Summary Ecotypic variations in leaf conductance, soil-to-leaf hydraulic conductance, components of tissue water poten-tial, hydraulic architecture parameters and xylem embolism were examined in greenhouse-grown two-year-old Aleppo pine (Pinus halepensis Mill.) seedlings from six origins repre-senting the geographic range of the species in Italy. Cortical resin composition of the seedlings was also determined. Meas-urements were made on well-watered seedlings and on seed-lings subjected to recurring severe drought. Drought-stressed seedlings had lower mean leaf conductances, transpiration rates and soil-to-leaf hydraulic conductances than well-wa-tered seedlings. They also exhibited more negative osmotic potentials, higher relative water deficit at incipient plasmolysis, but a similar maximum modulus of elasticity. Drought-stressed seedlings showed a higher degree of xylem embolism, a lower Huber value, lower leaf specific conductivity and lower spe-cific conductivity than well-watered seedlings.
Drought-stressed seedlings of provenances from more xeric habitats (Tremiti, Porto Pino and Mottola) had greater leaf conductances, transpiration rates and soil-to-leaf hydraulic conductances than drought-stressed seedlings of provenances from more mesic habitats (Imperia, Otricoli and Vico del Gargano). They also showed higher osmotic adjustment and a lower degree of xylem embolism. Among provenances, there were no significant differences in hydraulic architecture pa-rameters in response to the drought treatment; however, Tre-miti and Porto Pino seedlings displayed smaller drought-induced reductions in specific conductivity and leaf specific conductivity, respectively, than seedlings from other provenances. These differences suggest that seedlings from xeric provenances, especially Tremiti, have greater resistance to desiccation than seedlings from mesic provenances. No clear association was found between terpene variability and the other traits investigated, although terpene composition was related to the geographical distribution of the provenances. We conclude that the drought-tolerance responses of Tremiti make it a more suitable provenance than the others for establishment on sites prone to severe soil water deficits.
Keywords: drought, ecotypic variation, embolism, monoter-pene.
Introduction
Aleppo pine (Pinus halepensis Mill.) grows in parts of south-ern Europe, Asia Minor and northsouth-ern Africa, adjacent to the Mediterranean Sea (Mirov 1967). The native range of Aleppo pine includes a wide range of forest sites throughout the Mediterranean, especially dry habitats (Nahal 1981). Aleppo pine populations are often disjunct, resulting in prolonged isolation and complex geographic patterns of genetic variation (Schiller et al. 1986).
The species is selected for afforestation because of its high drought tolerance. The major components of drought resis-tance, including stomatal response to drought, soil-to-leaf hy-draulic conductance, osmotic potential, relative water deficit at incipient plasmolysis, maximum modulus of elasticity, loss of hydraulic conductivity and hydraulic architecture, are known to vary among tree species (Abrams 1988, Braatne et al. 1992, Cochard 1992, Wang et al. 1992, Jackson et al. 1995). How-ever, there are few data on intraspecific variation in the major components of drought resistance in woody species (Bon-garten and Teskey 1986, Abrams et al. 1990, Shumway et al. 1991, Borghetti et al. 1993), although variation in stem and leaf morphology, seed physiology, phenological stages and growth have been reported among Aleppo pine populations raised from different seed sources (Eccher et al. 1982, Falusi et al. 1983, Calamassi 1986, Weinstein 1989, Schiller and Brunori 1992). We examined several aspects of drought resistance in Aleppo pine seedlings from six provenances representing the spectrum of soil water conditions in Italy. Specifically, we investigated variability in the major components of drought resistance in well-watered seedlings and in seedlings precon-ditioned with recurring drought.
Adaption of species to geographic variation in the environ-ment often depends on genetic variation among seed sources. For example, in loblolly pine, genetic variation is closely related with the habitat from which plants originate (Bon-garten and Teskey 1986). In the present study, we used terpenes as biochemical markers of genetic variability among prove-nances to determine if variation in the major components of drought resistance is associated with genetic variation. Ter-penes have been widely used in chemosystematic studies to characterize species, determine provenances and identify
Geographical variation in water relations, hydraulic architecture and
terpene composition of Aleppo pine seedlings from Italian provenances
ROBERTO TOGNETTI, MARCO MICHELOZZI and ALESSIO GIOVANNELLI
Istituto Miglioramento Genetico delle Piante Forestali, Consiglio Nazionale delle Ricerche, via Atto Vannucci 13, I 50134, Firenze, Italy
Received February 8, 1996
clones, because they are strongly inherited and little influenced by environmental factors (Baradat and Yazdani 1988, Hanover 1992, Adams et al. 1993, Lang 1994, Baradat et al. 1996). Terpenes also offer the opportunity to select trees for resistance to pests or diseases because they are involved in the defense mechanisms of plants (Michelozzi et al. 1990, Stone and Bacon 1994, Michelozzi et al. 1995, Raffa and Smalley 1995).
Materials and methods
Plant material and growth conditions
The experiment was conducted on two-year-old seedlings of Aleppo pine from six geographical origins (Table 1) that were chosen to represent the natural geographic range of this species in Italy (Figure 1). In 1991, seeds were collected from mature trees in natural stands of each provenance (at least 30 trees per stand were sampled) and sown in a forest nursery at Pisa, Italy, in March 1992. The following year, seedlings were moved to the experimental nursery of the Institute of Silviculture, Uni-versity of Florence, Italy, and transplanted to 2.5-liter plastic pots filled with a mixture of sandy-loam soil and peat. The seedlings were watered daily and fertilized at the beginning of the growing season with Osmocote, a commercial slow-release fertilizer (18,18,18, N,P,K).
In October 1993, 100 plants (total of all provenances) were selected for dimensional uniformity (average height and root collar diameter were 32.25 ± 5.19 cm (SE) and 0.51 ± 0.11 cm, respectively) and moved to a greenhouse. The day/night tem-perature in the greenhouse was maintained at 27--30/22--25 °C, but relative humidity and photoperiod were not regulated. The seedlings were watered every other day, but no fertilizer was applied in order to simulate the nutrient-poor soil conditions of Aleppo pine natural forests. After a 5-month acclimation period in the greenhouse, the seedlings from each seed source were divided into two groups and the treatments applied. One group was watered every other day (well-watered seedlings), whereas the other group was exposed to recurring cycles of drought by watering only when predawn water po-tentials reached about −3 MPa (drought-stressed seedlings). The drought-stressed seedlings had completed six drought cycles (corresponding roughly to the dry season in the
Medi-terranean climate where Aleppo pine grows) when the meas-urements were made in June 1994.
