Biofiltrasi Menggunakan Kultur
Saccharomyces Cerevisiae ATCC
9763 dan Ragi
Kering Instan dengan Media Komposit Karbon aktif dan Onggok untuk
Mengurangi gas ammonia pada Industri Karet
Winda Marthalia dan Devi Oktiani
Balai Riset dan Standardisasi Industri Bandar Lampung
Jl. By Pass Soekarno Hatta KM.1 Rajabasa Bandar Lampung Indonesia Telp. 0721-706353 e-mail: winda_agawa@yahoo.com
ABSTRAK
Penelitian ini bertujuan untuk memberikan alternatif penyelesaian permasalahan bau amonia pada industri karet. Biofiltrasi dalam skala laboratorium menggunakan Saccharomyces cerevisiae ATCC 9763 dan khamir kering instan menggunakan media campuran karbon aktif dan onggok (limbah padat industri tapioka) digunakan untuk menurunkan kadar amonia di udara. Penelitian ini mempelajari pengaruh jumlah inokulum terhadap efektivitas penurunan kadar amonia. Penelitian in membandingkan antara penggunaan isolat Saccharomyces cerevisiae ATCC 9763dan ragi kering instan yang mengandung Saccharomyces cerevisiae yang ada di pasaran. Dilakukan pengujian terhadap kadar amonia udara setelah proses biofiltrasi. Dilakukan penjerapan udara yang telah melewati kolom biofiltrasi menggunakan larutan asam sulfat dan selanjutnya dilakukan pengukuran kadar amonia pada larutan penjerap menggunakan metoda indofenol secara spektrofotometri. Hasil penelitian menunjukkan bahwa semakin banyak jumlah inokulum ragi yang digunakan maka semakin efektif penurunan amonia. Ragi kering instan lebih efektif dibandingkan dengan kultur
Saccharomyces cerevisiae ATCC 9763.Saccharomyces cerevisiae baik dalam bentuk kultur murni maupun dalam bentuk instan dapat menurunkan kadar amonia di udara.
BiofiltrationwithCultureof Saccharomyces cerevisiae ATCC 9763 andInstant
Dried Yeast withComposite Media ofActivated Carbon andTapioca Solid Waste
for ReductionofAmmonia Gas in Rubber Industry
Winda Marthalia and Devi Oktiani
Balai Riset dan Standardisasi Industri Bandar Lampung
Jl. By Pass Soekarno Hatta KM.1 Rajabasa Bandar Lampung Indonesia Telp. 0721-706353 e-mail: winda_agawa@yahoo.com
ABSTRACT
The objective of this research is to give the alternative solution related to ammonia gas odor in rubber industry. Biofiltration in laboratory scale with the culture of Saccharomyces cerevisiae ATCC 9763 and instant dried yeast with activated carbon and tapioca solid waste as media applied for the reduction of ammonia content in air. This research analyzed the effect of yeast inoculums volume on ammonia reduction. This research compared culture of Saccharomyces cerevisiae ATCC 9763 and instant dried yeast of Saccharomyces cerevisiae which commonly sold in the market. Ammonia gas in outlet from biofiltration process was absorbed in sulfuric acid solution, and ammonia content was analyzed based on indophenol method using spectrophotometry. The result indicated that the number of yeast increases the effectiveness of ammonia reduction. The instant dried yeast is more effective than culture of Saccharomyces cerevisiae ATCC 9763. Saccharomyces cerevisiae in culture formula and instant dried yeast can be used for lowering ammonia content in the air.
INTRODUCTION
Indonesia is one of the natural rubber production and exporting country. Indonesia produces
natural rubber intermediate products includes ribbed smoked sheets (RSS), air dried sheets (ADS),
block rubber, crepe rubber, crumb rubber, and concentrated rubber latex. The production process of
these products causes water and air pollution. The air pollution, especially the ammonia (NH3) odor,
arises from the production process and waste water. The manufacturing and storage process emit
various gases, vapors, fumes, and aerosols due to the leaching out of chemicals and high
temperature vulcanization (Jagadaleetal., 2015).
There are two variety of rubber sheet, RSS and ADS, its production process causes odor. The
main difference of ADS and RSS is in the drying process, ADS exploits air, while RSS uses smokes
(Dasetal., 2016). The main environment problem in the rubber sheet industry is the smoke from
fuel wood burning which caused by the presence of hazardous components such as polycyclic
aromatic hydrocarbons, while in the rubber latex industry, the waste water and the odor arises from
ammonia used for latex preservation (Tekasakul & Tekasakul, 2006). In rubber glove industry the
problem is the wastewater which is treated in the same way in rubber latex industry. The latex is
usually treated with 0.2% or 0.7% ammonia solution. The ammonia solution added to the rubber
latex is the source of a strong ammonia smell (Tekasakul & Tekasakul, 2006).
