included in guidelines published by the Word Health Organisation (WHO, 1998, 2002). Questionnaires were originally written in English and translated into Spanish.
2.4. School survey and data collection
The project team arranged a survey day at each school once all parents had been informed of the study and the majority of informed consent forms had been received. Child assent was obtained on the day of the survey. Surveys were conducted in the morning at each school. Information that was obtained from the students on the survey day included: socio-demographic information, height (cm), weight (lbs), finger-prick blood for haemoglobin testing and thick and thin malaria smears and one stool sample. Haemoglobin levels were determined using Hemocuew
and stool samples were analysed by the Kato–Katz method for presence and intensity of helminth parasites by enumeration and identification of eggs that were characteristic for each parasite type. Presence of either hookworm species (Ancylostoma duodenale or Necator americanus) was con-sidered a positive result for hookworm infection. Giemsa stained thick and thin smears were examined for malaria positivity. WHO intensity levels were used to classify infections into light, moderate and heavy categories; anaemia was defined as a haemoglobin level!11 g/dL (WHO, 1998).
2.5. Data analysis
Means (continuous) and frequency (categorical) procedures were used to describe the characteristics of the study
population. Stunting and underweight were determined using EpiInfoe 2005 software (Version 3.3.2) and was defined as !K2 standard deviations from height-for-age of National Center for Health Statistics/WHO reference population. Height was entered in centimetres (cm) and weight in pounds. For comparability purposes, weight was converted to kilograms (kg) for all other analyses. Potential risk factors for stunting only and stunting and underweight were determined using a reference group of those that were neither underweight nor stunted. Only 13 individuals were underweight and were excluded from analyses. Risk factors were examined in a univariate logistic regression model and those variables that had aPvalue of 0.10 or lower were included in further model building. Variables remained in the final multivariate model if they had a P value of 0.05 or lower (SAS version 9.0, SAS Institute Inc., Cary, NC, USA).
2.6. Ethics approval
Ethics approval was obtained from the Research Ethics Board of the Research Institute of the McGill University Health Centre in Montreal, Canada, and the Ministerio de Salud in Iquitos, Peru.
3. Results
3.1. Study population
A total of 18 schools were identified from the 2004 listing provided by the Ministerio de Educacio´n that met the inclusion criteria. All schools were contacted and permission was obtained from each principal for the participation of the school. One school was subsequently excluded because of difficult access. Of the 17 selected schools, a total of 1421 grade 5 students were eligible to participate in the study. We received informed consent from 1145 parents and students (participation rateZ80.6%). Reasons for non-participation included not attending information sessions and unwillingness of parents to have their child participate. Of the students with informed consent, 71 were unable to provide a stool sample. The final total population of students who were able to provide complete information (questionnaire, stool sample, finger-prick blood) was 1074 from 17 schools.
3.2. Characteristics of the study population
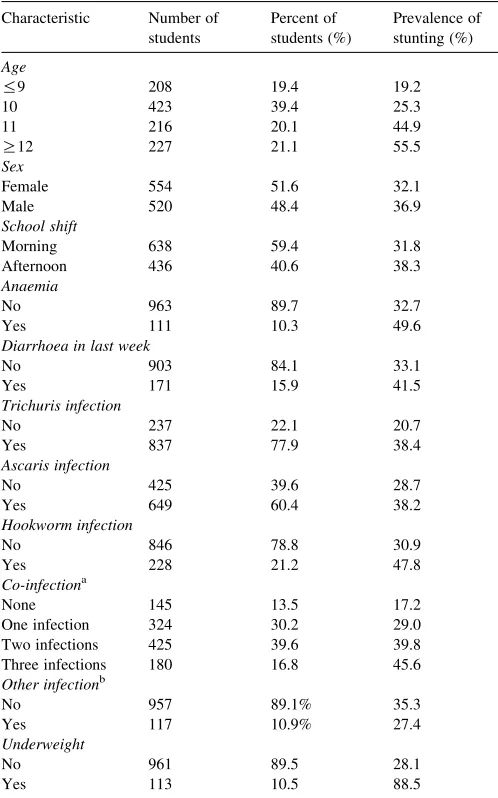
10.9% of students. Based on smears, no students were infected with malaria. Self-reported diarrhoea in the last week was present in 15.9% of students, of which 11.7% reported associated blood. Prevalence of anaemia (Hb!11 g/dL) was 10.3%. No students had severe anaemia (Hb!7 g/dL). Prevalence of underweight and stunting was 10.5 and 34.5%, respectively. Co-occurrence of stunting and underweight was 9.3%. Table 2shows the frequency of stunting according to selected population characteristics. Although information on school characteristics was gathered, these characteristics were too highly correlated with one another and appeared to mask the relationship of individual characteristics; therefore, they were not included in model building.