Transpiration, leaf conductance and soil-to-leaf hydraulic conductance
Transpiration (E) and leaf conductance (gs) were measured under steady-state conditions (high soil water content and equal evaporative demands). Before measurements, each seed-ling was watered and placed in the dark for 12 h (until 0800 h). Eight seedlings from each seed source × drought treatment combination were randomly sampled. Because photosyntheti-cally active radiation reached a maximum of about 1300 µmol m−2 s−1 (midday, 1100--1400 h), at least two shoots (upper canopy) were sampled on each seedling at a time with a steady-state porometer and a conifer foliage cuvette (LI-1600, Li-Cor Inc., Lincoln, NE). Precautions were taken to avoid large differences between external environmental conditions and those inside the cuvette. Leaf conductance and transpira-tion were expressed in molar units, taking into account tem-perature and atmospheric pressure changes due to altitude. We
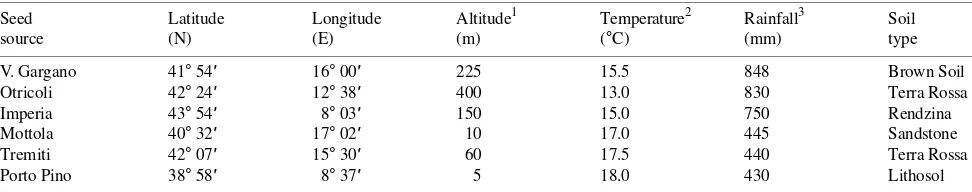
Table 1. Geographical origin and habitat characteristics of the studied Aleppo pine provenances listed in order of the amount of rainfall. Seed Latitude Longitude Altitude1 Temperature2 Rainfall3 Soil
source (N) (E) (m) (°C) (mm) type
V. Gargano 41° 54′ 16° 00′ 225 15.5 848 Brown Soil Otricoli 42° 24′ 12° 38′ 400 13.0 830 Terra Rossa Imperia 43° 54′ 8° 03′ 150 15.0 750 Rendzina Mottola 40° 32′ 17° 02′ 10 17.0 445 Sandstone Tremiti 42° 07′ 15° 30′ 60 17.5 440 Terra Rossa Porto Pino 38° 58′ 8° 37′ 5 18.0 430 Lithosol
1 Above sea level. 2 Mean annual temperature. 3 Total annual rainfall.
determined all-sided leaf area as h (2πr + 6r) (Bongarten and Teskey 1986) for two-needle fascicles, where h = needle length and r = average fascicle radius.
For each seedling, soil-to-leaf hydraulic conductance (gL) was estimed as Ohm’s law analogy (assuming predawn water potential is an estimate of the soil potential) by means of the following equation (Koide et al. 1989):
gL=E/(Ψp−Ψx), (1)
where Ψp = shoot xylem predawn water potential and Ψx = minimum shoot xylem water potential, both measured with a pressure chamber (PMS Instruments Co., Corvallis, OR). Minimum shoot xylem water potential was measured as E reached its maximum at about midday.
Pressure--volume curves
Components of tissue water potential were measured by the drying method (Talbot et al. 1975). The apical shoots were severed from the seedlings under water and allowed to resatu-rate in a covered container in distilled water for approximately 12 h in the dark. Before taking measurements, the excised shoots were recut under water above the level to which they had been submerged during the rehydration period. Then, the shoots were weighed and immediately placed in a pressure chamber to determine their initial xylem water potentials. The shoots were left to desiccate by free transpiration on the labo-ratory bench at room temperature. The shoots were then peri-odically reweighed and measured for xylem water potential as they dried until xylem water potentials reached −5 MPa, well below the turgor loss point. For each xylem water potential, two fresh weight measurements were obtained, one immedi-ately preceding and one immediimmedi-ately following the xylem water potential determination, and averaged. Shoot dry weights were subsequently determined after drying for 48 h at 70 °C. Pressure--volume data were analyzed as described by Schulte and Hinckley (1985). Pressure--volume curves defined the osmotic potential at saturation (Ψπo), the osmotic potential at incipient plasmolysis (Ψπp), the relative water deficit at incipient plasmolysis (RWDp) and the maximum modulus of elasticity (εmax ).
Hydraulic architecture
Hydraulic conductivity was measured before and after remov-ing air embolisms as described by Sperry et al. (1988). After steady-state treatment, the stem of each seedling was cut under water and then, while still submerged, recut to avoid further embolism. Both ends of the stem section (5--10 cm long) were fitted with rubber gaskets and trimmed in distilled water. The perfusing solution was 10 mM oxalic acid in distilled water (pH 1.8) to minimize microbial occlusions of xylem vessels. The perfusing solution was degassed by agitating under vac-uum for 60 min, and then introduced into an air-free plastic ball enclosed in a compressed gas tank. The solution, passing through a 0.2 µm inline filter (POLYCAP-36AS, Arbor Tech-nologies Inc., Ann Arbor, MI), perfused the samples under a constant pressure gradient maintained by a pressure regulator.
Stopcocks allowed selective influx for measuring the initial conductivity (Ki) on one sample at a time under a pressure gradient of 9--10 kPa. Maximum conductivity (Km) was deter-mined by pressurizing the solution through all the segments at 150 kPa for 60--90 min. The permeating solution flowed from the pressurizing reservoir across the samples to a container on an analytical balance linked to a computer that was pro-grammed to make the calculations. Measurements of hydraulic conductivity were recorded every 30 s and calculated by aver-aging ten readings after steady-state had been achieved.
Embolism causing per cent loss of hydraulic conductivity (Kl) was assessed from:
Kl= 100(Km−Ki)/Km. (2)
Specific hydraulic conductivity (Ks), as a measure of the porosity of the xylem on a cross-sectional area basis, was calculated from:
Ks= Ki/Aw, (3)
where Aw is the cross-sectional area of xylem tissue.
Leaf specific hydraulic conductivity (LSC), providing infor-mation about the hydraulic efficiency of seedling stems on a leaf dry weight basis, was derived from:
LSC = Ki/DL, (4)
where DL is the dry weight of whole foliage fed by the stem. The Huber value (HV), which provides information about the investment in stem tissue per distal foliage, was computed as the conducting cross-sectional area of the stem divided by the weight of oven-dried leaves:
HV = Aw/DL. (5)
Terpene composition
the carrier gas at a flow rate of 1 ml min−1, an injector tempera-ture of 130 °C and a detector temperature of 230 °C. The column oven was maintained at 50 °C isothermal for 3 min after injection and then increased in 3 °C increments to 170 °C, which were held constant for 5 min. Terpenes were identified by comparison of their retention times with those of standards under the same conditions.