Ammonia is a colorless, toxic, reactive, and corrosive gas with a prickly odor. Ammonia
vapor is an irritant to the eyes and the respiratory tract, and acute exposure to high concentration
can cause death (Ganduetal., 2015).Previous studies about ammonia in air concerned about its odor,
environmental acidification, and pollution of ground and surface water because of its high
deposition velocity (Nanda etal., 2012). Ammonia, quantitatively, is the largest emission from
agricultural operations (Anejaetal., 2008). According to the ministry of environment’s regulation,
the ammonia content in air should be less than 2 ppm (KLH, 1996). The traditional methods for
treatment of ammonia are based on physical and chemical processes. Recently, biological process
has received much attention (Gandu etal., 2015). When ammonia is discharged into open
atmosphere, the odor mitigated within short distance through dispersion and dilution (Joshi etal.,
2000).
A biofilter is a column filled with the porous and humid packing material and indigenous
microorganisms or inoculated microorganisms that are able to degrade pollutants to the filter bed
media (Chetpattananondh etal., 2005). Biofilter is widely used for odor and air pollution treatment,
with high flow rates and pollutant concentration less than 1000 ppm (Zhuetal.,2016). Air pollutants
In gas biofiltration, the contaminated gas pass through biofilter, the pollutants are transported
into the biofilm where they are utilized by microbes as a carbon source, or an energy source, or
both. The organic contaminants are converted to odorless compounds such as CO2, water vapor, and
organic biomass (Chan, 2006). Biofiltration is classified into open or closed and vertical or
horizontal by gas flow direction (Chen & Hoff, 2009). This research was applied in laboratory
scale vertical open biofilter. The success of biofilter used for controlling odors is based on
absorption and regeneration process (Chen & Hoff, 2009). Odor gasses, passing through a biofilter,
are absorbed on the surface of the biofilter medium particles.
The performance of biofiltration depends on the medium that provide the optimal
environment conditions for the growth or living of microbial population. A good filter material
should have a large surface area, high water retention capacity without becoming saturated, low
bulk density, high porosity, structural integrity, and a buffer capacity towards acidification and high
contaminant loads (Chan, 2006).
The quality of the filter media has been reported as one of the key factors in biofilter
performance. Composite based media has been extensively used because of its cheap price and has
several microbial communities for degrading various pollutants (Pagans, Font, & Sanchez, 2007).
The choice and preparation of a proper inoculums to obtain a healthy population of
microorganisms is a fundamental for successful biofilter operation (Chan, 2006). When treating a
gas mixture with many components, different microbial species are active, and it is difficult to
anticipate the biofilter treatment result. Microorganisms with various substrates will convert the
easily degradable compounds at the inlet of the filter. The contaminants must be biodegradable and
non-toxic for the microbes. The highly soluble organic compounds with simple bond structures are
easy to remove compare to inorganic compounds (Chan, 2006).
The following are previous studies about biofiltration. The bacteria used in biofiltration, in
example Nitrosomonas europea(Chung, Ho, & Tseng, 2007), Nitrosomonas sp., Pseudomonas sp.,
Nitrosomonassp. for biofiltration (Kimet al., 2015), Planctomycetes for biofiltration (Keuter, 2011),
Thiobacillus sp. (Kim etal., 2003), active sludge (Lebreroetal., 2011), gas phase compost biofilter
(Maiaetal., 2012). Various media and packing bed used in biofiltration in example, ceramic beads
and molasses with bacterial consortium (Pseudomonas sp., Nitrosomonas sp., Nitrobacter sp.)
(Muter etal., 2014), microbial soil in biofiltration (Nelson & Bohn, 2011). The industrial waste
which contains organics and nutrients can be used as media or substrate, as an example commercial
The application of biofiltration in industry: the application of ammonia gas reduction in
livestock farm (Jinanan & Leungprasert, 2015), the application of biofiltration in cattle manure and
rice husk mixture (Kavyashree, Ramya, Sanjay, Chandan, Shilpa, & Rashmi, 2015), the biofilter
packed with mixed of top soil and rubber leaves at crumb rubber industry (Yanietal., 2012).