3.3. Risk factors for stunting and underweight
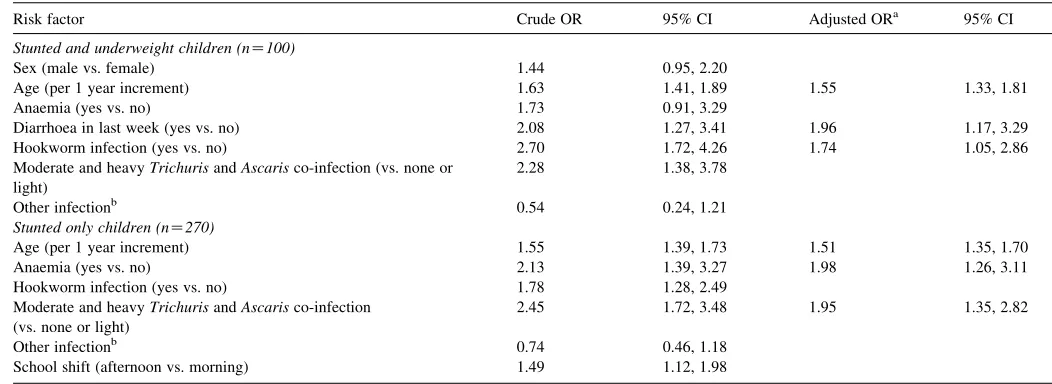
Statistically significant independent risk factors for stunting only were: co-infection with moderate and heavy intensity Trichuris and Ascaris infections (ORZ1.95; 95% CI: 1.35, 2.82); increasing age (ORZ1.51; 95% CI: 1.35, 1.70); having anaemia (ORZ1.98; 95% CI: 1.26, 3.11). Statistically significant independent risk factors for stunting and under-weight were: increasing age (ORZ1.55; 95% CI: 1.33, 1.81); diarrhoea in the last week (ORZ1.96; 95% CI: 1.17, 3.29); and hookworm infection (ORZ1.74; 95% CI: 1.05, 2.86) (Table 3).
4. Discussion
The study population of school-age children in Peru show high and moderate prevalences of stunting and underweight, respectively, (WHO, 1997). In addition, other adverse health indicators such as intestinal helminth infections and anaemia were high in this population.
Although stunting is thought to be the result of chronic undernutrition originating in infancy, our results suggest that the risk of stunting continues to grow with increasing age. This corroborates previous cross-sectional studies in school-age children (Parraga et al., 1996; Tshikuka et al., 1997; Partner-ship for Child Development, 1998; Ordo´n˜ez and Angulo, 2002; Khuwaja et al., 2005). Longitudinal studies have also demonstrated that stunted children continue to deviate from international growth standards as they get older (Friedman et al., 2005). Although this relationship has been demonstrated in the literature, it is also possible that the older children have increased risk of stunting because they represent a cohort of children with delayed enrolment or who have repeated grades. Therefore, this group of children could represent those already at risk for stunting due to low socio-economic status and other adverse health and social conditions. Although the literature generally demonstrates the importance of targeting infants and preschool-age children for stunting (Crompton and Nesheim, 2002), these results highlight the importance of targeting school-age children who continue to be at-risk for stunting and malnutrition.