Statistical analysis
Data were subjected to analysis of variance and Duncan’s multiple range test with P < 0.05, using individual seedlings as observations.
Ten monoterpenes and one sesquiterpene (β-caryophyllene) were present in sufficient quantities to be considered in the statistical analysis. The amount of each monoterpene was expressed as a percentage of total monoterpenes, whereas the β-caryophyllene concentration was expressed as a percentage of total terpenes. Percentages of various components were transformed to arcsin-square root functions on the plot mean basis to fulfil the normality assumption. The transformed plot means were used for analysis of variance and discriminant function analysis.
Results
Transpiration, leaf conductance and soil-to-leaf hydraulic conductance
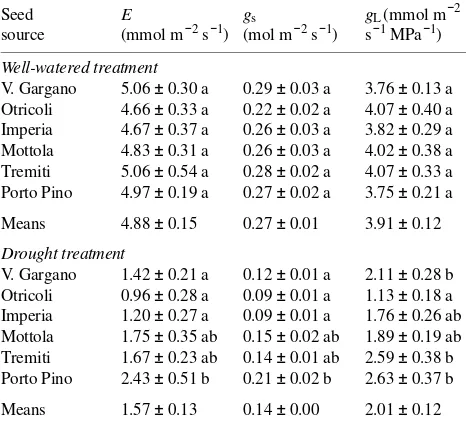
Taking all provenances together, values of E and gs were more than twice as large for well-watered seedlings (4.88 and 0.27 mol m−2 s−1, respectively) as for drought-stressed seedlings (1.57 and 0.06 mol m−2 s−1, respectively) (P < 0.05) (Table 2). There were also significant differences in E and gs between drought-stressed seedlings and well-watered seedlings of the same seed origin. Seed source differences in E and gs were not significant among well-watered seedlings. Among drought-stressed seedlings, those of xeric origins (Tremiti, Mottola and Porto Pino) displayed higher E and gs than seedlings from mesic origins (Imperia, Otricoli and Vico del Gargano). Among the xeric provenances, seedlings originating from the southernmost provenance, Porto Pino, showed the smallest drought-induced reductions in E and gs.
For all provenances combined, well-watered seedlings had significantly greater gL than drought-stressed seedlings (3.91 versus 2.01 mmol m−2 s−1 MPa−1) (P < 0.05) (Table 2) as a result of lower E in the drought-stressed seedlings. There were also significant differences in gL between drought-stressed seedlings and well-watered seedlings of the same seed origin. Water potential gradients were similar in seedlings of both treatments because xylem water potentials were lower in drought-stressed seedlings than in well-watered seedlings. Al-though seed source differences in gL were not significant among the well-watered seedlings, differences were detected among seed sources in the drought treatment. Among prove-nances, seedlings of the Porto Pino provenance showed the smallest drought-induced reductions in gL.
Pressure--volume curves
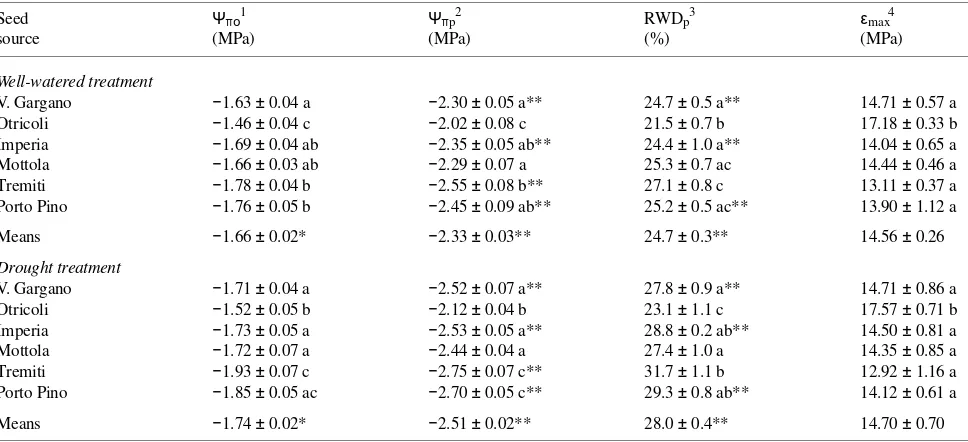
For all provenances combined, drought-stressed seedlings dif-fered significantly (P < 0.05) from well-watered seedlings in Ψπp (−2.33 versus −2.51 MPa) and RWDp (25 versus 28%) (Table 3), whereas treatment differences in Ψπo were small (P = 0.05) (−1.66 versus −1.74 MPa), and there were no sig-nificant treatment effects on εmax (14.56 versus 14.70 MPa).
Provenance differences in the measured parameters were detected in both well-watered and drought-stressed seedlings (Table 3). Among provenances, seedlings from the interior and mesic seed source Otricoli showed the highest Ψπo, Ψπp and εmax and the lowest RWDp. No differences in Ψπp were found between well-watered and drought-treated seedlings from the Otricoli and Mottola seed sources, and no differences in RWDp were detected between well-watered and drought-stressed seedlings from the Otricoli, Tremiti and Mottola seed sources. However, none of these traits showed a clear geographic pat-tern, although the insular and xeric provenances (Tremiti and Porto Pino) displayed an opposite tendency from the mesic seed sources (Imperia, Otricoli and Vico del Gargano). Among provenances, drought-stressed seedlings of the Tremiti prove-nance displayed the greatest osmotic adjustment at saturation (0.15 MPa). The treatments affected Ψπo and εmax of seedlings from each provenance similarly.