Previous studies about yeast in biofiltration, yeast for metal removal (Fogarty, 1998),
Saccharomyces cerevisiae for metal removal(Norris & Kelly, 1977), yeast biofilter and abattoir
wastewater (Rabahetal., 2011).
The biological treatment techniques for odor control have gained popularity in comparison to
traditional physical and chemical removal methods (Gopal etal., 2014). Biological treatment is an
attractive alternative for low concentration gas streams because of its low energy consumption,
relatively moderate operating cost, and minimal by-products generation (Kumaretal., 2011).
Biofiltration is advantageous because it does not require large amounts of energy during operations
and produce a relatively low toxicity waste stream (Kumar etal., 2013).
MATERIALS AND METHODS
Materials
Biofilter media was powder activated carbon and cassava solid waste. Cassava solid waste was
collected from the tapioca industry.
This experiment compared two different yeast, culture of Saccharomyces cerevisiaeATCC 9763
and instant dried yeast of Saccharomyces cerevisiaethat sold in the market and commonly used in
bakery.
Ammonia gas in this experiment was generated from Ammonia glacial 85% produced by
MERCK.
The chemical materials for analysis of ammonia content in biofilter gas outlet and chamber
included sodium nitropruside (Na2Fe(CN)5NO.2H2O 2%, sodium hydroxide 6.75M, sodium
hypochlorite 3.7%, phenol (C6H5OH) 45% v/v, buffer (50 gr Na3PO4.12H2O and 74 mL, NaOH
6.75 M diluted to 1000 mL), ammonia stock solution (3.18 gr NH4Cl diluted to 1000 mL with
distilled water). The air sampler absorbent made from sulfuric acid, the 3 mL sulfuric acid 97%
diluted to 1000 mL. The material based on SNI 19-7119.1-2005 (Badan Standardisasi Nasional,
Glassware, analytical laboratory equipment, and experiment tools
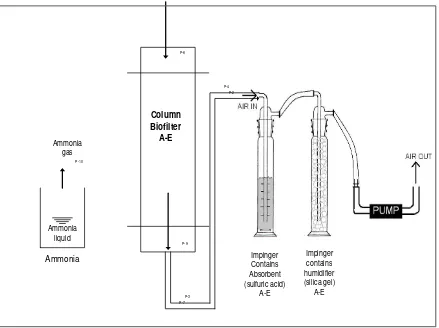
Biofilter columns made of polyvinyl chloride (PVC) pipe, 3 cm in diameter, and 20 cm in
height. The columns or pipes are opened in the upper end so that it is possible to come into constant
with the air contaminated with ammonia which represented the environment air. The lower end of
the columns or pipes are closed with the small hole which enough for the hose connecting the gas
outlet with the impinge air sampler.
Impinge air sampler, a set consists of 5 individual air samplers, each air sampler includes
pump 0.5, flow regulator, rotameter impinge for absorbent, and impinge for humidifier. Range of
air sampler was 0.5 – 3 L/minutes.
Glassware for analytical testing included erlenmeyer flask, pipette, volumetric flask,
magnetic stirrer, and petri dishes. UV visible spectrophotometer is needed for measure ammonia
content.
Methods
Starter yeast culture yeast preparation
This experiment compared two different yeasts: Saccharomyces cerevisiaeATCC 9763 and instant
dried yeast. Preparation Saccharomyces: 1 ose Saccharomyces cerevisiaeATCC 9763culture added
to 100 mL potato dextrose broth (PDB) media, incubated for 24 hours in 25°C. Preparation of
instant dried yeast: 1 gram of instant dried yeast added to 100 mL potato dextrose broth (PDB)
media, incubated for 24 hours in 25°C.
Calculated the colony number of yeast
1 mL starter yeast mixed with 9 mL Buffered Peptone Water (BPW) and then diluted to 10-9 times.
1 mL of each diluted culture transferred to 20 mL potato dextrose agar (PDA) media and then
incubated for 2 x 24 hours in temperature 25°C. The colony number of yeast calculated with
counting chamber.
The composit of media and yeast
There are four volume variations of Saccharomyces cerevisiae ATCC 9763 culture and instant dried
waste added to each sample (four variations and two types of Saccharomyces cerevisiae), then
incubated for 12 hours. Activated carbon is added to each sample and then homogenized. The
composite is transferred into biofilter column.
The Analysis of ammonia content
Ammonia concentration from the outlet gas biofilter was measured by indophenol testing
method based on SNI 19-7119.1-2005 (Badan Standardisasi Nasional, 2005).