Stunting was also found to be related to moderate and heavy TrichurisandAscarisco-infection. This finding is consistent with previous studies, which have foundTrichuris,Ascarisor co-infection with both to be associated with stunting and malnutrition (Watkins and Pollitt, 1996; Tshikuka et al., 1997; Oberhelman et al., 1998; Saldiva et al., 1999; Beltrame et al., 2002; Quihui-Cota et al., 2004). Our study, however, appears to be one of only a few that examined intensity of parasitic infection (Beltrame et al., 2002). It is possible that although current infection was found to be a risk factor for a chronic condition, current infection parallels infection throughout the earlier lifespan and can therefore be used as a proxy measurement for past infection. It has been demonstrated that individuals are predisposed to certain intensities of infection such that even after treatment, intensity of infection returns to pre-treatment levels (Crompton and Nesheim, 2002).
Table 2
Characteristics and prevalence of stunting of 1074 grade 5 students from Belen, Peru, April–June 2005
One infection 324 30.2 29.0
Two infections 425 39.6 39.8
Three infections 180 16.8 45.6
Other infectionb
a One infection, infection with eitherAscaris,Trichurisor hookworm; two
infections, infection withAscarisandTrichuris,Ascarisand hookworm, or Trichurisand hookworm; three infections, infection withAscaris,Trichurisand hookworm.
b Other infection, infection with Hymenolepis nana or Enterobius
Anaemia was also found to be associated with stunting, independently of intestinal helminth infection. This may represent the indirect relationship between anaemia from poor nutrient intake and stunting. Anaemia may also lead directly to stunting. We believe this is the first study to demonstrate this association and future studies, especially regarding causative mechanisms, will be needed to further examine this relationship.
In contrast to stunting only, risk factors for stunting and underweight may be predictors of current and non-current malnutrition. This is due to the fact that underweight can be an indicator of both acute and chronic malnutrition. Age was found to be a risk factor for stunting and underweight, which once again demonstrates the importance of targeting school-age children, especially those that may be at a higher risk of malnutrition.
Having had diarrhoea in the last week was also a risk factor for underweight and stunting. This appears to be an indication of current malnutrition due to the recent timeframe of the question asked. However, this risk factor may also identify individuals that are prone to chronic diarrhoea and overall adverse health effects. Hookworm may also represent a more recent indicator of infection as this is not very common in young children and has peak prevalence in the late teens and early adulthood (Bundy, 1990).
As no other studies were identified that examined risk factors for school-age children who were both stunted and underweight, we are unable to compare these results. However, our overall results demonstrate a high prevalence of malnutrition in the study population, both in terms of stunting and underweight, as well as adverse health effects in terms of intestinal helminth infections, anaemia and diarrhoea, all of which are interrelated conditions. Therefore, potential inter-ventions at the school and community level need to be
developed and evaluated. Potential interventions include mass de-worming, feeding programs and health and hygiene education for both children and teachers (del Rosso and Marek, 1996). Early prevention is also needed in the newborn to preschool-age groups through prenatal care and other family and community based programs. It is important to focus on maternal health to decrease the prevalence of low birth-weight babies who are more likely to suffer from long-term stunting and underweight. In addition to specific interventions for malnutrition in children, it is important to target underlying health and social problems in the community such as female literacy, immunisation rates and income distribution, to ensure a long-term reduction in stunting (Milman et al., 2005).