Hydraulic architecture
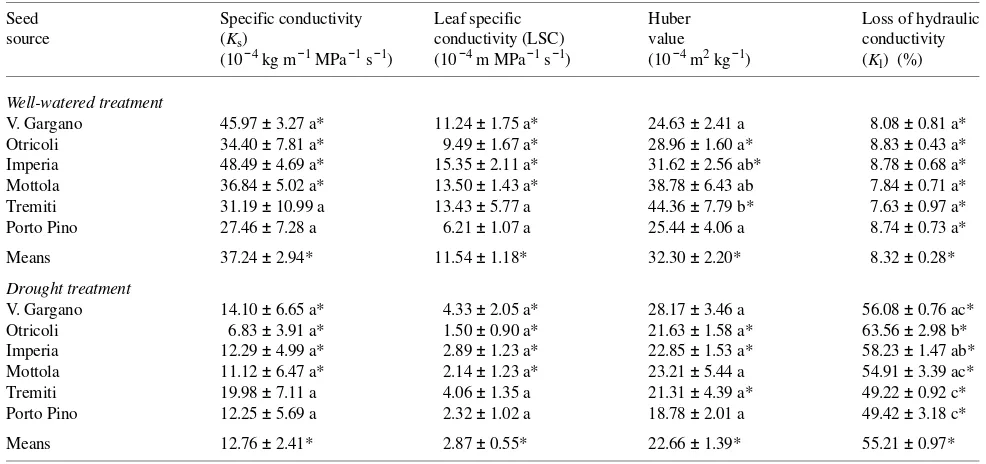
For all provenances combined, well-watered seedlings differed significantly (P < 0.05) from drought-stressed seedlings in Ks (37.24 × 10−4 versus 12.76 × 10−4 kg m−1 MPa−1 s−1), LSC
Table 2. Leaf transpiration rate (E), leaf conductance (gs) and
soil-to-leaf hydraulic conductance (gL) for well-watered and drought-stressed
seedlings of Aleppo pine. Means± SE (n = 8; at least two shoots per plant were sampled concurrently). Statistically significant seed source differences (P < 0.05) within a treatment are indicated by different letters. Within seed sources, the difference between treatments is significant in all cases (P < 0.05).
(11.54 × 10−4 versus 2.87 × 10−4 kg m−1 MPa−1 s−1), HV (32.30 × 10−4 versus 22.66 × 10−4 m2 kg−1) and Kl (8.32 versus 55.21%) (Table 4).
No seed source differences were detected among seedlings in either treatment for Ks and LSC (Table 4), although Otricoli seedlings usually had the lowest Ks and LSC values, and the Tremiti and Porto Pino seedlings displayed smaller drought-induced reductions in specific conductivity and leaf specific conductivity, respectively, than seedlings from other prove-nances. Seed source differences in HV were present only in well-watered trees, whereas provenance differences in Kl were detected only in drought-stressed seedlings. Among prove-nances, seedlings from insular and xeric seed sources (Tremiti and Porto Pino) were the least embolized, whereas seedlings from internal and mesic seed source (Otricoli) were the most embolized.
Monoterpene composition
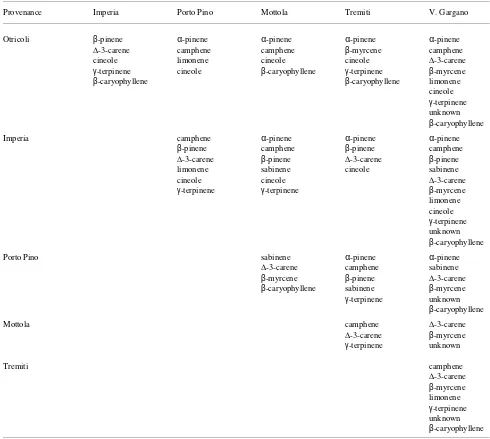
Among the terpenes, α-pinene (40.70%), β-myrcene (31.04%) and ∆-3-carene (19.70%) were the major constituents, whereas β-pinene, an unknown compound and β-caryophyllene were minor constituents, respectively with 4.10, 1.92 and 1.46% (Table 5). None of the other components detected represented more than 1% of the total terpene fraction of cortical oleoresin. There were highly significant differences among the nances for all of the compounds (Table 6). Among prove-nances, seedlings of the southernmost provenances (Tremiti, Vico del Gargano, Mottola and Porto Pino) contained higher proportions of α-pinene, limonene and β-caryophyllene,
whereas a large number of seedlings from the Imperia and Otricoli provenances had high amounts of β-myrcene.
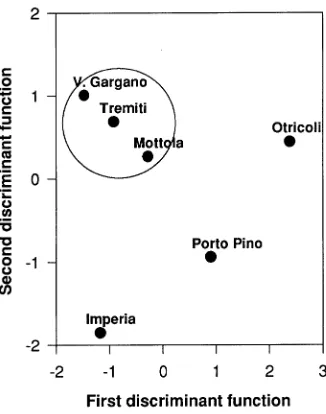
Duncan’s test indicated clear discrimination among prove-nances for many compounds (Table 7). The positions of the six seed sources on the plane of the first two canonical axes suggest four groupings, corresponding to the pooled southeast-ern provenances (Tremiti, Vico del Gargano and Mottola) and the other individually separated provenances (Figure 2).
Discussion
Regardless of origin, seedlings repeatedly exposed to drought had lower leaf conductances and rates of transpiration than well-watered seedlings. Because all measurements were re-corded under steady-state conditions of high xylem pressure potential, the differences in E and gs (and in components of water potential and hydraulic architecture parameters) be-tween well-watered and drought-stressed seedlings reflect physiological or morphological responses to soil water avail-ability during the drought treatment, rather than subsequently when measurements were made (Seiler and Johnson 1985, Bongarten and Teskey 1986, Teskey et al. 1987). Reduced stomatal aperture in response to drought is consistent with the hypothesis that complete stomatal closure is not the optimal response to drought and that limited embolism allows a maxi-mization of gas exchange (Jones and Sutherland 1991).
Differences in transpiration rates among drought-stressed seedlings from the various provenances provide evidence that, in Aleppo pine, drought resistance increased from mesic to
Table 3. Shoot tissue water relations for well-watered and drought-stressed seedlings of Aleppo pine. Means ± SE (n = 8). Statistically significant seed source differences (P < 0.05) within a treatment are indicated by different letters. Significant differences between treatments are indicated by asterisks: * = P = 0.05; ** = P < 0.05.