Experimental Design
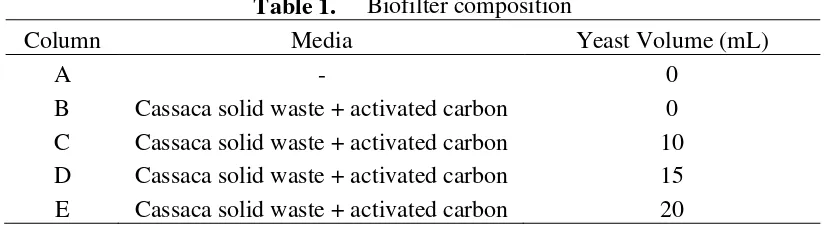
The objective of this experiment is to measure the outlet gas from 4 different composition of
biofilter with four volume variations of yeast. There are five biofilter columns (A-E) with different
biofilter composition.
Table 1. Biofilter composition
Column Media Yeast Volume (mL)
A - 0
B Cassaca solid waste + activated carbon 0
C Cassaca solid waste + activated carbon 10
D Cassaca solid waste + activated carbon 15
E Cassaca solid waste + activated carbon 20
Column A, column without media and bacteria, represents the air in the environment
without biofiltration, the contaminated ammonia air. The biofiltration process was operated for 90
minutes simultaneously for A-E in a chamber. Five impinge air sampler connected to biofilter gas
outlet (Figure 1), one impinger for each biofilter column. Ammonia in chamber generated from
liquid ammonia solution. Gas outlet was absorbed with sulfuric acid in impinge. The ammonia
absorbed was measured with indophenol methods based on SNI 19-7119.1-2005.
RESULT AND DISCUSSION
This research investigated the effect of microbial colony number which represents as
inoculums yeast volume on ammonia removal. The target of the biofiltration was that gas outlet
contains low ammonia, less than 2 ppm. The ammonia content of air in the chamber was
conditioned between 3-4 ppm by generating ammonia gas form liquid ammonia, this number
represents the common ammonia content in process and storage room rubber industry.
The experiment design included 5 biofilter columns: 1 column without biofiter (media)
which represents air without biofiltration process, and four columns which different in inoculums
bacteria volume (0 mL, 10 mL, 15 mL, and 20 mL). The media in this biofilter was 10 gram
activated carbon and 10 gram of cassava waste. The reason of choosing cassava waste as media was
because it contains carbon and nutrient for yeast and it is a natural mateial, low price resource,
available in a big quantity.
The air flow was set in 1 L/ minute based on the National Standard method of ambient air
sampling (SNI 19-7119.1-2005). The 1 L/minute represents the outdoor wind velocity which
mitigates air in outdoor environment or ambient air.
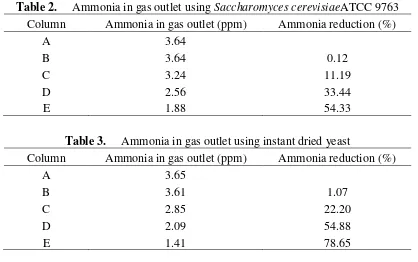
The finding indicates that the volume of inoculum bacteria has an effect on ammonia
absorption (Table 2). The ammonia concentration decreases as the additional volume of yeast. The
Table 2. Ammonia in gas outlet using Saccharomyces cerevisiaeATCC 9763
Column Ammonia in gas outlet (ppm) Ammonia reduction (%)
A 3.64
B 3.64 0.12
C 3.24 11.19
D 2.56 33.44
E 1.88 54.33
Table 3. Ammonia in gas outlet using instant dried yeast
Column Ammonia in gas outlet (ppm) Ammonia reduction (%)
A 3.65
B 3.61 1.07
C 2.85 22.20
D 2.09 54.88
E 1.41 78.65
Table 4. The number of yeast colony in biofilter after biofiltration process
Column Saccharomyces CerevisiaeATCC 9763 Instant Dried Yeast
B 1.59 x 108 1.72 x 108
C 1.62 x 109 1.84 x 109
D 2.40 x 109 2.70 x 109
E 3.10 x 109 3.21 x 109
In this biofilter system, the effectiveness of ammonia removal is affected by number or
volume yeast added, while activated carbon has no significant effect on ammonia removal. There is
no significant difference between ammonia content in experiment A and experiment B. The
function of cassava solid waste in this system is not as ammonia absorber, but as carbon source and
nutrient for bacteria and as water absorbent for maintaining moisture content, as water content has
effect on biofilter effectiveness.