The strengths of this study include a large sample size of school children, the selection of children from a large number of schools and representative area of Belen, a high participation rate of children, and in-depth multivariable analyses to take into account potential confounding variables such as age, gender and infection. Limitations of this study include the exclusion of individuals who did not have parental informed consent, and those who were not able to provide a stool sample. Individuals in both of these categories may be at an increased risk of stunting and overall poor health indicators and socio-economic status. The prevalence of stunting and underweight was higher (59.2 and 21.1%, respectively), in those who were unable to provide a stool sample. Additionally, we did not have in-depth socio-economic information or other family infor-mation from the children, which have been found to be associated with malnutrition (e.g. maternal education, father’s occupation, socio-economic status) (Watkins and Pollitt, 1996; Pegelow et al., 1997; Tshikuka et al., 1997; Saldiva et al., 1999; Ordo´n˜ez and Angulo, 2002; de Onis, 2003; Khuwaja et al., 2005). Lastly, the study design was that of a cross-sectional survey, therefore, a direct cause-and-effect relationship Table 3
Risk factors for stunting, and for stunting and underweight, in 1074 grade 5 schoolchildren living in Belen, Peru, April–June 2005
Risk factor Crude OR 95% CI Adjusted ORa 95% CI
Stunted and underweight children (nZ100)
Sex (male vs. female) 1.44 0.95, 2.20
Age (per 1 year increment) 1.63 1.41, 1.89 1.55 1.33, 1.81
Anaemia (yes vs. no) 1.73 0.91, 3.29
Diarrhoea in last week (yes vs. no) 2.08 1.27, 3.41 1.96 1.17, 3.29
Hookworm infection (yes vs. no) 2.70 1.72, 4.26 1.74 1.05, 2.86
Moderate and heavyTrichurisandAscarisco-infection (vs. none or light)
2.28 1.38, 3.78
Other infectionb 0.54 0.24, 1.21
Stunted only children (nZ270)
Age (per 1 year increment) 1.55 1.39, 1.73 1.51 1.35, 1.70
Anaemia (yes vs. no) 2.13 1.39, 3.27 1.98 1.26, 3.11
Hookworm infection (yes vs. no) 1.78 1.28, 2.49
Moderate and heavyTrichurisandAscarisco-infection (vs. none or light)
2.45 1.72, 3.48 1.95 1.35, 2.82
Other infectionb 0.74 0.46, 1.18
School shift (afternoon vs. morning) 1.49 1.12, 1.98
Thirteen children who were underweight but not stunted were excluded from analyses; schoolchildren who were neither stunted nor underweight (nZ691) were the
reference group. OR, odds ratio; CI, confidence interval.
a Multivariate model is controlled for sex.
between the risk factors and the outcome cannot be determined and reverse causality (i.e. malnutrition leading to increased susceptibility to infection) cannot be discounted. However, it is possible that the current health status of individuals are proxy measurements for chronic health indicators in a community such as this of extreme poverty. Therefore, the results of this study highly suggest that older age, intestinal helminth infections, anaemia and diarrhoea are risk factors for malnutrition in the school-age population. These results can have an important impact on improving health outcomes by providing targets for health promotion activities. Future research can provide additional insight on other determinants of stunting by examining the role of confounders not included in the present study, such as socio-economic status.
Acknowledgements
We are grateful for the support of the Ministerio de Educacio´n de Loreto, Peru, the participating school principals, the teacher-liaison for health and all Grade 5 students and their parents. Input into the survey was provided by all participants at the April 2005 workshop (Taller Participativo 1: Un Enfoque Multidisciplinario para Reducir la Pobreza Extrema) in Iquitos, Peru. The expert laboratory and field support of the Proyecto Canada´-Peru´-Bele´n is also gratefully recognised. The Cana-dian Institutes of Health Research (CIHR) (grant number GLP 67580) funded this research.
References
Beach, M.J., Streit, T.G., Addiss, D.G., Prospere, R., Roberts, J.M., Lammie, P.J., 1999. Assessment of combined ivermectin and albendazole for treatment of intestinal helminth andWuchereria bancroftiinfections in Haitian schoolchildren. Am. J. Trop. Med. Hyg. 60, 479–486.
Beltrame, A., Scolari, C., Torti, C., Urbani, C., 2002. Soil transmitted helminth (STH) infections in an indigenous community in Ortigueira, Parana, Brazil and relationship with its nutritional status. Parassitologia 44, 137–139.
Bundy, D.A.P., 1990. Is the hookworm just another geohelminth? In: Schad, G.A., Warren, K.S. (Eds.), Hookworm disease: current status and new directions. Taylor and Francis, London, pp. 147–164.