Seed Ψπο1 Ψπp2 RWDp3 εmax4
source (MPa) (MPa) (%) (MPa)
Well-watered treatment
V. Gargano −1.63 ± 0.04 a −2.30 ± 0.05 a** 24.7 ± 0.5 a** 14.71 ± 0.57 a Otricoli −1.46 ± 0.04 c −2.02 ± 0.08 c 21.5 ± 0.7 b 17.18 ± 0.33 b Imperia −1.69 ± 0.04 ab −2.35 ± 0.05 ab** 24.4 ± 1.0 a** 14.04 ± 0.65 a Mottola −1.66 ± 0.03 ab −2.29 ± 0.07 a 25.3 ± 0.7 ac 14.44 ± 0.46 a Tremiti −1.78 ± 0.04 b −2.55 ± 0.08 b** 27.1 ± 0.8 c 13.11 ± 0.37 a Porto Pino −1.76 ± 0.05 b −2.45 ± 0.09 ab** 25.2 ± 0.5 ac** 13.90 ± 1.12 a Means −1.66 ± 0.02* −2.33 ± 0.03** 24.7 ± 0.3** 14.56 ± 0.26
Drought treatment
V. Gargano −1.71 ± 0.04 a −2.52 ± 0.07 a** 27.8 ± 0.9 a** 14.71 ± 0.86 a Otricoli −1.52 ± 0.05 b −2.12 ± 0.04 b 23.1 ± 1.1 c 17.57 ± 0.71 b Imperia −1.73 ± 0.05 a −2.53 ± 0.05 a** 28.8 ± 0.2 ab** 14.50 ± 0.81 a Mottola −1.72 ± 0.07 a −2.44 ± 0.04 a 27.4 ± 1.0 a 14.35 ± 0.85 a Tremiti −1.93 ± 0.07 c −2.75 ± 0.07 c** 31.7 ± 1.1 b 12.92 ± 1.16 a Porto Pino −1.85 ± 0.05 ac −2.70 ± 0.05 c** 29.3 ± 0.8 ab** 14.12 ± 0.61 a Means −1.74 ± 0.02* −2.51 ± 0.02** 28.0 ± 0.4** 14.70 ± 0.70
1 Ψ
πο = osmotic potential at saturation.
2 Ψ
πp = osmotic potential at incipient plasmolysis. 3
RWDp = relative water deficit at incipient plasmolysis. 4 ε
xeric sites of origin. Drought-stressed seedlings of prove-nances originating from regions of severe summer drought (Tremiti, Mottola and Porto Pino) had higher leaf conduc-tances and transpiration rates than seedlings originating from seed sources from wetter locations (Imperia, Otricoli and Vico
del Gargano), indicating that seedlings of xeric provenances may be better able to adjust their osmotic potentials. Among provenances, seedlings of the Porto Pino provenance showed the smallest drought-induced reductions in leaf conductance and transpiration rate. Because of the threshold-type
relation-Table 5. Average terpene composition (%) of cortical oleoresin from seedlings of different Aleppo pine provenances. The amount of each monoterpene is expressed as a percentage of total monoterpenes, whereas the β-caryophyllene concentration is expressed as a percentage of total terpenes.
Seed source α-Pinene1 Camphene β-Pinene Sabinene ∆-3-Careneβ-Myrcene Limonene Cineole γ-Terpinene Unknownβ-Caryophyllene
V. Gargano 50.67 0.71 4.12 0.45 24.74 15.07 1.30 0.45 0.27 2.09 2.05 Otricoli 30.52 0.49 4.14 0.30 18.65 42.22 0.37 0.99 0.31 1.93 1.23 Imperia 36.17 0.40 3.86 0.24 21.34 35.09 0.29 0.25 0.15 1.89 0.90 Mottola 42.62 0.82 4.16 0.44 20.87 27.55 0.67 0.53 0.31 1.95 1.79 Tremiti 46.36 0.42 4.01 0.29 18.10 27.86 0.43 0.45 0.16 1.81 1.55 Porto Pino 37.20 0.83 4.25 0.23 17.53 35.91 1.32 0.51 0.32 1.86 1.28 Means 40.70 0.60 4.10 0.32 19.70 31.04 0.72 0.55 0.26 1.92 1.46
1 Compounds are listed in order of elution.
Table 6. Results of analyses of variance (F tests) for transformed relative proportions of terpene in seedlings of different Aleppo pine provenances. Source of α-Pinene Camphene β-Pinene Sabinene ∆-3-Careneβ-Myrcene Limonene Cineole γ-Terpinene Unknownβ-Caryophyllene variation
Among 563.171 11.081 0.911 6.661 89.991 1149.801 23.671 25.021 5.081 1.05 22.041 provenances
Within 39.89 1.16 0.12 1.65 9.15 71.35 6.89 0.58 1.55 0.25 1.72 provenances
1 Significant at P < 0.01.
Table 4. Stem hydraulic architecture and loss of hydraulic conductivity for well-watered and drought-stressed seedlings of Aleppo pine. Means ± SE (n = 8). Statistically significant seed source differences (P < 0.05) within treatments are indicated by different letters. An asterisk indicates significant differences (P < 0.05) between well-watered and drought treatment means.
Seed Specific conductivity Leaf specific Huber Loss of hydraulic source (Ks) conductivity (LSC) value conductivity
(10−4 kg m−1 MPa−1 s−1) (10−4 m MPa−1 s−1) (10−4 m2 kg−1) (Kl) (%)
Well-watered treatment
V. Gargano 45.97 ± 3.27 a* 11.24 ± 1.75 a* 24.63 ± 2.41 a 8.08 ± 0.81 a* Otricoli 34.40 ± 7.81 a* 9.49 ± 1.67 a* 28.96 ± 1.60 a* 8.83 ± 0.43 a* Imperia 48.49 ± 4.69 a* 15.35 ± 2.11 a* 31.62 ± 2.56 ab* 8.78 ± 0.68 a* Mottola 36.84 ± 5.02 a* 13.50 ± 1.43 a* 38.78 ± 6.43 ab 7.84 ± 0.71 a* Tremiti 31.19 ± 10.99 a 13.43 ± 5.77 a 44.36 ± 7.79 b* 7.63 ± 0.97 a* Porto Pino 27.46 ± 7.28 a 6.21 ± 1.07 a 25.44 ± 4.06 a 8.74 ± 0.73 a* Means 37.24 ± 2.94* 11.54 ± 1.18* 32.30 ± 2.20* 8.32 ± 0.28*
Drought treatment
ship between leaf conductance and net photosynthesis in coni-fers (Teskey et al. 1986), photosynthetic capacity may be sacrificed as a result of reduced leaf conductance particularly in mesic seed sources. However, the well-watered seedlings did not show any geographical discrimination attributable to climate.
The soil-to-leaf hydraulic conductance measurements showed that, following recovery from drought, drought-stressed seedlings had lower rates of water movement from roots to leaves than well-watered seedlings. In whole or in part, this difference must reflect the observed difference between the treatments in leaf conductance. The reduction in leaf con-ductance caused by drought-stress may have been caused by cavitation in water conducting tracheids leading to a decrease in hydraulic conductance of roots, stem or leaves (Sperry and Tyree 1988). Among provenances, the greatest reduction in
soil-to-leaf hydraulic conductance caused by drought stress was in Otricoli seedlings.
Although the drought treatment had little effect on osmotic potential at saturation, drought-stressed seedlings had lower osmotic potentials and relative water deficits at incipient plas-molysis than well-watered seedlings (cf. Seiler and Johnson 1985, Bongarten and Teskey 1986), indicating that recurring droughts resulted in a persistent alteration of the osmotic potential of Aleppo pine seedlings. Well-watered seedlings had low osmotic potentials, indicating that Aleppo pine seedlings have intrinsically low osmotic potentials. Although osmotic potentials declined in drought-stressed seedlings, elastic modulus remained constant. Similar behavior has been re-ported in other conifers, e.g., Tsuga heterophylla (Raf.) Sarg. by Kandico et al. (1980). Because stomatal activity is closely related to turgor maintenance, turgor and turgor-related
proc-Table 7. Terpenes differing significantly (P < 0.05 ) in content between pairs of Aleppo pine provenances according to Duncan’s test.
Provenance Imperia Porto Pino Mottola Tremiti V. Gargano
Otricoli β-pinene α-pinene α-pinene α-pinene α-pinene
∆-3-carene camphene camphene β-myrcene camphene cineole limonene cineole cineole ∆-3-carene
γ-terpinene cineole β-caryophyllene γ-terpinene β-myrcene
β-caryophyllene β-caryophyllene limonene cineole
γ-terpinene unknown
β-caryophyllene Imperia camphene α-pinene α-pinene α-pinene
β-pinene camphene β-pinene camphene
∆-3-carene β-pinene ∆-3-carene β-pinene limonene sabinene cineole sabinene cineole cineole ∆-3-carene
γ-terpinene γ-terpinene β-myrcene limonene cineole
γ-terpinene unknown
β-caryophyllene
Porto Pino sabinene α-pinene α-pinene
∆-3-carene camphene sabinene
β-myrcene β-pinene ∆-3-carene
β-caryophyllene sabinene β-myrcene
γ-terpinene unknown
β-caryophyllene
Mottola camphene ∆-3-carene
∆-3-carene β-myrcene
γ-terpinene unknown
Tremiti camphene
∆-3-carene
β-myrcene limonene
γ-terpinene unknown
esses may be maintained at lower shoot xylem water potentials in drought-stressed Aleppo pine seedlings than in well-watered seedlings as a result of decreased osmotic potentials (Kramer 1983).
It is unclear whether osmotic adjustment delays stomatal closure during drought (Abrams 1988). Among provenances, drought-stressed Otricoli seedlings had the highest osmotic potentials and the lowest leaf conductances and rates of tran-spiration, suggesting that gas exchange may be partially lim-ited by a lack of osmotic adjustment. On the other hand, each provenance may have an inherent relative gas exchange capac-ity under drought conditions. Of the provenances studied, Tremiti, and to a lesser extent Porto Pino, seedlings displayed the greatest degree of drought tolerance, as evidenced by greater osmotic adjustment at saturation (0.15 MPa).
Relative water deficits at incipient plasmolysis differed among the provenances. In drought-stressed seedlings of mesic or xeric genotypes, higher or lower values of osmotic potential and maximum modulus of elasticity contributed to a lower or higher relative water deficit at incipient plasmolysis, respectively. In severely drought-stressed seedlings of Otricoli provenance, inelastic cell walls might increase water extrac-tion from dry soil by producing a steeper water potential gradient within the plant (Hinckley et al. 1983, Meinzer et al. 1986). If the low relative water deficit at incipient plasmolysis indicates a greater sensitivity to tissue desiccation in seedlings of the Otricoli provenance than in more xeric provenances, then a high modulus of elasticity might help these plants maintain a high leaf water content.
Seedlings responded to drought by producing less xylem tissue in the stem as indicated by decreases in Huber value, leaf specific conductivity and specific conductivity. However, in the most xeric Tremiti and Porto Pino provenances, the drought treatment did not significantly decrease specific conductivity and leaf specific conductivity, respectively, suggesting that
high hydraulic permeability and efficiency are associated with resistance to cavitation, because seedlings of these two nances were less embolized than seedlings of the other prove-nances. In contrast, Cochard (1992) found that Cedrus atlantica (Endl.) Carr. had a high permeability but a low vulnerability to xylem embolism.
Drought caused a marked reduction in Huber values of Tremiti seedlings, perhaps indicating a drought-induced in-crease in allocation of biomass to leaves and roots. Trees from xeric environments often allocate a larger portion of dry matter to roots than trees from mesic environments. Cuccui et al. (1996), who studied 1-year-old seedlings from the same prove-nances, found that Porto Pino seedlings had the lowest height increments and height/diameter ratios and that Otricoli seed-lings had the highest height increments and height/diameter ratios and also the largest root collar diameters. We found that drought increased Huber values of Vico del Gargano seedlings. Huber values may be positively related to leaf specific conduc-tivity, because both parameters are related to the amount of foliage sustained by the stem.
Drought-stressed seedlings showed a more than 50% loss of hydraulic conductivity and negligible nocturnal refilling (Bor-ghetti et al. 1989, Jackson et al. 1995). This high loss may be explained by the combined effects of recurring drought and high concentrations of dissolved carbon dioxide in the xylem sap, because measurements were made during the growth period when the transpiration stream is especially prone to cavitation (Peña and Grace 1986). Recurring drought may also have caused irreversible sealing of bordered pits (Sperry and Tyree 1990). Conifers have been found to refill tracheids within a few days after rewatering in several studies, but not in others (Peña and Grace 1986, Borghetti et al. 1991, Sobrado et al. 1992, Jackson et al. 1995). Sperry and Tyree (1990) suggest that conifers lack the ability to refill cavitated tracheids be-cause they cannot generate a positive xylem pressure. Well-watered seedlings showed a low percentage loss of hydraulic conductivity in the range of native and structural embolisms (Tyree and Ewers 1991), with no differences among geno-types. Differences in percent loss of hydraulic conductivity among drought-stressed seedlings were consistent with cli-matic differences among seed sources and with the pattern of reductions in transpiration rates. Variations in stem diameters, tracheid sizes and pit membrane flexibility, which are known to vary along geographical gradients, could account for the genotype differences (Tyree et al. 1984, Sperry and Tyree 1990, Cochard 1992, Borghetti et al. 1993, Jackson et al. 1995). For example, the lower growth rate of seedlings of xeric seed sources may have permitted a relatively higher flexibility of the margo’s strands (Cochard 1992).