The chamber temperature in this experiment was 35°C, it represents the natural temperature
of room in rubber industry, this experiment held on optimum temperature. The instant dried yeast
can be applied in the biofilter, especially in the warm tropical country without temperature
conditioning. The biofiltration in this experiment effectively reduced the ammonia content below 2
ppm. The Saccharomyces cerevisiaeATCC 9763 was not effective for reducing ammonia compared
to instant dried yeast, maybe because it cannot grow optimally in the experiment temperature 35°C,
while the instant dried yeast is processed to be applied in the warm temperature.
Biofiltration with instant dry yeast and the composite media of activated carbon and cassava
solid waste can be used for ammonia gas removal. The instant dried yeast is more applicable for the
biofilter system than Saccharomyces cerevisiae ATCC 9763culture.
REFERENCES
Aneja , V. P., Blunden, J., Roelle, P. A., Schlesinger, W. H., Knighton, R., Niyogi, D., et al. (2008).
Workshop on agricultural air quality: state of the science. Atmospheric Environment 42,
3195-3208.
Badan Standardisasi Nasional. (2005). Standar Nasional Indonesia (SNI) 19-7119.1-2005.. Udara
ambien- Bagian 1: Cara Uji kadar amoniak (NH3) dengan metoda indofenol menggunakan spektrofotometer. Jakarta: Badan Standardisasi Nasional.
Chan, A. A. (2006). Biofiltration of odours gas emissions (Doctoral Thesis). Lulea, Sweden: Lulea
University of technology.
Chen, L., & Hoff, S. (2009). Mitigating odors from agriculture facilities: a review of literature
concerning biofilters. American Society of Agricultural and Biological Engineers, 751-766.
Chetpattananondh, P., Nitipavachon, Y., & Bunyakan, C. (2005). Biofiltration of air contaminated
with methanol and toluene. Journal of Science and Technology 27(3), 761-773.
Chung, Y.-C., Ho, K.-L., & Tseng, C.-P. (2007). Two - stage biofilter for effective NH3 removal
from waste gases containing high concentration of H2S. Journal of the Air & Waste
Management Association 57(3), 337 - 347.
Das, D., Saha, A., & Bhattacharjee, H. (2016). Rubber processing is detrimental to environment: a
case study. International Journal of Scientific & Engineering Research 7(7), 369-376.
Fogarty, R. V. (1998). Toxicity and Biosorbtion of Metals by Saccharomyces cereviseae,
Amorphtheca resinae, and Azolla filiculoides (Master Thesis). Dublin 9, Ireland: School of Biological Sciences Dublin City University .
Gandu, B., Rao, G., Kranti K, & Sawmy, Y. (2015). Ammonia odours removal by gasphase
biofilter through nitrification and Anammox proceses. Journal of Chemical and
Pharmaceutical Research 7(10), 763-766.
Gopal, B., Rao, A., Devi, V., Rao, Y., & Babu, B. (2014). Biofilter for the purification of air
contaminated with tryethylamine (TEA). International Journal of Advanced Research,
295-302.
Jagadale, S. C., Rajkumar, K., Chavan, R. P., & Shinde, D. N. (2015). Environmental concern of
pollution in rubber industry. International Journal of Resesarch in Engineering and
Jinanan, J., & Leungprasert, S. (2015). Feasibility study of low cost biofilter to control ammonia
from livestock farms. International Journal of Research in Chemical, Metallurgical, and
Civil Engineering 2(1), 1-8.
Joshi, J. A., Hogan, J. A., Cowan, R. M., Strom , P. F., & Finstein , M. F. (2000). Biological
removal of gaseous ammonia in biofilters: space travel and earth-based applications. Journal
of
Kavyashree, B., Ramya, N., Sanjay, U. A., Chandan, K., Shilpa, B., & Rashmi, M. (2015).
Ammonia gas removal using biofilter. International Advanced Research Journal in Science,
Engineering, and Technology 2(7), 110 - 114.
Keuter, S. (2011). Characterization of nitrifying bacteria in marine recirculation aquaculture
systems with regard to process optimization (Dissertation). Hamburg: Departement Biologie der Universitat Hamburg.
Kim, B., Kim, J., Kim, D., & Park, J. (2015). Analysis of biofilter and microbial community change
under thetreatment of ammonia and toluene. International Conference on Biotechnology &
Environment Engineering (ICBNE '15) (pp. 32-33). Bangkok, Thailand: International Academy of Art, Science, and Technology.