Bundy, D.A.P., Cooper, E.S., Thompson, D.E., Anderson, R.M., Didier, J.M., 1987a. Age-related prevalence and intensity of Trichuris trichiura infection in a St Lucian community. Trans. R. Soc. Trop. Med. Hyg. 81, 85–94.
Bundy, D.A.P., Cooper, E.S., Thompson, D.E., Didier, J.M., Simmons, I., 1987b. Epidemiology and population dynamics ofAscaris lumbricoides andTrichuris trichiurainfection in the same community. Trans. R. Soc. Trop. Med. Hyg. 81, 987–993.
Chandrasena, T.G.A.N., de Alwis, A.C.D., de Silva, L.D.R., Morel, R.P., de Silva, N.R., 2004. Intestinal parasitoses and the nutritional status of veddah children in Sri Lanka. Southeast Asian J. Trop. Med. Public Health 35, 255–259.
Crompton, D.W.T., Nesheim, M.C., 2002. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu. Rev. Nutr. 22, 35–59. de Onis, M., 2003. Socioeconomic inequalities and child growth. Int.
J. Epidemiol. 32, 503–505.
del Rosso, J.M., Marek, T., 1996. Class action: improving school performance in the developing world through better health and nutrition. The World Bank, Washington.
Fernando, S.D., Paranavitane, S.R., Rajakaruna, J., Weerasinghe, S., De Silva, D., Wickremasinghe, A.R., 2000. The health and nutritional status of
school children in two rural communities in Sri Lanka. Trop. Med. Int. Health 5, 450–452.
Friedman, J.F., Phillips-Howard, P.A., Mirel, L.B., Terlouw, D.J., Okello, N., Vulule, J.M., Hawley, W.A., Nahlen, B.L., Kuile, F., 2005. Progression of stunting and its predictors among school-aged children in western Kenya. Eur. J. Clin. Nutr. doi.101038/sjejcn1602161
Hadju, V., Satriono, Abadi, K., Stephenson, L.S., 1997. Relationships between soil-transmitted helminthiases and growth in urban slum schoolchildren in Ujung Pandang, Indonesia. Int. J. Food Sci. Nutr. 48, 85–93.
Hagel, I., Lynch, N.R., Di Prisco, M.C., Perez, M., Sanchez, J.E., Pereyra, B.N., Sanabria, I.S., 1999. Helminthic infection and anthropometric indicators in children from a tropical slum: Ascaris reinfection after anthelminthic treatment. J. Trop. Pediatr. 45, 215–220.
Khuwaja, S., Selwyn, B.J., Shah, S.M., 2005. Prevalence and correlates of stunting among primary school children in rural areas of Southern Pakistan. J. Trop. Pediatr. 51, 72–77.
Kossmann, J., Nestel, P., Herrera, M.G., El Amin, A., Fawzi, W.W., 2000. Undernutrition in relation to childhood infections: a prospective study in the Sudan. Eur. J. Clin. Nutr. 54, 463–472.
Levav, M., Mirsky, A.F., Schantz, P.M., Castro, S., Cruz, M.E., 1995. Parasitic infection in malnourished school children: effects on behaviour and EEG. Parasitology 110, 103–111.
Mendez, M.A., Adair, L.S., 1999. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J. Nutr. 129, 1555–1562.
Milman, A., Frongillo, E.A., de Onis, M., Hwang, J.Y., 2005. Differential improvement among countries in child stunting is associated with long-term development and specific interventions. J. Nutr. 135, 1415–1422.
O’Lorcain, P.O., Holland, C.V., 2000. The public health importance ofAscaris lumbricoides. Parasitology 121, S51–S71.
Oberhelman, R.A., Guerrero, E.S., Fernandez, M.L., Silio, M., Mercado, D., Comiskey, N., Ihenacho, G., Mera, R., 1998. Correlations between intestinal parasitosis, physical growth, and psychomotor development among infants and children from rural Nicaragua. Am. J. Trop. Med. Hyg. 58, 470–475.
Ordo´n˜ez, L.E., Angulo, E.S., 2002. Desnutricio´n y su relacio´n con parasitismo intestinal en nin˜os de una poblacio´n de la Amazonia Colombiana. Biome´dica 22, 486–498.
Parraga, I.M., Assis, A.M., Prado, M.S., Barreto, M.L., Reis, M.G., King, C.H., Blanton, R.E., 1996. Gender differences in growth of school-aged children with schistosomiasis and geohelminth infection. Am. J. Trop. Med. Hyg. 55, 150–156.
Partnership for Child Development, 1998. The health and nutritional status of schoolchildren in Africa: evidence from school-based health programmes in Ghana and Tanzania. Trans. R. Soc. Trop. Med. Hyg. 92, 254–261. Partnership for Child Development, 1999. Short stature and the age of
enrolment in Primary School studies in two African countries. Soc. Sci. Med. 48, 675–682.
Pegelow, K., Gross, R., Pietrzik, K., Lukito, W., Richards, A.L., Fryauff, D.J., 1997. Parasitological and nutritional situation of school children in the Sukaraja District, West Java, Indonesia. Southeast Asian J. Trop. Med. Public Health 28, 173–190.
Quihui-Cota, L., Valencia, M.E., Crompton, D.W.T., Phillips, S., Hagan, P., Diaz-Camacho, S.P., Tejas, A.T., 2004. Prevalence and intensity of intestinal parasitic infections in relation to nutritional status in Mexican schoolchildren. Trans. R. Soc. Trop. Med. Hyg. 98, 653–659.
Sackey, M.E., Weigel, M.W., Armijos, R.X., 2003. Predictors and nutritional consequences of intestinal parasitic infections in rural Ecuadorian children. J. Trop. Pediatr. 49, 17–23.
Saldiva, S.R., Silveira, A.S., Philippi, S.T., Torres, D.M., Mangini, A.C., Dias, R.M.S., da Silva, R.M., Buratini, M.N., Massad, E., 1999. Ascaris-Trichuris association and malnutrition in Brazilian children. Paediatr. Perinat. Epidemiol. 13, 89–98.
Santos, J.I., 1994. Nutrition, infection, and immunocompetence. Infect. Dis. Clin. N. Am. 8, 243–267.
Stephenson, L.S., Latham, M.C., Adams, E.J., Kinoti, S.N., Pertet, A., 1993. Physical fitness, growth and appetite of Kenyan school boys with hookworm,Trichuris trichiuraand Ascaris lumbricoides infections are improved four months after a single dose of albendazole. J. Nutr. 123, 1036–1046.
Stephenson, L.S., Holland, C.V., Cooper, E.S., 2000. The public health significance ofTrichuris trichiura. Parasitology 121, S73–S95.
Stoltzfus, R.J., Albonico, M., Tielsh, J.M., Chwaya, H.M., Savioli, L., 1997. Linear growth retardation in Zanzibari school children. J. Nutr. 127, 1099–1105.
Tshikuka, J.G., Gray-Donald, K., Scott, M., Olela, K.N., 1997. Relationship of childhood protein-energy malnutrition and parasite infections in an urban African setting. Trop. Med. Int. Health 2, 374–382.
Watkins, W.E., Pollitt, E., 1996. Effect of removingAscarison the growth of Guatemalan schoolchildren. Pediatrics 97, 871–876.
Wilson, W.M., Dufour, D.L., Staten, L.K., Barac-Nieto, M., Reina, J.C., Spurr, G.B., 1999. Gastrointestinal parasitic infection, anthropometrics, nutritional status and physical work capacity in Colombian boys. Am. J. Hum. Biol. 11, 763–771.
World Health Organization, 1986. Use and interpretation of anthropometric indicators of nutritional status. Bull. World Health Organ. 64, 929–941. World Health Organization. Global Database on Child Growth and
Malnutrition. Geneva: World Health Organization, 1997. WHO/NUT/97.4. World Health Organization. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level. A guide for managers of control programmes. Geneva: World Health Organization, 1998. WHO/CTD/SIP/98.1.