There was no clear relationship between terpene variability and the other traits investigated; however, a weak correlation was found between terpene variability and physiological pa-rameters of seedlings originating from seed sources from xeric habitats. The proportions of α-pinene, β-myrcene, limonene and the sesquiterpene β-caryophyllene showed a tendency for clinal patterns with the geographical distribution of the prove-nances. This trend was less evident when climate rather than
latitude of origin was considered. Adaption to local environ-mental conditions might be important in determining prove-nance genetic structure, and the observed variation in our study material could be interpreted as ecotypic variation. However, a significant role of geographical isolation in influencing the observed pattern of genetic variation cannot be ruled out.
Discriminant analysis confirmed that southeastern seed sources (Tremiti, Vico del Gargano and Mottola) are closely related, whereas the other seed sources are distinct from each other (cf. Schiller et al. 1986, Baradat et al. 1989, Baradat et al. 1996). Seedlings of the southeastern provenances (Tremiti, Vico del Gargano and Mottola) had lower amounts of β -myr-cene and larger amounts of limonene than seedlings of the nothern provenances (Imperia and Otricoli), whereas seedlings of the Porto Pino provenance had higher amounts of both monoterpenes. In Aleppo pine, both of these monoterpenes are associated with susceptibility to attack by Matsucoccus josephi (Bodenh.) Harpaz (Homoptera: Matsucoccidae) (Schiller and Grunwald 1987, Mendel and Schiller 1993). The provenances with lower amounts of β-myrcene and higher amounts of limonene have reduced susceptibility to the pest (Schiller and Grunwald 1987). However, Schiller and Grun-wald (1987) found that other terpenes, which were not detected in the trees we sampled, are also related to increased injury by this phytophagous insect.
In conclusion, although we found geographic variations in Aleppo pine seedlings along a latitudinal pattern, as revealed by terpene markers, seed source differences in the examined physiological traits were minor compared to the effects of the drought treatment. Many of the drought-induced physiological differences were consistent with the geographic location of the seed sources, particularly when seedlings from Otricoli (mesic extreme of the species’ range in Italy) were compared with seedings from Tremiti and Porto Pino (xeric extremes of the species’ range in Italy). These drought-induced traits may result in greater resistance to desiccation in seedlings of xeric provenances than in seedlings of mesic provenances.
Acknowledgments
We thank C. Boggi for her kind assistance in the laboratory. We are indebted to A. Tani, A. Maltoni and A. Pierguidi (Institute of Silvicul-ture, University of Florence, Italy) for assisting in seedling estab-lishment and maintenance, and for encouraging the research. We also thank A.E. Squillace and J.D. Johnson (School of Forest Resources and Conservation, University of Florida, USA) for helpful discussion and comments on the manuscript.
References
Abrams, M.D. 1988. Sources of variation in osmotic potentials with special reference to North American tree species. For. Sci. 34:1030--1046.
Abrams, M.D., M.E. Kubiske and K.C. Steiner. 1990. Drought adap-tations and responses in five genotypes of Fraxinus pennsylvanica
Marsh.: photosynthesis, water relations and leaf morphology. Tree Physiol. 6:305--315.
Adams, R.P., T. Demeke and H.A. Abulfatith. 1993. RAPD DNA fingerprints and terpenoids: clues to past migrations of Juniperus in Arabia and east Africa. Theor. Appl. Genet. 87:22--26.
Baradat, Ph. and R. Yazdani. 1988. Genetic expression for monoter-penes in clones of Pinus sylvestris grown on different sites. Scand. J. For. Res. 3:25--36.
Baradat, Ph., M. Michelozzi and M. Lambardi. 1989. Terpene compo-sition in four Italian provenances of Aleppo pine (Pinus halepensis
Mill.). J. Genet. Breed. 43:195--200.
Baradat, Ph., M. Michelozzi, R. Tognetti, M.L. Khouja and A. Khaldi. 1996. Geographical variation of Pinus halepensis Mill. In Popula-tion Genetics and Genetic ConservaPopula-tion of Forest Trees. Eds. Ph. Baradat, W.T. Adams and G. Müller-Starck. SPB Academic Pub-lishing, Amsterdam, pp 141--158.
Bongarten, B.C. and R.O. Teskey. 1986. Water relations of loblolly pine seedlings from diverse geographic origins. Tree Physiol. 1:265--276.
Borghetti, M., A. Raschi and J. Grace. 1989. Ultrasound emission after cycles of water stress in Picea abies. Tree Physiol. 5:229--237. Borghetti, M., W.R.N. Edwards, J. Grace, P.J. Jarvis and A. Raschi.
1991. The refilling of embolised xylem in Pinus sylvestris L. Plant Cell Environ. 14:357--369.
Borghetti, M., S. Leonardi, A. Raschi, D. Snyderman and R. Tognetti. 1993. Ecotypic variation of xylem embolism, phenological traits, growth parameters and allozyme characteristics in Fagus sylvatica. Funct. Ecol. 7:713--720.
Braatne, J.H., T.M. Hinckley and R.F. Stettler. 1992. Influence of soil water on the physiological and morphological components of plant water balance in Populus trichocarpa, Populus deltoides and their F1 hybrids. Tree Physiol. 11:325--339.
Calamassi, R. 1986. Characterisation de quelques provenances de
Pinus halepensis sur la base de la structure anatomique et mor-phologique des aguilles. Ann. Sci. For. 43:281--289.
Cochard, H. 1992. Vulnerability of several conifers to air embolism. Tree Physiol. 11:73--83.
Cuccui, I., A. Maltoni and A. Tani. 1996. Indagini su semi e semenzali di provenienze Italiane di Pinus halepensis Mill. Monti e Boschi. 5:55--62.
Eccher, A., E. Fusaro and F. Righi. 1982. Primi risultati di prove a dimora sui pini Mediterranei della ‘‘sezione halepensis,’’ con parti-colare riferimento a Pinus eldarica Medw. Cellulosa e Carta 33:3--30.
Falusi, M., R. Calamassi and A. Tocci. 1983. Sensitivity of seed germination and seedling root growth to moisture stress in four provenances of Pinus halepensis Mill. Silvae Genet. 32:4--9. Hanover, J.W. 1992. Applications of terpene analysis in forest
genet-ics. New For. 6:159--178.
Hinckley, T.M., F. Duhme, A.R. Hinckley and H. Richter. 1983. Drought relations of shrub species: assessment of the mechanisms of drought resistance. Oecologia 59:344--350.
Jackson, G.E., J. Irvine and J. Grace. 1995. Xylem cavitation in Scots pine and Sitka spruce saplings during water stress. Tree Physiol. 15:783--790.
Jones, H.G. and R.A. Sutherland. 1991. Stomatal control of xylem embolism. Plant Cell Environ. 14:607--612.
Kandico, R.A., T. Timmis and J. Warral. 1980. Pressure--volume curves of shoots and roots of normal and drought conditioned western hemlock seedlings. Can. J. For. Res. 10:10--16.
Kramer, R.J. 1983. Water Relations of Plants. Academic Press, New York, 489 p.
Lang, K.J. 1994. Abies alba Mill.: differentiation of provenances and provenance groups by the monoterpene patterns in the cortex resin of twigs. Biochem. System. Ecol. 22:53--63.
Meinzer, F., P.W. Rundel, M.R. Sharifi and E.T. Nilson. 1986. Turgor and osmotic relations of the desert shrub Larrea tridentata. Plant Cell Environ. 9:467--475.
Mendel, Z. and G. Schiller. 1993. Biogeography of Matsucoccus josephi Bodenheimer et Harpaz in Crete and mainland Greece. Ann. Sci. For. 50:383--388.
Michelozzi, M., A.E. Squillace and T.L. White. 1990. Monoterpene composition and fusiform rust resistance in slash pine. For. Sci. 36:470--475.
Michelozzi, M., T.L. White, A.E. Squillace and W.J. Lowe. 1995. Monoterpene composition and fusiform rust resistance in slash and loblolly pines. Can. J. For. Res. 25:193--197.
Mirov, N.T. 1967. The genus Pinus. Ronald Press, New York, 602 p. Nahal, I. 1981. The Mediterranean climate from a biological
view-point. In Ecosystems of the World. Ed. F. Di Castri. Elsevier, Amsterdam, pp 63--86.
Peña, J. and J. Grace. 1986. Water relations and ultrasound emissions of Pinus sylvestris before, during and after a period of water stress. New Phytol. 103:515--524.
Raffa, K.F. and E.B. Smalley. 1995. Interaction of pre-attack and induced monoterpene concentrations in host conifer defense against bark beetle-fungal complexes. Oecologia 102:285--295.
Schiller, G. and C. Grunwald. 1987. Resin monoterpene in range wide provenance trials of Pinus halepensis Mill. in Israel. Silvae Genet. 36:109--114.
Schiller, G. and A. Brunori. 1992. Aleppo pine (Pinus halepensis
Mill.) in Umbria (Italy) and its relation to native Israeli populations. Isr. J. Bot. 41:123--127.
Schiller, G., M.T. Conkle and C. Grunwald. 1986. Local differentia-tion among Mediterranean populadifferentia-tions of Aleppo pine in their isoenzymes. Silvae Genet. 35:11--19.
Schulte, P.J. and T.M. Hinckley. 1985. A comparison of pressure--vol-ume curve data analysis techniques. J. Exp. Bot. 36:1590--1602. Seiler, J.R. and J.D. Johnson. 1985. Photosynthesis and transpiration
of loblolly pine seedlings as influenced by moisture-stress condi-tioning. For. Sci. 31:742--749.
Shumway, D.L., K.C. Steiner and M.D. Abrams. 1991. Effects of drought stress on hydraulic architecture of seedlings from five populations of green ash. Can. J. Bot. 69:2158--2164.
Sobrado, M.A., J. Grace and P.G. Jarvis. 1992. The limits of xylem embolism recovery in Pinus sylvestris L. Plant Cell Environ. 43:831--836.
Sperry, J.S. and M.T. Tyree. 1988. Mechanism of water-stress-induced xylem embolism. Plant Physiol. 88:581--587.
Sperry, J.S. and M.T. Tyree. 1990. Water-stress-induced xylem embo-lism in three species of conifers. Plant Cell Environ. 13:427--436. Sperry, J.S., J.R. Donnelly and M.T. Tyree. 1988. A method for
measuring hydraulic conductivity and embolism in xylem. Plant Cell Environ. 11:35--40.
Squillace, A.E. 1976. Analyses of monoterpenes of conifers by gas-liquid chromatography. In Modern Methods in Forest Genetics. Ed. J.P. Miksche. Springer-Verlag, Berlin, pp 120--157.
Stone, C. and P.E. Bacon. 1994. Relationship among moisture stress, insect herbivory, foliar cineole and the growth of river red gum
Eucalyptus camaldulensis. J. Appl. Ecol. 31:604--612.
Talbot, A.J.B., M.T. Tyree and J. Dainty. 1975. Some notes concerning the measurement of water potentials of leaf tissue with specific reference to Tsuga canadensis and Picea abies. Can. J. Bot. 53:784--788.
Teskey, R.O., J.A. Fites, L.J. Samuelson and B.C. Bongarten. 1986. Stomatal and nonstomatal limitations to net photosynthesis in Pinus taeda L. under different environmental conditions. Tree Physiol. 2:131--142.
Teskey, R.O., B.C. Bongarten, B.M. Cregg, P.M. Dougherty and T.C. Hennessey. 1987. Physiology and genetics of tree growth response to moisture and temperature stress: an examination of the charac-teristics of loblolly pine (Pinus taeda L.). Tree Physiol. 3:41--61. Tyree, M.T. and F.W. Ewers. 1991. The hydraulic architecture of trees
and other woody plants. New Phytol. 119:345--360.
Tyree, M.T., M.A. Dixon, E.L. Tyree and R. Johnson. 1984. Ultrasonic acoustic emissions from the sapwood of cedar and hemlock: an examination of three hypotheses regarding cavitations. Plant Physiol. 75:988--992.
Wang, J., N.E. Ives and M.J. Lechowicz. 1992. The relation of foliar phenology to xylem embolism in trees. Funct. Ecol. 6:469--475. Weinstein, A. 1989. Geographical variation and phenology of Pinus