Kim, C.-W., Park, J.-S., Cho, S.-K., Oh, K.-J., Kim, Y.-S., & Kim, D. (2003). Removal of hydrogen
sulfide, ammonia,and benzena by fluidized bed reactor and biofilter. Journal of
Microbiology and Biotechnology 13(2), 301-304.
Kumar, K. V., Sridevi, V., Harsha, N., Lakshmi, M. C., & Rani, K. (2013). Biofiltration and its
application in treatment of air and water polutants- a review. International Journal of
Application or Innovation in Engineering & Management 2(9), 226-231.
Kumar, T. P., Rahul, Kumar, M. A., & Chandrajit, B. (2011). Review paper. Biofiltration of volatile
Organic Compounds (VOCs)- an averview. Reearch Journal of Chemical Sciences 1(8),
83-92.
Kumar, V. R., Sukumaran, V., Achuthan, C., Joseph, V., Philip, R., & Singh, I. B. (2013).
Molecular characterization of the nitrifying bacterial consortia employed for the activation
of bioreactors used in brackish and marine aquaculture systems. International
Biodeterioration & Biodegradation 78, 74-81.
Lebrero, R., Rodriguez, E., Garcia-Encina, P., & Munoz, R. (2011). A comparative assessment of
biofiltration and activated sludge for odour abatement. Journal of Hazardous Materials,
622-630.
Maia, G., Day V, G., Gates, R., Taraba, J., & Coyne, M. (2012). Moisture effects on greenhouse
gases generation in nitrifying gas-phase compost biofilters. Water Research, 3023 - 3031.
Muter, O., Mihallova, A., Berzins, A., Shvirksts, K., Patmalnieks, A., Strikauska, S., et al. (2014). Optimization of nitrification process by a bacterial consortium in the submerged biofiltration
Nanda, S., Sarangi, P. K., & Abraham, J. (2012). Microbial biofiltration technology for odour
abatement: an introductory review. Journal of Soil Science and Environmental Management
3(2), 28-35.
Nanda, S., Sarangi, P. K., & Abraham, J. (2012). MIcrobial biofiltration technology for odour
abatement: ann introductory review. Journal of Soil Science and Environmental
Management 3(2), 28-35.
Nelson , M., & Bohn, H. (2011). Soil-based biofiltration for air purification: potentials for
environmental and space life support application . Journal of Environmental Protection 2,
1084 - 1094.
Norris, P., & Kelly, D. (1977). Accumulation of cadmium and cobalt by Saccharomyces cerevisiae.
Journal of General Microbiology (99), 317-324.
Pagans, E., Font, X., & Sanchez, A. (2005). Biofiltration for ammonia removal from composting
exhaust gas. Chemical Engineering Journal 113, 105-110.
Pagans, E., Font, X., & Sanchez, A. (2005). Biofiltration for ammonia removal from composting
exhaust gas. Chemical Engineering Journal, 105 - 110.
Pagans, E., Font, X., & Sanchez, A. (2007). Coupling composting and biofiltration for ammonia
and volatile organic compounds removal. Biosystems Engineering, 281-284.
Rabah, A. B., Ibrahim , M. L., Ijah, U. J., & Manga, S. B. (2011). Assessment of the efficient of a
yeast biofilter in the treatment of abattoir wastewater. African Journal of Biotechnologi,
9347-9352.
Rattanapan, C., & Ounsaneha, W. (2012). Removal of hydrogen sulfide gas using biofiltration -
Tekasakul , P., & Tekasakul, S. (2006). Environmental problems related to natural rubber
production in Thailand. Journal of Aerosol Research 21(2), 122-129.
Yani, M., Nurcahyani, P. R., & Rahayuningsih, M. (2013). Ammonia removal by biofiltration technique packed with coral and granulated activated carbon (GAC) inoculated with
enriched nitrifying bacteria. Jurnal Teknologi Industri pertanian 23(1), 22-29.
Yani, M., Purwoko, Ismayana, A., Nurcahyani, P. R., & Pahlevi, D. (2012). Penghilangan bau amoniak dari tempat penumpukan leum pada industri karet remah dengan menggunakan
teknik biofilter. Jurnal Ilmu Pertanian Indonesia 17(1), 58 -64.
Zhu, Y., Li, S., Luo, Y., Ma, H., & Wang, Y. (2016). A biofilter for treating toluene vapors: